Soursop (Annona muricata) Properties and Perspectives for Integral Valorization
Abstract
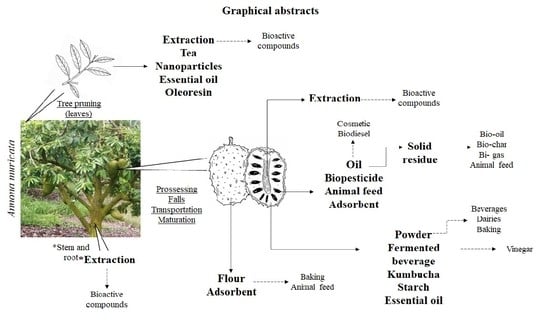
:1. Introduction
2. Characteristics of Soursop By-Products
3. Food Products from Soursop By-Products
3.1. Fermented Products
3.2. Dried Products
3.3. Tea
3.4. Essential Oil and Oleoresin
3.5. Oil
3.6. Starch
4. Non-Food Applications for Soursop By-Products
4.1. Pharmaceutical Products
4.2. Cosmetics
4.3. Animal Feed
4.4. Bio-Diesel, Bio-Oil, Bio-Char and Gas
4.5. Biopesticides
4.6. Adsorbers
5. Projection of the Recovery of Residues from the Soursop Production Chain and Perspectives for Future Research
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Badrie, N.; Schauss, A.G. Chapter 39—Soursop (Annona muricata L.): Composition, Nutritional Value, Medicinal Uses, and Toxicology. In Bioactive Foods in Promoting Health. Fruits and Vege-Tables; Watson, R.R., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA, 2010; pp. 621–643. [Google Scholar]
- Gajalakshmi, S.; Vijayalakshmi, S.; Rajeswari, V.D. Phytochemical and pharmacological properties of Annona muricata: A review. Int. J. Pharm. Sci. 2012, 4, 3–6. [Google Scholar]
- Coria-Téllez, A.V.; Montalvo-Gónzalez, E.; Yahia, E.M.; Obledo-Vázquez, E.N. Annona muricata: A comprehensive review on its traditional medicinal uses, phytochemicals, pharmacological activities, mechanisms of action and toxicity. Arab. J. Chem. 2018, 11, 662–691. [Google Scholar] [CrossRef] [Green Version]
- de Lima, M.C.; Alves, R.E. Soursop (Annona muricata L.). In Postharvest Biology and Technology of Tropical and Subtropical Fruits; Woodhead Publishing: Sawston, UK, 2011; pp. 363–392e. [Google Scholar]
- Gentile, C.; Mannino, G.; Palazzolo, E.; Gianguzzi, G.; Perrone, A.; Serio, G.; Farina, V. Pomological, Sensorial, Nutritional and Nutraceutical Profile of Seven Cultivars of Cherimoya (Annona Cherimola Mill). Foods 2021, 10, 35. [Google Scholar] [CrossRef]
- Sacramento, C.K.D.; Faria, J.C.; Cruz, F.L.D.; Barretto, W.D.S.; Gaspar, J.W.; Leite, J.B.V. Caracterização física e química de frutos de três tipos de gravioleira (Annona muricata L.). Rev. Brasil. Fruticult. 2003, 25, 329–331. [Google Scholar] [CrossRef] [Green Version]
- Nolasco-González, Y.; Hernández-Fuentes, L.M.; González, E.M. Caracterización morfológica y físicoquímica de frutos de acessiones de guanábanas selecionadas in Nayarit. Rev. Mex. Cienc. Agric. 2019, 23, 223–237. [Google Scholar]
- de Oliveira, E.N.; Santos, D.D.C.; Gomes, J.P.; Rocha, A.; Albuquerque, E. Estabilidade física e química de licores de graviola durante o armazenamento em condições ambientais. Rev. Brasil. Eng. Agríc. Amb. 2015, 19, 245–251. [Google Scholar] [CrossRef] [Green Version]
- São José, A.R.; Pires, M.D.M.; Freitas, A.L.G.E.D.; Ribeiro, D.P.; Perez, L.A. Atualidades e perspectivas das Anonáceas no mundo. Rev. Brasil. Fruticult. 2014, 36, 86–93. [Google Scholar] [CrossRef] [Green Version]
- Siqueira, A.M.O.; Moreira, A.C.C.G.; de Melo, E.A.; Stamford, T.C.M.; Stamford, T.L.M. Fibre dietary content, phenolic compounds and antioxidant activity in soursops (Annona muricata, L.). Rev. Brasil. Fruticult. 2015, 37, 1020–1026. [Google Scholar] [CrossRef]
- Calzavara, B.B.G.; Muller, C.H. Fruticultura Tropical: A Gravioleira (Annona muricata L). Embrapa Amazônia Oriental-Documentos (INFOTECA-E). 1987. Available online: https://www.infoteca.cnptia.embrapa.br/bitstream/doc/382561/1/DOCUMENTOS47CPATU.pdf (accessed on 2 July 2022).
- de Moraes, I.V.; Rabelo, R.S.; Júlia, A.D.L.; Hubinger, M.D.; Schmidt, F.L. Concentration of hydroalcoholic extracts of graviola (Annona muricata L.) pruning waste by ultra and nanofiltration: Recovery of bioactive compounds and prediction of energy consumption. J. Clean. Prod. 2018, 174, 1412–1421. [Google Scholar] [CrossRef] [Green Version]
- Caldeira, C.; Vlysidis, A.; Fiore, G.; De Laurentiis, V.; Vignali, G.; Sala, S. Sustainability of food waste biorefinery: A review on valorisation pathways, techno-economic constraints, and environmental assessment. Bioresour. Technol. 2020, 312, 123575. [Google Scholar] [CrossRef]
- de Souza, J.R.C.L.; Villanova, J.C.O.; de Souza, T.D.S.; Maximino, R.C.; Menini, L. Vegetable fixed oils obtained from soursop agro-industrial waste: Extraction, characterization and preliminary evaluation of the functionality as pharmaceutical ingredients. Environ. Technol. Innov. 2021, 21, 101379. [Google Scholar] [CrossRef]
- Pinto, A.D.Q.; Cordeiro, M.C.R.; Andrade, S.R.M.; Ferreira, F.R.; Filgueiras, H.A.; Alves, R.E. Annona Species. International Centre for Underutilised Crops; University of Southampton: Southampton, UK, 2005. [Google Scholar]
- Behl, S.; Inbanathan, A.; Sundaran, M.K.; Hussain, A. Plants of the genus Annona: Source of potential anti-cancer therapeutics. In Functional Foods and Nutraceuticals in Metabolic and Non-Communicable Diseases; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Jiménez, V.M.; Gruschwitz, M.; Schweiggert, R.M.; Carle, R.; Esquivel, P. Identification of phenolic compounds in soursop (Annona muricata) pulp by high-performance liquid chromatography with diode array and electrospray ionization mass spectrometric detection. Food Res. Int. 2014, 65, 42–46. [Google Scholar] [CrossRef]
- Meinhart, A.D.; Caldeirão, L.; Damin, F.M.; Teixeira Filho, J.; Godoy, H.T. Analysis of chlorogenic acids isomers and caffeic acid in 89 herbal infusions (tea). J. Food Comp. Anal. 2018, 73, 76–82. [Google Scholar] [CrossRef]
- Aguilar-Hernández, G.; García-Magaña, M.D.L.; Vivar-Vera, M.D.L.Á.; Sáyago-Ayerdi, S.G.; Sánchez-Burgos, J.A.; Morales-Castro, J.; Anaya-Esparza, L.; González, E.M. Optimization of ultrasound-assisted ex-traction of phenolic compounds from Annona muricata by-products and pulp. Molecules 2019, 24, 904. [Google Scholar] [CrossRef] [Green Version]
- Mesquita, P.C.; Rodrigues, L.G.G.; Mazzutti, S.; Silva, M.; Vitali, L.; Lanza, M. Intensified green-based extraction process as a circular economy approach to recover bioactive compounds from soursop seeds (Annona muricata L.). Food Chem. 2021, 12, 100164–100174. [Google Scholar] [CrossRef]
- Cárdenas, C.; Torres-Vargas, J.A.; Cárdenas-Valdivia, A.; Jurado, N.; Quesada, A.R.; García-Caballero, M.; Martínez-Poveda, B.; Medina, M.Á. Non-targeted metabolomics characterization of Annona muricata leaf extracts with an-ti-angiogenic activity. Biomed. Pharm. 2021, 144, 112263. [Google Scholar] [CrossRef]
- Nam, J.-S.; Park, S.-Y.; Jang, H.-L.; Rhee, Y.H. Phenolic compounds in different parts of young Annona muricata cultivated in Korea and their antioxidant ac-tivity. Appl. Biol. Chem. 2017, 60, 535–543. [Google Scholar] [CrossRef]
- Justino, A.B.; Miranda, N.C.; Franco, R.R.; Martins, M.M.; Silva, N.M.; Espindola, F.S. Annona muricata Linn. leaf as a source of antioxidant com-pounds with in vitro antidiabetic and inhibitory potential against α-amylase, α-glucosidase, lipase, non-enzymatic glycation and lipid peroxidation. Biomed. Pharm. 2018, 100, 83–92. [Google Scholar] [CrossRef]
- Ojo, O.A.; Grant, S.; Amanze, J.C.; Oni, A.I.; Ojo, A.B.; Elebiyo, T.C.; Obafemi, T.O.; Ayokunle, D.I.; Ogunlakin, A.D. Annona muricata L. peel extract in-hibits carbohydrate metabolizing enzymes and reduces pancreatic β-cells, in-flammation, and apoptosis via upregulation of PI3K/AKT genes. PLoS ONE 2022, 17, 1–10. [Google Scholar] [CrossRef]
- Adaramoye, O.A.; Oladipo, T.D.; Akanni, O.O.; Abiola, O.J. Hexane fraction of Annona muricata (Soursop) seed ameliorates testosterone-induced benign prostatic hyperplasia in rats. Biom. Pharmacotherapy 2019, 111, 403–413. [Google Scholar] [CrossRef]
- Afroz, N.; Hoq, M.A.; Jahan, S.; Islam, M.M.; Ahmed, F.; Shahid-Ud-Daula, A.F.M.; Hasanuzzaman, M.D. Methanol soluble fraction of fruits of Annona muricata possesses significant antidiarrheal activities. Heliyon 2020, 6, 3112–3122. [Google Scholar] [CrossRef] [Green Version]
- Agu, K.C.; Eluehike, N.; Ofeimun, R.O.; Abile, D.; Ideho, G.; Ogedengbe, M.O.; Onose, P.O.; Elekofehinti, O.O. Possible anti-diabetic potentials of Annona muricata (soursop): Inhibition of α-amylase and α-glucosidase activi-ties. Clin. Phytosci. 2019, 5, 21. [Google Scholar] [CrossRef]
- Adefegha, S.A.; Oyeleye, S.I.; Oboh, G. Distribution of Phenolic Contents, Antidiabetic Potentials, Antihypertensive Properties, and Antioxidative Ef-fects of Soursop (Annona muricata L.) Fruit Parts In Vitro. Biochem. Res. Int. 2015, 2015, 347673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizwana, H.; Bokahri, N.A.; Alsahli, S.A.; Al Showiman, A.S.; Alzahrani, R.M.; Aldehaish, H.A. Postharvest disease management of Alternaria spots on tomato fruit by Annona muricata fruit extracts. Saudi J. Biol. Sci. 2021, 28, 2236–2244. [Google Scholar] [CrossRef] [PubMed]
- Raybaudi-Massilia, R.; Suárez, A.; Arvelo, F.; Sojo, F.; Mosqueda-Melgar, J.; Zambrano, A.; Calderón-Gabaldón, M. An Analysis In-vitro of the Cytotoxic, Antioxidant and Antimicrobial Activity of Aqueous and Alcoholic Extracts of Annona muricata L. Seed and Pulp. Br. J. Appl. Sci. Technol. 2015, 5, 333–341. [Google Scholar] [CrossRef]
- Agu, K.C.; Okolie, N.P.; Falodun, A.; Engel-Lutz, N. In vitro anticancer as-sessments of Annona muricata fractions and in vitro antioxidant profile of fractions and isolated acetogenin (15-acetyl guanacone). J. Cancer Res. Pract. 2018, 5, 53–66. [Google Scholar] [CrossRef]
- Kadir, A.; Moghadamtousi, S.Z.; Rouhollahi, E.; Karimian, H.; Abdulla, M.A.; Fadaeinasab, M. Gastroprotective activity of Annona muricata leaves against ethanol-induced gastric injury in rats via Hsp70/Bax involvement. Drug Des. Dev. Ther. 2014, 8, 2099–2111. [Google Scholar]
- Kisani, A.I.; Saganuwan, S.A. Investigation of anaesthetic potentials of various extracts of Annona muricata (soursop) in Wister albino rat and dog. J. King Saud Univ. Sci. 2022, 34, 102225. [Google Scholar] [CrossRef]
- Badmus, J.A.; Oyemomi, S.A.; Adedosu, O.T.; Yekeen, T.A.; Azeez, M.A.; Adebayo, E.A.; Lateef, A.; Badeggi, U.M.; Botha, S.; Hussein, A.A.; et al. Photo-assisted bio-fabrication of silver nanoparticles using Annona muricata leaf extract: Exploring the antioxidant, anti-diabetic, anti-microbial, and cytotoxic activities. Heliyon 2020, 6, e05413. [Google Scholar] [CrossRef]
- Balderrama-Carmona, A.P.; Silva-Beltrán, N.P.; Gálvez-Ruiz, J.-C.; Ruíz-Cruz, S.; Chaidez-Quiroz, C.; Morán-Palacio, E.F. Antiviral, Antioxidant, and An-tihemolytic Effect of Annona muricata L. Leaves Extracts. Plants 2020, 9, 1650. [Google Scholar] [CrossRef]
- Byun, E.B.; Song, H.Y.; Kim, W.S. Polysaccharides from Annona muricata leaves protect normal human epidermal keratinocytes and mice skin from ra-diation-induced injuries. Rad. Phys. Chem. 2020, 170, 108672. [Google Scholar] [CrossRef]
- Liu, N.; Yang, H.L.; Wang, P.; Lu, Y.C.; Yang, Y.J.; Wang, L.; Lee, S.C. Functional proteomic analysis revels that the ethanol extract of Annona muricata L. induces liver cancer cell apoptosis through endoplasmic reticulum stress pathway. J. Ethnopharm. 2016, 189, 210–217. [Google Scholar]
- Ferreira, L.E.; Castro, P.M.N.; Chagas, A.C.S.; França, S.C.; Beleboni, R.O. In vitro anthelmintic activity of aqueous leaf extract of Annona muricata L. (Annonaceae) against Haemonchus contortus from sheep. Exp. Para-sitol. 2013, 134, 327–332. [Google Scholar]
- Viera, G.H.F.; Mourão, J.A.; Ângelo, Â.M.; Costa, R.A.; Vieira, R.H.S.F. Antibacterial effect (in vitro) of Moringa oleifera and Annona muricata against Gram positive and Gram negative bacteria. Rev. Inst. Med. Trop. 2010, 52, 129–132. [Google Scholar]
- Rodrigues, A.M.; Silva, A.A.S.; Pinto, C.C.C.; Santos, D.L.; Freitas, J.C.C.; Martins, V.E.P.; Morais, S.M. Larvicidal and enzymatic inhibition effects of Annona muricata seed extract and main constituent annonacin against Aedes aegypti and Aedes albopictus (Diptera: Culicidae). Pharmaceuticals 2019, 12, 112–122. [Google Scholar]
- Agbai, E.O.; Njoku, C.J.; Nwanegwo, C.O.; Nwafor, A. Effect of aqueous extract of Annona muricata seed on atherogenicity in streptozotocin-induced diabetic rats. Afr. J. Pharm. Pharmacol. 2015, 9, 745–755. [Google Scholar] [CrossRef] [Green Version]
- Doe, P.; Iddrisu, A.; Lartey, P.; Elijah, K.; Issaka, S.; Enock, D.A. Evaluation of the Anti-Diarrheal activity of the ethanolic seed extract of An-nona muricata. J. Phytopharmacol. 2019, 8, 199–202. [Google Scholar] [CrossRef]
- Parthiban, E.; Arokiyaraj, C.; Ramanibai, R. Annona muricata: An alternate mosquito control agent with special reference to inhibition of detoxifying en-zymes in Aedes aegypti. Ecotoxic. Environ. Saf. 2020, 189, 110050. [Google Scholar]
- Tovar-Gómez, B.; Mata-Montes, M.; García-Galindo, H.S.; Montalvo-González, E. Efecto de emulsiones de cera y 1-metilciclopropeno en la conservación poscosecha de guanabana. Rev. Chapingo. Ser. Hortic. 2011, 17, 53–61. [Google Scholar] [CrossRef]
- da Silva, L.M.R.; De Figueiredo, E.A.T.; Ricardo, N.M.P.S.; Vieira, I.G.P.; De Figueiredo, R.W.; Brasil, I.M.; Gomes, C.L. Quantification of bioactive com-pounds in pulps and by-products of tropical fruits from Brazil. Food Chem. 2014, 143, 398–404. [Google Scholar]
- Jiménez-Zurita, J.O.; Balois-Morales, R.; Alia-Tejacal, I.; Juárez-López, P.; Sumaya-Martínez, M.T.; Bello-Lara, J.E. Caracterización de frutos de guanábana (Annona muricata L.) en Tepic, Nayarit, México. Rev. Mex. De Cienc. Agrícolas 2016, 7, 1261–1270. [Google Scholar]
- Onimawo, I.A. Proximate composition and selected physicochemical properties of the seed, pulp and oil of soursop (Annona muricata). Plant Foods Human Nut. 2002, 57, 165–171. [Google Scholar]
- Gavamukulya, Y.; Wamunyokoli, F.; El-Shemy, H.A. Annona muricata: Is the natural therapy to most disease conditions including cancer growing in our backyard? A systematic review of its research history and future pro-spects. Asian Pac. J. Trop. Med. 2017, 10, 835–848. [Google Scholar]
- Lee, W.Z.; Chang, S.K.; Khoo, H.E.; Sia, C.M.; Yim, H.S. Influence of different extraction conditions on antioxidant properties of soursop peel. Acta Sci. Pol. Technol. Aliment. 2016, 15, 419–428. [Google Scholar] [CrossRef] [Green Version]
- Jagtap, U.B.; Bapat, V.A. Wines from fruits other than grapes: Current status and future prospectus. Food Biosci. 2015, 9, 80–96. [Google Scholar] [CrossRef]
- Joshi, V.K.; Panesar, P.S.; Rana, V.S.; Kaur, S. Science and technology of fruit wines: An overview. Sci. Technol. Fruit Wine Prod. 2017, 1–72. [Google Scholar] [CrossRef]
- Okigbo, R.N.; Obire, O. Mycoflora and production of wine from fruits of soursop (Annona muricata L.). Int. J. Wine Res. 2008, 1, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Ho, C.W.; Lazim, A.; Fazry, S.; Zaki, U.K.H.; Massa, S.; Lim, S.J. Alcoholic fermentation of soursop (Annona muricata) juice via an alternative fermentation technique. J. Sci. Food Agric. 2019, 100, 1012–1021. [Google Scholar]
- Soares, M.G.; de Lima, M.; Schmidt, V.C.R. Technological aspects of Kum-bucha, its applications and the symbiotic culture (SCOBY), and extraction of compounds of interest: A literature review. Trends Food Sci. Technol. 2021, 110, 539–550. [Google Scholar]
- Tan, W.C.; Muhialdin, B.J.; Meor Hussin, A.S. Influence of storage conditions on the quality, metabolites, and biological activity of soursop (Annona muricata. L.) kumbucha. Front. Microbiol. 2020, 11, 2982. [Google Scholar] [CrossRef]
- Isham, N.K.M.; Mokhtar, N.; Fazry, S.; Lim, S.J. The development of an al-ternative fermentation model system for vinegar production. LWT-Food Sci. Technol. 2019, 100, 322–327. [Google Scholar] [CrossRef]
- Ho, C.W.; Chang, L.S.; Muzni, S.K.S.; Fazry, S.; Lazim, A.; Zaki, U.K.H.H.; Lim, S.J. Functional beverage production using acetous fermentation of sour-sop: Physicochemical, toxicity and organoleptic properties. Food Biosci. 2021, 39, 100812. [Google Scholar] [CrossRef]
- Neta, M.T.S.L.; de Jesus, M.S.; da Silva, J.L.A.; Araujo, H.C.S.; Sandes, R.D.D.; Shanmugam, S.; Narain, N. Effect of spray drying on bioactive and volatile compounds in soursop (Annona muricata) fruit pulp. Food Res. Int. 2019, 124, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Ceballos, A.M.; Giraldo, G.I.; Orrego, C.E. Effect of freezing rate on quality parameters of freeze dried soursop fruit pulp. J. Food Eng. 2012, 111, 360–365. [Google Scholar] [CrossRef]
- Telis-Romero, J.; Beristain, C.I.; Gabas, A.L.; Telis, V.R.N. Effect of apparent viscosity on the pressure drop during fluidized bed drying of soursop pulp. Chem. Eng. Proc. Process Intensif. 2007, 46, 684–694. [Google Scholar] [CrossRef]
- Saavedra, A.; Almendariz, D.; Navarrete, D.; Vernaza, M.G. A new bread formulation based on a partial substitution of soursop residues flour through Mixolab and a process mixture design. Food Sci. Technol. 2021, 42, e63420. [Google Scholar] [CrossRef]
- Resende, L.M.; Franca, A.S. Flours based on exotic fruits and their processing residues—features and potential applications to health and disease prevention. In Flour and Breads and Their Fortification in Health and Disease Prevention; Academic Press: Cambridge, MA, USA, 2019; pp. 387–401. [Google Scholar]
- Osathanunkul, M. Bar-HRM for authenticating soursop (Annona muricata) tea. Scient. Rep. 2018, 8, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Innocent-Ukachi, A.C.; Onukwugha, U.C. Quality Evaluation of Tea Brewed from Blends of Soursop (Annona muricata) and Moringa (Moringa oleifera) Leaves. Eur. J. Nut. Food Saf. 2019, 10, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Gavamukulya, Y.; Abou-Elella, F.; Wamunyokoli, F.; AEl-Shemy, H. Phyto-chemical screening, anti-oxidant activity and in vitro anticancer potential of ethanolic and water leaves extracts of Annona muricata (Graviola). Asian Pac. J. Trop. Med. 2014, 7, S355–S363. [Google Scholar] [CrossRef] [Green Version]
- Smith, R.E.; Shejwalkar, P. Potential neurotoxicity of graviola (Annona muricata) juice. In Safety Issues in Beverage Production; Academic Press: Cambridge, MA, USA, 2020; pp. 429–449. [Google Scholar]
- Roduan, M.R.M.; Abd Hamid, R.; Sulaiman, H.; Mohtarrudin, N. Annona muricata leaves extracts prevent DMBA/TPA-induced skin tumorigenesis via modulating antioxidants enzymes system in ICR mice. Biom. Pharm.-Apy. 2017, 94, 481–488. [Google Scholar] [CrossRef]
- Quílez, A.M.; Fernández-Arche, M.A.; García-Giménez, M.D.; De la Puerta, R. Potential therapeutic applications of the genus Annona: Local and traditional uses and pharmacology. J. Ethnopharm. 2018, 225, 244–270. [Google Scholar] [CrossRef]
- Primiani, C.N.; Mumtahanah, M.; Ardhi, W. Kumbucha fermentation test used for various types of herbal Teas. J. Physics Conf. Ser. 2018, 1025, 012073. [Google Scholar] [CrossRef] [Green Version]
- Candra, A.; Prasetyo, B.E.; Tarigan, J.B. Study of vitamin C level of soursop leaves (Annona muricata L.) and galactomannan utilization in kumbucha during fermentation. In AIP Conference Proceedings; AIP Publishing LLC.: Melville, NY, USA, 2021; Volume 2342, p. 100007. [Google Scholar] [CrossRef]
- Gyesi, J.N.; Opoku, R.; Borquaye, L.S. Chemical composition, total phenolic content, and antioxidant activities of the essential oils of the leaves and fruit pulp of Annona muricata L. (Soursop) from Ghana. Biochem. Res. Int. 2019, 2019, 4164576. [Google Scholar] [CrossRef] [Green Version]
- Jirovetz, L.; Buchbauer, G.; Ngassoum, M.B. Essential Oil Compounds of the Annona muricata Fresh Fruit Pulp from Cameroon. J. Agric. Food Chem. 1998, 46, 3719–3720. [Google Scholar] [CrossRef]
- Kossouoh, C.; Moudachirou, M.; Adjakidje, V.; Chalchat, J.-C.; Figuérédo, G. Essential oil chemical composition of Annona muricata L. Leaves from Benin. J. Essent. Oil Res. 2007, 19, 307–309. [Google Scholar] [CrossRef]
- Rodrigues, V.H.; Melo, M.M.R.; Tenberg, V.; Carreira, R.; Portugal, I.; Silva, C.M. Similarity analysis of essential oils and oleoresins of Eucalyptus globulus leaves produced by distinct methods, solvents and operating conditions. Ind. Crops Prod. 2021, 164, 113339–113349. [Google Scholar] [CrossRef]
- Cagnini, C.Z.; Dias, A.B.; Boas, M.R.V.; Batista, F.P.R.; Faria, M.G.I.; Glamočlija, J.; Soković, M.; Tełević, V.; Ferreira, E.S.; Colauto, N.B. Antimi-crobial activity of Annona muricata leaf oleoresin. Nat. Prod. Res. 2021, 36, 4781–4787. [Google Scholar] [CrossRef]
- Ravaomanarivo, L.H.R.; Razafindraleva, H.A.; Raharimalala, F.N.; Rasoa-hantaveloniaina, B.; Ravelonandro, P.H.; Mavingui, P. Efficacy of seed extracts of Annona squamosa and Annona muricata (Annonaceae) for the control of Aedes albopictus and Culex quinquefasciatus (Culicidae). Asian Pac. J. Trop. Biomed. 2014, 4, 798–806. [Google Scholar] [CrossRef] [Green Version]
- Silva, A.C.; Jorge, N. Bioactive compounds of the lipid fractions of agro-industrial waste. Food Res. Int. 2014, 66, 493–500. [Google Scholar] [CrossRef]
- Su, C.-H.; Nguyen, H.; Pham, U.; Nguyen, M.; Juan, H.-Y. Biodiesel Production from a Novel Nonedible Feedstock, Soursop (Annona muricata L.) Seed Oil. Energies 2018, 11, 2562. [Google Scholar] [CrossRef] [Green Version]
- Chew, S.C. Cold-pressed rapeseed (Brassica napus) oil: Chemistry and func-tionality. Food Res. Int. 2020, 131, 108997. [Google Scholar] [CrossRef] [PubMed]
- Dorado, D.J.; Hurtado-Benavides, A.M.; Martínez-Correa, H. Extracción con CO2 Supercrítico de Aceite de Semillas de Guanábana (Annona muricata): Cinética, perfil de ácidos grasos y esteroles. Inf. Tecnológica 2016, 27, 37–48. [Google Scholar] [CrossRef] [Green Version]
- Pinto, L.C.; Cerqueira-Lima, A.T.; dos Santos Suzarth, S.; de Souza, R.; Tosta, B.R.; da Silva, H.B.; de Pires, A.O.; de Queiroz, G.A.; Texeira, T.O.; Dourado, K.M.C.; et al. Anonna muricata L. (soursop) seed oil improves type 1 diabetes parameters in vivo and in vitro. Pharma Nut. 2018, 6, 1–8. [Google Scholar] [CrossRef]
- Menezes, E.G.T.; Queiroz, F.; Araújo, A.C.M.A. Extraction of soluble solids of soursop (Annona muricata) and marolo (Annona crassiflora Mart.) seeds using Different Solvents and Processes. In International Joint Conference on Indus-Trial Engineering and Operations Management; Springer: Cham, Switzerland, 2020; pp. 243–255. [Google Scholar]
- Pinto, L.C.; Cerqueira-lima, A.T.; dos Santos, S.; Souza, R.; Tosta, B.; Silva, H.B.F.; Pires, A.O.; Queiroz, G.; Silva, R.R.; Teixeira, T.; et al. Effects of Anonna muricata L. (soursop) seeds oil improves in model in vivo and in vitro of type 1 diabetes mellitus. J. Allergy Clin. Immun. 2017, 139, AB15. [Google Scholar] [CrossRef]
- Nwokocha, L.M.; Williams, P.A. New starches: Physicochemical properties of sweetsop (Annona squamosa) and soursop (Anonna muricata) starches. Carb. Polym. 2009, 78, 462–468. [Google Scholar] [CrossRef] [Green Version]
- Teigiserova, D.A.; Hamelin, L.; Tomsen, M. Towards transparent valorization of food surplus, waste and loss: Clarifying definitions, food waste hierarchy, and role in the circular economy. Sci. Total Environ. 2020, 706, 136033. [Google Scholar] [CrossRef]
- Kshtriya, V.; Koshti, B.; Gour, N. Green synthesized nanoparticles: Classifi-cation, synthesis, characterization, and applications. Compreh. Anal. Chem. 2021, 94, 173–222. [Google Scholar]
- Gavamukulya, Y.; Maina, E.N.; Meroka, A.M.; El-Shemy, H.A.; Magoma, G.; Wamunyokoli, F. In search of new anticancer drugs: Data for cytotoxic activi-ties of green synthesized silver nanoparticles from ethanolic extracts of fruits and leaves of Annona muricata and 5-Fluorouracil against HeLa, PC3 and PNT1A cell lines. Data Brief 2019, 26, 104442. [Google Scholar] [CrossRef]
- Meenakshisundaram, S.; Krishnamoorthy, V.; Jagadeesan, Y.; Vilwanathan, R.; Balaiah, A. Annona muricata assisted biogenic synthesis of silver nanoparticles regulates cell cycle arrest in NSCLC cell lines. Bioorg. Chem. 2020, 95, 103451. [Google Scholar] [CrossRef]
- Agu, K.C.; Okolie, P.N. Proximate composition, phytochemical analysis, and in vitro antioxidant potentials of extracts of Annona muricata (Soursop). Food Sci. Nut. 2017, 5, 1029–1036. [Google Scholar] [CrossRef] [Green Version]
- Vijayakumar, S. Eco-friendly synthesis of gold nanoparticles using fruit ex-tracts and in vitro anticancer studies. J. Saudi Chem. Soc. 2019, 23, 753–761. [Google Scholar] [CrossRef]
- Fasakin, A.O.; Fehintola, E.O.; Obijole, O.A.; Oseni, O.A. Compositional analyses of the seed of soursop, Annona muricata L., as a potential animal feed supplement. Scient. Res. Assays. 2008, 3, 521–523. [Google Scholar]
- Abdualrahman, M.A.Y.; Ma, H.; Zhou, C.; Yagoub, A.E.A.; Ali, A.O.; Tahir, H.E.; Wali, A. Postharvest physicochemical properties of the pulp and seed oil from Annona squamosa L.(Gishta) fruit grown in Darfur region, Sudan. Arab. J. Chem. 2019, 12, 4514–4521. [Google Scholar] [CrossRef] [Green Version]
- Pinto, C.S.; Magalhães, A.L.R.; Teodoro, A.L.; Gois, G.C.; Véras, R.M.L.; Campos, F.S.; Nascimento, D.B.; Andrade, A.P.; Oliveira, L.P.; Lima, I.E. Potential alternative feed sources for ruminant feeding from the biodiesel production chain by-products. S. Afr. J. Anim. Sci. 2020, 50, 69–77. [Google Scholar] [CrossRef]
- Haider, A.; Shafique, A.; Nadeem, H.U.; Azeem, F.; Siddique, M.H.; Afzal, M.; Irshad, A.; Rasul, I. Nonedible oil. In Green Sustainable Process for Chemical and Environmental Engineering and Science; Elsevier: Amsterdam, The Netherlands, 2021; pp. 127–155. [Google Scholar]
- Mohiddin, M.N.B.; Tan, Y.H.; Seow, Y.X.; Kansedo, J.; Mubarak, N.M.; Abdullah, M.O.; Chan, Y.S.; Khalid, M. Evaluation on feedstock, technologies, catalyst and reactor for sustainable biodiesel production: A review. J. Ind. Eng. Chem. 2021, 98, 60–81. [Google Scholar] [CrossRef]
- Schroeder, P.; do Nascimento, B.P.; Romeiro, G.A.; Figueiredo, M.K.K.; da Cunha Veloso, M.C. Chemical and physical analysis of the liquid fractions from soursop seed cake obtained using slow pyrolysis conditions. J. Anal. Appl. Pyrol. 2017, 124, 161–174. [Google Scholar] [CrossRef]
- Dike, C.C.; Shahsavari, E.; Surapaneni, A.; Shah, K.; Ball, A.S. Can biochar be an effective and reliable biostimulating agent for the remediation of hydrocarbon-contaminated soils? Environ. Int. 2021, 154, 106553. [Google Scholar] [CrossRef]
- Ghodake, G.S.; Shinde, S.K.; Kadam, A.A.; Saratale, R.G.; Saratale, G.D.; Kumar, M.; Palem, R.R.; Al-Shawainam, H.A.; Elgorban, A.M.; Syed, A.; et al. Review on biomass feedstocks, pyrolysis mechanism and physicochemical properties of biochar: State-of-the-art framework to speed up vision of circular bioeconomy. J. Clean. Prod. 2021, 297, 126645. [Google Scholar] [CrossRef]
- Hossain, A.K.; Davies, P.A. Pyrolysis liquids and gases as alternative fuels in internal combustion engines–A review. Renew. Sustain. En. Rev. 2013, 21, 165–189. [Google Scholar] [CrossRef]
- Kan, T.; Strezov, V.; Evans, T.J. Lignocellulosic biomass pyrolysis: A review of product properties and effects of pyrolysis parameters. Renew. Sustain. En. Rev. 2016, 57, 1126–1140. [Google Scholar] [CrossRef]
- Struelens, Q.; Silvie, P. Orienting insecticide research in the tropics to meet the sustainable development goals. Cur. Op. Insect Sci. 2020, 40, 24–30. [Google Scholar] [CrossRef]
- Santhosh, S.B.; Ragavendran, C.; Natarajan, D. Spectral and HRTEM analysis of Annona muricata leaf extract mediated silver nanoparticles and its larvicidal efficacy against three mosquito vectors Anopheles stephensi, Culex quinque-fasciatus, and Aedes aegypti. J. Photochem. Photobio. B Biol. 2015, 153, 184–190. [Google Scholar] [CrossRef]
- Amarasinghe, L.D.; Wickramarachchi, P.A.S.R.; Aberathna, A.A.A.U.; Sith-ara, W.S.; De Silva, C.R. Comparative study on larvicidal activity of green synthesized silver nanoparticles and Annona glabra (Annonaceae) aqueous extract to control Aedes aegypti and Aedes albopictus (Diptera: Cu-licidae). Heliyon 2020, 6, e04322. [Google Scholar] [CrossRef]
- Chen, M.; Wang, X.; Zhang, H. Comparative research on selective adsorption of Pb (II) by biosorbents prepared by two kinds of modifying waste biomass: Highly-efficient performance, application and mechanism. J. Environ. Manag. 2021, 288, 112388. [Google Scholar] [CrossRef]
- Meili, L.; Lins, P.V.S.; Costa, M.T.; Almeida, R.L.; Abud, A.K.S.; Soletti, J.I.; Dotto, G.L.; Tanabe, E.H.; Sellaqui, L.; Carvalho, S.H.V.; et al. Adsorption of methylene blue on agroindustrial wastes: Experimental investigation and phenomenological modelling. Prog. Biophys. Mol. Biol. 2019, 141, 60–71. [Google Scholar] [CrossRef]
- Pang, X.; Sellaoui, L.; Franco, D.; Netto, M.S.; Georgin, J.; Dotto, G.L.; Shayeb, M.K.A.; Belmabrouk, H.; Bonilla-Petriciolet, A.; Li, Z. Preparation and characterization of a novel mountain soursop seeds powder adsorbent and its application for the removal of crystal violet and methylene blue from aqueous solutions. Chem. Eng. J. 2020, 391, 123617. [Google Scholar] [CrossRef]
- Ndamitso, M.M.; Mustapha, S.; Etsuyankpa, M.B.; Jacob, J.O.; Adeshina, I.O.; Ekor, L. Removal of lead, cadmium and cobalt from oil spill water onto soursop (Annona muricata) peel. Science 2016, 4, 7–11. [Google Scholar]
- Velidandi, A.; Pabbathi, N.P.P.; Baadhe, R.R. Study of parameters affecting the degradation of rhodamine-B and methyl orange dyes by Annona muricata leaf extract synthesized nanoparticles as well as their recyclability. J. Mol. Struc. 2021, 1236, 130287. [Google Scholar] [CrossRef]
- São Paulo. 2022. Available online: https://ceagesp.gov.br/cotacoes/#cotacao (accessed on 22 January 2022).
- Walmart. 2022. Available online: https://super.walmart.com.mx/frutas/guanabana-por-kilo/00000000003381 (accessed on 22 January 2022).


| Bioactive Compounds | Leaf | Peel | Pulp | Seed |
|---|---|---|---|---|
| References | ||||
| 4-Aminobenzoic acid | [20] | |||
| β-amyrin | [20] | |||
| p-Anisic acid | [20] | |||
| Annoionoside | [21] | |||
| Anonaine | [21] | |||
| Apeginin | [20] | |||
| Argentinine | [21] | |||
| Benzoic acid | [19] | |||
| 3,4 Dihydroxybenzoic acid | [20] | |||
| 4-Hydroxybenzoic acid | [19] | [19] | [20] | |
| Blumenol C glucoside | [21] | |||
| Caffeic acid | [22] | [19,20] | ||
| Caffeic acid derivative | [17] | |||
| 5-Caffeoylquinic acid | [17] | |||
| Dicaffeoylquinic acid | [17] | |||
| Carnosol | [20] | |||
| Catechin | [21,22] | [20] | ||
| Catechin gallate | [22] | |||
| Chlorogenic acid | [21,22,23] | [19] | [19] | [19,20] |
| Neochlorogenic acid | [19] | [19] | [19] | |
| Chrysin | [20] | |||
| Cinnamic acid | [19,24] | [17,19] | [19,20] | |
| Cinnamic acid derivative | [19,20] | [17] | ||
| Citroside A | [21] | |||
| Coclaurine | [21] | |||
| Coniferaldehyde | [20] | |||
| Corossolone | [21] | |||
| Coumaric acid | [19] | [19] | [19] | |
| Coumaric acid hexose | [17] | |||
| p-Coumaric acid | [22] | [17] | [19,20] | |
| p-Coumaric acid methyl ester | [17] | |||
| Cyanidin | [24] | |||
| 9,19-cyclolanostan-3-ol,24-methlene-,(3β) | [25] | |||
| 9,19-cyclolanost-24-en-3-ol,(3β) | [25] | |||
| 2,8-dimethyl-2-(3E,7E)-,8,12-trimethyltrideca-3,7,11-trien-1-yl)chroman-6-ol | [25] | |||
| Datiscetin | [21] | |||
| Ellagic acid | [20] | |||
| Epicatechin | [22,23] | [20] | ||
| Epicatechin gallate | [22] | |||
| Epigallocatechin | [22] | |||
| Eriodictyol | [20] | |||
| Ferulic acid | [22] | [20] | ||
| 4-Feruloyl-5-caffeoylquinic acid | [17] | |||
| Feruloyl-glucoside | [17] | |||
| Fustin | [20] | |||
| Galangin | [20] | |||
| Gallic acid | [19] | [19] | [19,20] | |
| Gallocatechin gallate | [22] | |||
| Hispidulin | [20] | |||
| Kaempferol | [21] | [20] | ||
| Dihydrokaempferol-hexoside | [17] | |||
| Kaempferol-rhamnoside | [26] | |||
| Kaempferol 3-o-rutinoside | [21] | |||
| Lanost-7-en-3-one,(9β, 13α, 14β,17α) | [25] | |||
| Loliolide | [21] | |||
| Isolaureline | [21] | |||
| Lupeol | [25] | |||
| Luteolin | [24] | |||
| Mandelic acid | [20] | |||
| Myricetin | [20] | |||
| Naringenin | [20] | |||
| Naringin | [22] | |||
| Norcorydine | [21] | |||
| Pinocembrin | [20] | |||
| Procyanidin B2 | [23] | |||
| Procyanidin C1 | [23] | |||
| Protocatechuic acid | [19] | [19] | [19] | |
| Quercetin | [21,23] | [24] | [19] | |
| Isoquercetin | [21] | |||
| Quercetin-diglucoside | [23] | |||
| Quercetin-glucosyl-pentoside | [23] | |||
| Quercetin-glucoside | [23] | |||
| Quercetin-rhamnoside | [23] | |||
| Quercetin-xylosyl-rutinoside | [23] | |||
| Resorcinol | [24] | |||
| Reticuline | [21] | |||
| Rosmarinic acid | [20] | |||
| Rutin | [21,22,23] | [20] | ||
| Salicylic acid | [20] | |||
| Scopoletin | [20] | |||
| Sinapaldehyde | [20] | |||
| Sinapic acid | [20] | |||
| Stepharine | [21] | |||
| Syringaldehyde | [20] | |||
| Syringic acid | [19] | [19] | [19,20] | |
| Taxifolin | [20] | |||
| Tirucallol | [25] | |||
| Umbelliferone | [20] | |||
| Vanillic acid | [20] | |||
| Vanillin | [24] | [20] | ||
| Vitexina | [20] | |||
| Vomifoliol | [21] | |||
| Xylopine | [21] | |||
| In Vitro | In Vivo | ||
|---|---|---|---|
| Soursop fruit | |||
| Antibacterial Methanol extract from dehydrated whole soursop fruit, Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 15422, Micrococcus luteus ATCC 4698, and Staphylococcus aureus ATCC 2592 [26] | Trombolytic Swiss albino mice of either sex (male and female), treated with crude methanol extract from dehydrated whole soursop fruit [26] | ||
| Antioxidant Methanol extract of dried fruit and leaves as well as isolated 15-acetyl guanacone were evaluated for antioxidant activity by DPPH, ABTS, and ferric reducing in comparison to control (ascorbic acid) [31] | Antidiarrheal activity Castor oil induced method; the control group received vehicle (normal saline solution, post orally), the positive control group received loperamide, and the test group received soursop pulp methanol extracts [26] | ||
| Soursop pulp | |||
| Antioxidant DPPH radical-scavenging activity of methanol pulp extract [30] | Antidiabetic Methanol extracts of pulverized soursop pulp and leaf to male albino Wistar rats in different doses. At the end of the 28-day experimental period, serum was collected separately and used for serum amylase assay [27] | ||
| Antitumor Human tumor cell lines from MCF-7 (breast carcinoma without over-expression of the HER2/c-erb-2 gene), SKBr3 (breast carcinoma, in which the HER2/c-erb-2 gene is overexpressed), PC3 (prostate carcinoma), and HeLa (cervix epithelial carcinoma). Human dermis fibroblasts were used as control cells. Aqueous and ethanol extracts of soursop pulp [30] | Antifungal Methanol and aqueous extracts (dried and applied as aqueous solution) from soursop pulp controlling blackspots of Alternaria alternata in tomatoes [29] | ||
| Antimicrobial Agar disc diffusion method for screening the antimicrobial activity of ethanol and aqueous pulp extract against Salmonella enterica ser. Enteritidis, Staphylococcus aureus, and Listeria monocytogenes [30] | |||
| Antidiabetic Pulp extract amylase inhibition assay; pancreatic alpha-amylase of porcine origin [27,28] | |||
| Antihypertensive Angiotensin-I converting enzyme (ACE). Inhibition assay [28] | |||
| Antifungal Inhibitory activity of methanol and aqueous soursop pulp extract on the radial growth of A. alternata [29] | |||
| Soursop leaf | |||
| Antidiabetic Pulp and leaf extract amylase inhibition assay; pancreatic α-amylase of porcine origin [27] Ethanol extract of soursop leaf evaluated about inhibitory against α-amylase, α-glucosidase, and lipase [23,27] | Gastroprotective Sprague Dawley strain rats (gastric injury induced) were treated with ethyl acetate extract of A. muricata leaves. Results evaluated by histopathology and immunohistochemistry [32] | ||
| Antioxidant Methanol extract of dried leaves and isolated 15-acetyl guanacone, evaluated for antioxidant activity by DPPH, ABTS, and ferric reducing in comparison to control (ascorbic acid) [31] | Anaesthetic Wister albino rats and mongrel dogs were used for the study. They were induced for local and general anesthesia with different doses of soursop leaf methanol extracts [33] | ||
| Soursop leaf nanoparticles as antioxidant assayed by DPPH, ABTS, and inhibition of lipid peroxidation [34] Soursop leaf extracts (80% methanol) and aqueous extracts were evaluated for antioxidant activity by FRAP, ABTS, DPPH, and nitrite [22] Soursop leaf ethanol extract, ORAC, FRAP, DPPH. Inhibition tests for the formation of advanced glycation end products; inhibition of non-enzymatic lipid peroxidation [23] Soursop leaf ethanol:acetic acid extract, DPPH, ABTS assay [34] | |||
| Antibacterial Soursop leaf nanoparticles evaluated against Staphylococcus aureus, Escherichia coli, Serratia marcescens, Bacillus cereus, Pseudomonas aeruginosa, and Salmonella enterica by using a microdilution assay. The growth of bacterial isolates was considered as optical density at 530 nm [35] | |||
| Anti-angiogenic 3-(4,5-dimethylthiazol-2-yl)− 2,5-diphenyltetrazolium bromide (MTT) dye reduction assay in microplates. The assay is dependent on the reduction of MTT by mitochondrial dehydrogenases of viable cell to a blue formazan product, measured spectrophotometrically (550 nm). Incubated with serial dilutions of aqueous or DMSO of leaf soursop extracts [21] | |||
| Antiparasitic T. gondii proliferation, NIH/3T3 fibroblasts were cultured in well plates and infected with tachyzoites of T. gondii RH-2F1 strain cells and treated with different concentrations of soursop leaf extracts. Chlorophenol red–β-D-galactopyranoside was utilized for measuring the T. gondii growth [35] | |||
| Enzymes inhibition Ethanolic extract of soursop leaves, α-amylase, α-glucosidase, and pancreatic lipase inhibition [23] Protection and treatment of radiation-induced skin damage Soursop leaves polysaccharides were tested as a protector of irradiated human cells (keratinocytes); evaluation of the effect by measuring cell viability and oxidant enzyme activity [36] | |||
| Anticancer The various soursop leaf methanolic extracts were used to several fractions: hexane, hexane-ethyl acetate, ethyl acetate, ethyl acetate-methanol, methanol, and methanol-water. Each fraction was dissolved in dimethyl sulfoxide (DMSO) to generate the desired stock solution. Effects of these fractions on cancer cell viability [31] Ethanol extract of soursop leaf effect on liver cancer HepG2 and colon cancer HCT116 cells. Cell viability and apoptosis assays, bioinformatics, and proteomics [37] | |||
| Anthelmintic Eggs to perform the egg hatch test (EHT) and for culture of infective larvae for larval motility test (LMT) were obtained from fecal samples collected rectally from a monospecifically H. contortus infected sheep. The effect of crude A. muricata leaf aqueous extract was evaluated [38] | |||
| Soursop peel | |||
| Antihypertensive Angiotensin-I converting enzyme (ACE) inhibition assay [28] | Restoration of pancreatic cells Aqueous extract of Annona muricata peels were tested in alloxan-induced diabetic male Wistar rats. Effect evaluated by biochemical parameters in serum and liver, antioxidant biomarkers, activity of glycolytic enzymes, and metabolomic analysis [24] | ||
| Bactericidal The bactericidal effect of the soursop peel aqueous and ethanol extracts was evaluated with the modified Kirby–Bauer disk diffusion method. The effect on S. aureus ATCC25923, Vibrio cholerae classic 569B, S. Enteritidis, and E. coli was evaluated [39] | |||
| Soursop seed | |||
| Antioxidant DPPH radical-scavenging activity of methanol seed extract [30] Aqueous extract of soursop seed, DPPH, ABTS, and hydroxyl (OH) radical scavenging assay [28] | Antifungal Methanol and aqueous extracts (dried and applied as aqueous solution) from soursop seed controlling blackspots of Alternaria alternata in tomatoes [29] | ||
| Antitumor Human tumor cell lines from MCF-7 (breast carcinoma, without over-expression of the HER2/c-erb-2 gene), SKBr3 (breast carcinoma, in which the HER2/c-erb-2 gene is overexpressed), PC3 (prostate carcinoma), and HeLa (cervix epithelial carcinoma). Human dermis fibroblasts were used as control cells. Aqueous and ethanol extracts of soursop seed [30] | Attenuation in benign prostatic hyperplasia Adult male Wistar rats treated with testosterone and soursop seed n-hexane extract. Effect evaluated by immunohistochemical on the expression of proteins and histology of prostate, markers of inflammation, and antioxidants [25] | ||
| Antimicrobial Agar disk diffusion method for screening the antimicrobial activity of ethanol and aqueous seed extract against Salmonella enterica ser. Enteritidis, Staphylococcus aureus, and Listeria monocytogenes [30] | Antiatherogenic Male albino Wistar rats streptozotocin-induced diabetics, treated with soursop seed aqueous extract. The effect on biochemical markers was determined [40] | ||
| Antidiabetic Soursop seed extract amylase inhibition assay; pancreatic alpha-amylase of porcine origin [28] Antihypertensive Angiotensin-I converting enzyme (ACE) inhibition assay [28] | Antidiarrhea Wistar albino rats with castor oil induced diarrhea were treated with soursop ethanol seed extracts. Gastro-intestinal mobility was evaluated [41] | ||
| Larvicidal Acetogenin-rich fraction of A. muricata seeds and annonacin effects on the larvae of Ae. aegypti and Ae. Albopictus were verified from the analysis of the main enzymes of the Culicidae larvae metabolism [42] Soursop seed kernel powder extracts (hexane, chloroform, ethyl acetate, and ethanol). Early fourth instars of A. aegypti, A. stephensi, and C. quinquefasciatus larvae were introduced into water containing each solvent extract. Assay of biochemical constituents; threshold time for lethal effect was evaluated [43] | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, I.L.; Rodrigues, A.M.d.C.; Amante, E.R.; Silva, L.H.M.d. Soursop (Annona muricata) Properties and Perspectives for Integral Valorization. Foods 2023, 12, 1448. https://doi.org/10.3390/foods12071448
Santos IL, Rodrigues AMdC, Amante ER, Silva LHMd. Soursop (Annona muricata) Properties and Perspectives for Integral Valorization. Foods. 2023; 12(7):1448. https://doi.org/10.3390/foods12071448
Chicago/Turabian StyleSantos, Ivone Lima, Antonio Manoel da Cruz Rodrigues, Edna Regina Amante, and Luiza Helena Meller da Silva. 2023. "Soursop (Annona muricata) Properties and Perspectives for Integral Valorization" Foods 12, no. 7: 1448. https://doi.org/10.3390/foods12071448