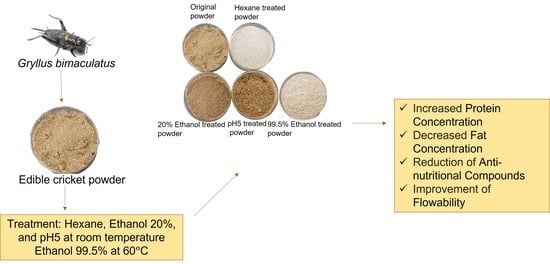
Improvements in Visual Aspects and Chemical, Techno-Functional and Rheological Characteristics of Cricket Powder (Gryllus bimaculatus) by Solvent Treatment for Food Utilization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Effect of the pH and Ethanol Concentration on Protein Behavior
2.2. Solvent Treatment
2.3. Color Difference after Treatments
2.4. Moisture Content
2.5. Ash Concentration
2.6. Fat Concentration
2.7. Protein Concentration
2.8. Chitin Concentration
2.9. Total Phenolic Concentration
2.10. Osborne Classification
2.11. Tannins Concentration
2.12. Phytates Concentration
2.13. pH
2.14. Zeta Potential
2.15. Water-Holding Capacity (WHC) and Oil-Holding Capacity (OHC)
2.16. Flowability
2.17. Viscosity
2.18. Total Recovery and Recoveries of Each Compound
2.19. Statistical Analysis
3. Results and Discussion
3.1. Effect of the pH and Ethanol Concentration on Protein Behavior
3.2. Osborne’s Classification of Proteins
3.3. Total Powder Recovery
3.4. Chemical Characterization of the Powders
3.4.1. Proximate Analyses
3.4.2. Total Phenolic Compounds and Antinutritional Compounds
3.5. Visual Aspects, Techno-Functional and Rheological Characterization of Powders
3.5.1. Color Change after Powder Treatment
3.5.2. Water-Holding Capacity (WHC)
3.5.3. Oil-Holding Capacity (OHC)
3.5.4. Zeta Potential and pH
3.5.5. Flowability
3.5.6. Paste Properties—Viscosity
3.5.7. Relationship between the Chemical Composition of the Powders and Visual, Techno-Functional, and Rheological Parameters
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mishra, G. Omkar Entomoceuticals. In Industrial Entomology; Omkar, Ed.; Springer: Singapore, 2017; pp. 435–449. ISBN 978-981-10-3303-2. [Google Scholar]
- Churchward-Venne, T.A.; Pinckaers, P.J.M.; van Loon, J.J.A.; van Loon, L.J.C. Consideration of Insects as a Source of Dietary Protein for Human Consumption. Nutr. Rev. 2017, 75, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- FAO Key Facts and Findings. Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/news/story/en/item/197623/icode/ (accessed on 2 February 2023).
- Aravind, L. Alternative Food Production and Consumption: Evolving and Exploring Alternative Protein Supplement Potential through Entomoceuticals. Int. J. Chem. Stud. 2019, 7, 1393–1397. [Google Scholar]
- Mishra, G. Omkar Insects as Food. In Industrial Entomology; Omkar, Ed.; Springer: Singapore, 2017; pp. 413–434. ISBN 978-981-10-3303-2. [Google Scholar]
- De Almeida, J.C.; Perassolo, M.S.; Camargo, J.L.; Bragagnolo, N.; Gross, J.L. Fatty Acid Composition and Cholesterol Content of Beef and Chicken Meat in Southern Brazil. Rev. Bras. Ciênc. Farm. 2006, 42, 109–117. [Google Scholar] [CrossRef] [Green Version]
- Moruzzo, R.; Mancini, S.; Boncinelli, F.; Riccioli, F. Exploring the Acceptance of Entomophagy: A Survey of Italian Consumers. Insects 2021, 12, 123. [Google Scholar] [CrossRef] [PubMed]
- Nino, M.C.; Reddivari, L.; Ferruzzi, M.G.; Liceaga, A.M. Targeted Phenolic Characterization and Antioxidant Bioactivity of Extracts from Edible Acheta Domesticus. Foods 2021, 10, 2295. [Google Scholar] [CrossRef]
- Kim, D.-H.; Kim, E.-M.; Chang, Y.-J.; Ahn, M.-Y.; Lee, Y.-H.; Park, J.J.; Lim, J.-H. Determination of the shelf life of cricket powder and effects ofstorage on its quality characteristics. Korean J. Food Preserv. 2016, 23, 211–217. [Google Scholar] [CrossRef] [Green Version]
- Wadhera, D.; Capaldi-Phillips, E.D. A Review of Visual Cues Associated with Food on Food Acceptance and Consumption. Eat. Behav. 2014, 15, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Severini, C.; Azzollini, D.; Albenzio, M.; Derossi, A. On Printability, Quality and Nutritional Properties of 3D Printed Cereal Based Snacks Enriched with Edible Insects. Food Res. Int. 2018, 106, 666–676. [Google Scholar] [CrossRef]
- Nijdam, J.J.; Agarwal, D.; Schon, B.S. Assessment of a Novel Window of Dimensional Stability for Screening Food Inks for 3D Printing. J. Food Eng. 2021, 292, 110349. [Google Scholar] [CrossRef]
- Séré, A.; Bougma, A.; Bazié, B.S.R.; Traoré, E.; Parkouda, C.; Gnankiné, O.; Bassolé, I.H.N. Chemical Composition, Energy and Nutritional Values, Digestibility and Functional Properties of Defatted Flour, Protein Concentrates and Isolates from Carbula Marginella (Hemiptera: Pentatomidae) and Cirina Butyrospermi (Lepidoptera: Saturniidae). BMC Chem. 2021, 15, 46. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Johnson, L.A.; Wang, T. Preparation of Soy Protein Concentrate and Isolate from Extruded-Expelled Soybean Meals. J. Am. Oil Chem. Soc. 2004, 81, 713–717. [Google Scholar] [CrossRef]
- Horwitz, W. Official Methods of Analysis of AOAC International. In Volume I, Agricultural Chemicals, Contaminants; William, H., Ed.; AOAC International: Gaithersburg, MD, USA, 2010; ISBN 0-935584-67-6. [Google Scholar]
- Thiex, N.; Novotny, L.; Crawford, A. Determination of Ash in Animal Feed: AOAC Official Method 942.05 Revisited. J. AOAC Int. 2012, 95, 1392–1397. [Google Scholar] [CrossRef] [PubMed]
- Thiex, N.J.; Manson, H.; Anderson, S.; Persson, J.Å.; Anderson, S.; Bogren, E.; Bolek, G.; Budde, D.; Ellis, C.; Eriksson, S.; et al. Determination of Crude Protein in Animal Feed, Forage, Grain, and Oilseeds by Using Block Digestion with a Copper Catalyst and Steam Distillation into Boric Acid: Collaborative Study. J. AOAC Int. 2002, 85, 309–317. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Sun, J.; Yu, L.; Zhang, C.; Bi, J.; Zhu, F.; Qu, M.; Jiang, C.; Yang, Q. Extraction and Characterization of Chitin from the Beetle Holotrichia Parallela Motschulsky. Molecules 2012, 17, 4604–4611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, A.; Nayak, R.; Purohit, S.R.; Rao, P.S. Impact of UV-C Irradiation on Solubility of Osborne Protein Fractions in Wheat Flour. Food Hydrocoll. 2021, 110, 105845. [Google Scholar] [CrossRef]
- Tandon, M. Research Gate: Tannin Assay. Available online: https://www.researchgate.net/publication/309212455_Tannin_Assay?channel=doi&linkId=5806054308ae03256b764cbc&showFulltext=true (accessed on 20 January 2023).
- Raboy, V.; Gibson, R.S.; Bailey, K.B.; King, J.C. Comparison of Four Methods for Phytate Analysis in Plant-Based Foods. J. Food Compos. Anal. 2020, 90, 103481. [Google Scholar] [CrossRef]
- Nahar, M.K.; Zakaria, Z.; Hashim, U.; Bari, M. Effect of PH and Salt Concentration on Protein Solubility of Slaughtered and Non-Slaughtered Broiler Chicken Meat. Sains Malays. 2017, 46, 719–724. [Google Scholar] [CrossRef]
- Pelegrine, D.H.G.; Gasparetto, C.A. Whey Proteins Solubility as Function of Temperature and PH. LWT Food Sci. Technol. 2005, 38, 77–80. [Google Scholar] [CrossRef]
- Zidani, S.; Fahloul, D.; Bacha, A. Effects of PH, NaCl, Ethanol, and Drying Methods on the Solubility of Saccharomyces Cerevisiae Proteins. CyTA-J. Food 2012, 10, 42–47. [Google Scholar] [CrossRef] [Green Version]
- Radhika, V.; Rao, V.S.H. Computational Approaches for the Classification of Seed Storage Proteins. J. Food Sci. Technol. 2015, 52, 4246–4255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stone, A.K.; Tanaka, T.; Nickerson, M.T. Protein Quality and Physicochemical Properties of Commercial Cricket and Mealworm Powders. J. Food Sci. Technol. 2019, 56, 3355–3363. [Google Scholar] [CrossRef] [PubMed]
- Ndiritu, A.K.; Kinyuru, J.N.; Kenji, G.M.; Gichuhi, P.N. Extraction Technique Influences the Physico-Chemical Characteristics and Functional Properties of Edible Crickets (Acheta Domesticus) Protein Concentrate. J. Food Meas. Charact. 2017, 11, 2013–2021. [Google Scholar] [CrossRef]
- Liu, H.; Tan, B.; Kong, X.; Li, J.; Li, G.; He, L.; Bai, M.; Yin, Y. Dietary Insect Powder Protein Sources Improve Protein Utilization by Regulation on Intestinal Amino Acid-Chemosensing System. Animals 2020, 10, 1590. [Google Scholar] [CrossRef] [PubMed]
- Joint WHO/FAO/UNU Expert Consultation. Protein and Amino Acid Requirements in Human Nutrition: Report of a Joint WHO/FAO/UNU Expert Consultation; WHO Technical Report Series; Weltgesundheitsorganisation, FAO, Vereinte Nationen, Eds.; WHO: Geneva, Switzerland, 2007; ISBN 978-92-4-120935-9.
- Khatun, H.; Claes, J.; Smets, R.; De Winne, A.; Akhtaruzzaman, M.; Van Der Borght, M. Characterization of Freeze-Dried, Oven-Dried and Blanched House Crickets (Acheta Domesticus) and Jamaican Field Crickets (Gryllus Assimilis) by Means of Their Physicochemical Properties and Volatile Compounds. Eur. Food Res. Technol. 2021, 247, 1291–1305. [Google Scholar] [CrossRef]
- Udomsil, N.; Imsoonthornruksa, S.; Gosalawit, C.; Ketudat-Cairns, M. Nutritional Values and Functional Properties of House Cricket (Acheta Domesticus) and Field Cricket (Gryllus Bimaculatus). Food Sci. Technol. Res. 2019, 25, 597–605. [Google Scholar] [CrossRef]
- Gravel, A.; Marciniak, A.; Couture, M.; Doyen, A. Effects of Hexane on Protein Profile, Solubility and Foaming Properties of Defatted Proteins Extracted from Tenebrio Molitor Larvae. Molecules 2021, 26, 351. [Google Scholar] [CrossRef]
- Harris, G.K.; Marshall, M.R. Ash Analysis. In Food Analysis; Nielsen, S.S., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 287–297. ISBN 978-3-319-45776-5. [Google Scholar]
- Sun, K.; Nguyen, C.V.; Nguyen, N.N.; Nguyen, A.V. Flotation Surface Chemistry of Water-Soluble Salt Minerals: From Experimental Results to New Perspectives. Adv. Colloid Interface Sci. 2022, 309, 102775. [Google Scholar] [CrossRef]
- Navarro del Hierro, J.; Gutiérrez-Docio, A.; Otero, P.; Reglero, G.; Martin, D. Characterization, Antioxidant Activity, and Inhibitory Effect on Pancreatic Lipase of Extracts from the Edible Insects Acheta Domesticus and Tenebrio Molitor. Food Chem. 2020, 309, 125742. [Google Scholar] [CrossRef]
- Chung, K.-T.; Wong, T.Y.; Wei, C.-I.; Huang, Y.-W.; Lin, Y. Tannins and Human Health: A Review. Crit. Rev. Food Sci. Nutr. 1998, 38, 421–464. [Google Scholar] [CrossRef]
- Iwuozor, K.O. Qualitative and Quantitative Determination of Anti-Nutritional Factors of Five Wine Samples. Adv. J. Chem.-Sect. A 2019, 2, 136–146. [Google Scholar] [CrossRef] [Green Version]
- Meyer-Rochow, V.B.; Gahukar, R.T.; Ghosh, S.; Jung, C. Chemical Composition, Nutrient Quality and Acceptability of Edible Insects Are Affected by Species, Developmental Stage, Gender, Diet, and Processing Method. Foods 2021, 10, 1036. [Google Scholar] [CrossRef] [PubMed]
- Afify, A.E.-M.M.; El-Beltagi, H.S.; Abd El-Salam, S.M.; Omran, A.A. Bioavailability of Iron, Zinc, Phytate and Phytase Activity during Soaking and Germination of White Sorghum Varieties. PLoS ONE 2011, 6, e25512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGrath, J.R.; Beck, M.; Hill, M.E., Jr. Replicating Red: Analysis of Ceramic Slip Color with CIELAB Color Data. J. Archaeol. Sci. Rep. 2017, 14, 432–438. [Google Scholar] [CrossRef]
- Premi, S.; Brash, D.E. Unanticipated Role of Melanin in Causing Carcinogenic Cyclobutane Pyrimidine Dimers. Mol. Cell. Oncol. 2016, 3, e1033588. [Google Scholar] [CrossRef] [Green Version]
- Delgado-García, Y.I.; Luna-Suárez, S.; López-Malo, A.; Morales-Camacho, J.I. Effect of Supercritical Carbon Dioxide on Physicochemical and Techno-Functional Properties of Amaranth Flour. Chem. Eng. Process.-Process Intensif. 2022, 178, 109031. [Google Scholar] [CrossRef]
- Markovic, I.; Ilic, J.; Markovic, D.; Simonovic, V.; Kosanic, N. Color Measurement of Food Products Using CIE L* A* B* and RGB Color Space. J. Hyg. Eng. Des. 2013, 4, 50–53. [Google Scholar]
- Sioriki, E.; Lemarcq, V.; Alhakim, F.; Triharyogi, H.; Tuenter, E.; Cazin, C.S.J.; Nolan, S.P.; Pieters, L.; Van de Walle, D.; Dewettinck, K. Impact of Alkalization Conditions on the Phytochemical Content of Cocoa Powder and the Aroma of Cocoa Drinks. LWT 2021, 145, 111181. [Google Scholar] [CrossRef]
- Ludwig, I.A.; Bravo, J.; De Peña, M.P.; Cid, C. Effect of Sugar Addition (Torrefacto) during Roasting Process on Antioxidant Capacity and Phenolics of Coffee. LWT-Food Sci. Technol. 2013, 51, 553–559. [Google Scholar] [CrossRef]
- Gómez-Narváez, F.; Medina-Pineda, Y.; Contreras-Calderón, J. Evaluation of the Heat Damage of Whey and Whey Proteins Using Multivariate Analysis. Food Res. Int. 2017, 102, 768–775. [Google Scholar] [CrossRef]
- Mintah, B.K.; He, R.; Agyekum, A.A.; Dabbour, M.; Golly, M.K.; Ma, H. Edible Insect Protein for Food Applications: Extraction, Composition, and Functional Properties. J. Food Process Eng. 2020, 43, e13362. [Google Scholar] [CrossRef]
- Zielińska, E.; Karaś, M.; Baraniak, B. Comparison of Functional Properties of Edible Insects and Protein Preparations Thereof. LWT 2018, 91, 168–174. [Google Scholar] [CrossRef]
- Haque, M.A.; Timilsena, Y.P.; Adhikari, B. Food Proteins, Structure, and Function. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2016; p. B9780081005965031000. ISBN 978-0-08-100596-5. [Google Scholar]
- Aryee, A.N.A.; Agyei, D.; Udenigwe, C.C. Impact of Processing on the Chemistry and Functionality of Food Proteins. In Proteins in Food Processing; Elsevier: Amsterdam, The Netherlands, 2018; pp. 27–45. ISBN 978-0-08-100722-8. [Google Scholar]
- Kingwascharapong, P.; Chaijan, M.; Karnjanapratum, S. Ultrasound-Assisted Extraction of Protein from Bombay Locusts and Its Impact on Functional and Antioxidative Properties. Sci. Rep. 2021, 11, 17320. [Google Scholar] [CrossRef] [PubMed]
- Hiremath, P.G.; Rajashekhara, S.; Binnal, P.; Theodore, T. Chapter 9—Fluoride Contamination in Underground Water and Its Treatment. In Management of Contaminants of Emerging Concern (CEC) in Environment; Singh, P., Hussain, C.M., Rajkhowa, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 249–280. ISBN 978-0-12-822263-8. [Google Scholar]
- Mohammadi, K.; Movahhedy, M.R.; Khodaygan, S. Colloidal Particle Reaction and Aggregation Control in the Electrohydrodynamic 3D Printing Technology. Int. J. Mech. Sci. 2021, 195, 106222. [Google Scholar] [CrossRef]
- Hosokawa Micron Corporation. Powder Tester Model PT-X Operating Manual; A10082-OM01E. Available online: https://hosokawamex.com/catalogos/productos/hosokawa_micron-pt-x_catalogo.pdf (accessed on 21 February 2023).
- Groesbeck, C.; Goodband, R.; Tokach, M.; Nelssen, J.; Dritz, S.; Derouchey, J. Particle Size, Mill Type, and Added Fat Influence Angle of Repose of Ground Corn. Prof. Anim. Sci. 2006, 22, 120–125. [Google Scholar] [CrossRef]
- Tuohy, J. Some Physical Properties of Milk Powders. Ir. J. Food Sci. Technol. 1989, 13, 141–152. [Google Scholar]
- Barbosa-Cánovas, G.V.; Ortega-Rivas, E.; Juliano, P.; Yan, H. Food Powders: Physical Properties, Processing, and Functionality; Springer: Berlin/Heidelberg, Germany, 2005; Volume 86. [Google Scholar]
- Spierings, A.B.; Voegtlin, M.; Bauer, T.; Wegener, K. Powder Flowability Characterisation Methodology for Powder-Bed-Based Metal Additive Manufacturing. Prog. Addit. Manuf. 2016, 1, 9–20. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.; Guo, X.; Jin, Y.; Gong, X. Effect of Moisture on Flowability of Pulverized Coal. Chem. Eng. Res. Des. 2018, 133, 326–334. [Google Scholar] [CrossRef]
- Bourne, M.C. Food Texture and Viscosity: Concept and Measurement, 2nd ed.; Food Science and Technology International Series; Academic Press: San Diego, CA, USA, 2002; ISBN 978-0-12-119062-0. [Google Scholar]
- Liu, Z.; Bhandari, B.; Prakash, S.; Mantihal, S.; Zhang, M. Linking Rheology and Printability of a Multicomponent Gel System of Carrageenan-Xanthan-Starch in Extrusion Based Additive Manufacturing. Food Hydrocoll. 2019, 87, 413–424. [Google Scholar] [CrossRef]
- De Smet, J.; Lenaerts, S.; Borremans, A.; Scholliers, J.; Van Der Borght, M.; Van Campenhout, L. Stability Assessment and Laboratory Scale Fermentation of Pastes Produced on a Pilot Scale from Mealworms (Tenebrio Molitor). LWT 2019, 102, 113–121. [Google Scholar] [CrossRef]
- Pant, A.; Lee, A.Y.; Karyappa, R.; Lee, C.P.; An, J.; Hashimoto, M.; Tan, U.-X.; Wong, G.; Chua, C.K.; Zhang, Y. 3D Food Printing of Fresh Vegetables Using Food Hydrocolloids for Dysphagic Patients. Food Hydrocoll. 2021, 114, 106546. [Google Scholar] [CrossRef]









| Original Powder | pH 5 Treated Powder | Ethanol 20% Treated Powder | Ethanol 99.5% Treated Powder | Hexane Treated Powder | |
|---|---|---|---|---|---|
| Total powder recovery (%) | - | 79.1 ± 2.0 a | 72.3 ± 1.0 b | 63.3 ± 2.8 c | 67.1 ± 0.2 d |
| Protein (%) | 55.4 ± 1.0 a | 56.0 ± 2.2 a | 60.4 ± 0.6 b | 72.5 ± 0.4 c | 68.5 ± 1.9 d |
| Protein recovery (%) | - | 79.8 ± 3.1 a | 78.8 ± 0.8 a | 82.9 ± 0.4 a | 83.0 ± 2.3 a |
| Fat (%) | 33.0 ± 0.6 a | 29.3 ± 0.2 b | 23.0 ± 0.7 c | 6.8 ± 0.3 d | 7.8 ± 0.4 e |
| Fat recovery (%) | - | 70.3 ± 0.4 a | 50.4 ± 1.5 b | 13.0 ± 0.6 c | 16.0 ± 0.9 d |
| Chitin (%) | 7.3 ± 0.2 a | 8.7 ± 0.9 b | 9.8 ± 0.8 b | 9.5 ± 0.5 b | 9.8 ± 0.8 b |
| Chitin recovery (%) | - | 90.9 ± 10 a | 93.5 ± 6.3 a | 82.6 ± 4.3 a | 90.6 ± 7.4 a |
| Ash (%) | 3.2 ± 0.0 a | 1.5 ± 0.2 b | 2.2 ± 0.2 a | 4.1 ± 0.2 a | 2.8 ± 0.3 a |
| Ash recovery (%) | - | 41.7 ± 0.6 a | 50.2 ± 5.4 a | 85.5 ± 1.3 b | 62.4 ± 5.3 c |
| Moisture (%) | 3.7 ± 0.3 a | 3.5 ± 0.2 a | 2.7 ± 0.1 a | 4.5 ± 0.3 a | 4.6 ± 0.1 a |
| Total phenolic compounds (g/kg) | 6.0 ± 0.17 a | 4.3 ± 1.1 b | 4,6 ± 1.7 b | 7.7 ± 3.6 c | 5.6 ± 3.3 a |
| Total phenolic compounds recovery (%) | - | 57.3 ± 1.5 a | 55.6 ± 2.0 a | 82.0 ± 3.8 b | 62.5 ± 3.7 a |
| Tannins (g/kg) | 13.0 ± 1.7 a | 7.3 ± 2.5 b | 5.9 ± 1.9 b | 16.0 ± 4.6 a | 16.0 ± 2.6 a |
| Tannins recovery (%) | - | 57.3 ± 1.5 a | 55.6 ± 2.0 a | 82.0 ± 3.8 b | 62.5 ± 3.7 a |
| Phytates (mg/kg) | 9.32 ± 0.08 a | 8.92 ± 0.08 b | 9.12 ± 0.05 a | 8.75 ± 0.09 a | 9.19 ± 0.36 a |
| Phytates recovery (%) | - | 75.2 ± 0.1 a | 70.8 ± 0.4 b | 59.4 ± 0.6 c | 66.2 ± 2.5 d |
|
Original Powder |
pH 5 Treated Powder | Ethanol 20% Treated Powder | Ethanol 99.5% Treated Powder | Hexane Treated Powder | |
|---|---|---|---|---|---|
| pH | 7.0 a | 4.9 b | 7.3 a | 7.0 a | 6.8 a |
| Zeta potential (mV) | −27.9 (−33.8–−25.8) | 16.2 (15.4–17.0) | −31.5 (−31.5–−30.4) | −19.25 (−17.9–−20.3) | −27.5 (−28.0–−26.9) |
| Material | Cohesion | Carr Index |
|---|---|---|
| Original Powder | n/a | 0 |
| pH 5 | n/a | 0 |
| Ethanol 20% | 59.8 ± 2.6 | 2 |
| Ethanol 99.5% | 50.3 ± 3.5 | 7 |
| Hexane | 48.7 ± 1.0 | 7 |
| Comparison | Correlation? | p Value |
|---|---|---|
| Fat × Color | Yes | <0.01 |
| Fat × WHC | Yes | <0.01 |
| Fat × OHC | Yes | <0.01 |
| Fat × AoR | Yes | <0.01 |
| Protein × WHC | Yes | <0.01 |
| Protein × OHC | Yes | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Damasceno, B.C.; Nakajima, M.; Taarji, N.; Kobayashi, I.; Ichikawa, S.; Neves, M.A. Improvements in Visual Aspects and Chemical, Techno-Functional and Rheological Characteristics of Cricket Powder (Gryllus bimaculatus) by Solvent Treatment for Food Utilization. Foods 2023, 12, 1422. https://doi.org/10.3390/foods12071422
Damasceno BC, Nakajima M, Taarji N, Kobayashi I, Ichikawa S, Neves MA. Improvements in Visual Aspects and Chemical, Techno-Functional and Rheological Characteristics of Cricket Powder (Gryllus bimaculatus) by Solvent Treatment for Food Utilization. Foods. 2023; 12(7):1422. https://doi.org/10.3390/foods12071422
Chicago/Turabian StyleDamasceno, Barbara Catalano, Mitsutoshi Nakajima, Noamane Taarji, Isao Kobayashi, Sosaku Ichikawa, and Marcos A. Neves. 2023. "Improvements in Visual Aspects and Chemical, Techno-Functional and Rheological Characteristics of Cricket Powder (Gryllus bimaculatus) by Solvent Treatment for Food Utilization" Foods 12, no. 7: 1422. https://doi.org/10.3390/foods12071422