Effect of Processing and In Vitro Digestion on Bioactive Constituents of Powdered IV Range Carrot (Daucus carota, L.) Wastes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material
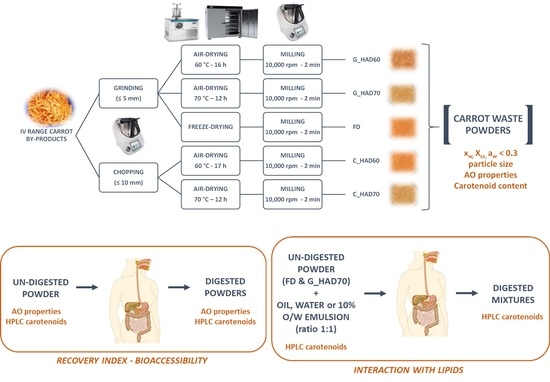
2.2. Powders Manufacturing
2.3. In Vitro Digestion Method
2.4. Analytical Determinations
2.4.1. Water Activity, Moisture Content, Total Soluble Solids, and Particle Size
2.4.2. Antioxidant Properties of Carrot Wastes and Powders
2.4.3. Carotenoids Content
2.5. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Properties of Carrot Waste Powder
3.2. Antioxidant Properties of Fresh and Powdered Carrot Waste
3.3. Carotenoid Content of Carrot Waste Powders
3.4. Simulated In Vitro Digestion of Carrot Waste Powders
3.4.1. Antioxidant Properties along Simulated In Vitro Digestion
3.4.2. Carotenoid Release along Simulated In Vitro Digestion
3.4.3. Interaction with Lipids during In Vitro Digestion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hernández Rendón, R.M.; Blanco Gómez, D.J. Evaluación de polvos de zanahoria obtenidos por deshidratación por aire forzado a diferentes temperaturas. Idesia 2015, 33, 75–80. [Google Scholar] [CrossRef]
- Sharma, H.K.; Kumar, N. Utilization of Carrot Pomace. In Food Processing By-Products and their Utilization; Anal, A.K., Ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2017; pp. 207–229. [Google Scholar]
- Sun, T.; Simon, P.W.; Tanumihardjo, S.A. Antioxidant Phytochemicals and Antioxidant Capacity of Biofortified Carrots (Daucus carota L.) of Various Colors. J. Agric. Food Chem. 2009, 57, 4142–4147. [Google Scholar] [CrossRef] [PubMed]
- Rawson, A.; Tiwari, B.K.; Tuohy, M.G.; O’Donnell, C.P.; Brunton, N. Effect of ultrasound and blanching pretreatments on polyacetylene and carotenoid content of hot air and freeze dried carrot discs. Ultrason Sonochem. 2011, 18, 1172–1179. [Google Scholar] [CrossRef]
- Barzee, T.J.; El- Mashad, H.M.; Zhang, R.; Pan, Z. Carrots. In Integrated Processing Technologies for Food and Agricultural By-Products; Pan, Z., Zhang, R., Zicari, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 297–330. [Google Scholar]
- Lin, T.; Durance, T.; Scaman, C. Characterization of vacuum microwave, air and freeze dried carrot slices. Food Res. Int. 1998, 31, 111–117. [Google Scholar] [CrossRef]
- Neacsu, M.; Vaughan, N.; Raikos, V.; Multari, S.; Duncan, G.J.; Duthie, G.G.; Russell, W.R. Phytochemical profile of commercially available food plant powders: Their potential role in healthier food reformulations. Food Chem. 2015, 179, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zhang, M.; Adhikari, B. Fruit and vegetable powders. In Handbook of Food Powders: Processes and Properties; Bhandari, B., Bansal, N., Zhang, M., Schuck, P., Eds.; Woodhead Publishing: Sawston, UK, 2013; pp. 532–552. [Google Scholar]
- Santos, P.H.S.; Silva, M.A. Retention of Vitamin C in Drying Processes of Fruits and Vegetables—A Review. Dry Technol. 2008, 26, 1421–1437. [Google Scholar] [CrossRef]
- Calín-Sánchez, A.; Lipan, L.; Cano-Lamadrid, M.; Kharaghani, A.; Masztalerz, K.; Carbonell-Barrachina, A.A.; Figie, A. Comparison of Traditional and Novel Drying Techniques and Its Effect on Quality of Fruits, Vegetables and Aromatic Herbs. Foods 2020, 9, 1261. [Google Scholar] [CrossRef] [PubMed]
- Anjani, G.; Ayustaningwarno, F.; Eviana, R. Critical review on the immunomodulatory activities of carrot’s β-carotene and other bioactive compounds. J. Funct. Foods 2022, 99, 105303. [Google Scholar] [CrossRef]
- Bobrowski Rodrigues, D.; Barros Mariutti, L.R.; Zerlotti Mercadante, A. An in vitro digestion method adapted for carotenoids and carotenoid esters: Moving forward towards standardization. Food Funct. 2016, 7, 4992–5001. [Google Scholar] [CrossRef]
- Adadi, P.; Barakova, N.V.; Krivoshapkina, E.F. Selected Methods of Extracting Carotenoids, Characterization, and Health Concerns: A Review. J. Agric. Food Chem. 2018, 66, 5925–5947. [Google Scholar] [CrossRef]
- Cui, Z.W.; Xu, S.Y.; Sun, D.W. Effect of Microwave-Vacuum Drying on the Carotenoids Retention of Carrot Slices and Chlorophyll Retention of Chinese Chive Leaves. Dry Technol. 2007, 22, 563–575. [Google Scholar] [CrossRef]
- Pinazo-Durán, M.D.; Bendala-Tufanisco, E. Capítulo IV. Las vitaminas, carotenoides y minerales. In Guía Sobre Alimentación en las Enfermedades de la Retina; BRUDYLAB S.L.: Barcelona, Spain, 2017; pp. 37–47. [Google Scholar]
- Müller-Maatsch, J.; Sprenger, J.; Hempel, J.; Kreiser, F.; Carle, R.; Schweiggert, R.M. Carotenoids from gac fruit aril (Momordica cochinchinensis [Lour.] Spreng.) are more bioaccessible than those from carrot root and tomato fruit. Food Res. Int. 2017, 99, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Maiani, G.; Castón, M.J.; Catasta, G.; Toti, E.; Cambrodón, I.G.; Bysted, A.; Granado-Lorencio, F.; Olmedilla-Alonso, B.; Knuthsen, P.; Valoti, M.; et al. Carotenoids: Actual knowledge on food sources, intakes, stability and bioavailability and their protective role in humans. Mol. Nutr. Food Res. 2009, 53, S194–S218. [Google Scholar] [CrossRef]
- Camargo Marques, M.; Hacke, A.; Camargo Neto, C.A.; Mariutti, L.R. Impact of phenolic compounds in the digestion and absorption of carotenoids. Curr. Opin. Food Sci. 2021, 39, 190–196. [Google Scholar] [CrossRef]
- Beltrán-de-Miguel, B.; Estévez-Santiago, R.; Olmedilla-Alonso, B. Assessment of dietary vitamin A intake (retinol, α-carotene, β-carotene, β-cryptoxanthin) and its sources in the National Survey of Dietary Intake in Spain (2009–2010). Int. J. Food Sci. Nutr. 2015, 66, 706–712. [Google Scholar] [CrossRef]
- Estévez-Santiago, R.; Olmedilla-Alonso, B.; Fernández-Jalao, I. Bioaccessibility of provitamin A carotenoids from fruits: Application of a standardised static in vitro digestion method. Food Funct. 2016, 7, 1354–1366. [Google Scholar] [CrossRef] [PubMed]
- Hornero-Méndez, D.; Mínguez-Mosquera, M.I. Bioaccessibility of carotenes from carrots: Effect of cooking and addition of oil. Innov. Food Sci. Emerg. Technol. 2007, 8, 407–412. [Google Scholar] [CrossRef]
- van het Hof, K.H.; West, C.E.; Weststrate, J.A.; Hautvast, J.G.A.J. Dietary factors that affect the bioavailability of carotenoids. J. Nutr. 2000, 130, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Hernández-Olivas, E.; Asensio-Grau, A.; Calvo-Lerma, J.; García-Hernández, J.; Heredia, A.; Andrés, A. Content and bioaccessibility of bioactive compounds with potential benefits for macular health in tiger nut products. Food Biosci. 2022, 49, 101879. [Google Scholar] [CrossRef]
- Bas-Bellver, C.; Barrera, C.; Betoret, N.; Seguí, L. Turning Agri-Food Cooperative Vegetable Residues into Functional Powdered Ingredients for the Food Industry. Sustainability 2020, 12, 1284. [Google Scholar] [CrossRef]
- Hedrén, E.; Diaz, V.; Svanberg, U. Estimation of carotenoid accessibility from carrots determined by an in vitro digestion method. Eur. J. Clin. Nutr. 2002, 56, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Ortega, N.; Macià, A.; Romero, M.P.; Reguant, J.; Motilva, M.J. Matrix composition effect on the digestibility of carob flour phenols by an in-vitro digestion model. Food Chem. 2011, 124, 65–71. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemist Official Methods of Analysis. AOAC Official Method 934.06, Moisture in Dried Fruits, 17th ed.; Association of Official Analytical Chemist Official Methods of Analysis: Rockville, MD, USA, 2000. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Luximon-Ramma, A.; Bahorun, T.; Soobrattee, M.A.; Aruoma, O.I. Antioxidant Activities of Phenolic, Proanthocyanidin, and Flavonoid Components in Extracts of Cassia fistula. J. Agric. Food Chem. 2002, 50, 5042–5047. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Bunea, A.; Andjelkovic, M.; Socaciu, C.; Bobis, O.; Neacsu, M.; Verhéc, R.; Van Camp, J. Total and individual carotenoids and phenolic acids content in fresh, refrigerated and processed spinach (Spinacia oleracea L.). Food Chem. 2008, 108, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Surles, R.; Weng, N.; Simon, P.; Tanumihardjo, S. Carotenoid profiles and consumer sensory evaluation of specialty carrots (Daucus carota, L.) of various colors. J. Agric. Food Chem. 2004, 52, 3417–3421. [Google Scholar] [CrossRef]
- Djantou, E.B.; Mbofung, C.M.F.; Scher, J.; Phambu, N.; Morael, J.D. Alternation drying and grinding (ADG) technique: A novel approach for producing ripe mango powder. LWT—Food Sci. Technol. 2011, 44, 1585–1590. [Google Scholar] [CrossRef]
- Gulati, T.; Datta, A.K. Mechanistic understanding of case-hardening and texture development during drying of food materials. J. Food Eng. 2015, 166, 119–138. [Google Scholar] [CrossRef]
- Xiao, H.; Gao, Z.; Lin, H.; Yang, W. Air impingement drying characteristics and quality of carrot cubes. J. Food Process. Eng. 2010, 33, 899–918. [Google Scholar] [CrossRef]
- van Buggenhout, S.; Lille, M.; Messagie, I.; von Loey, A.; Autio, K.; Hendrickx, M. Impact of pretreatment and freezing conditions on the microstructure of frozen carrots: Quantification and relation to texture loss. Eur. Food Res. Technol. 2006, 222, 543–553. [Google Scholar] [CrossRef]
- Raghavendra, S.N.; Ramachandra Swamy, S.R.; Rastogi, N.K.; Raghavarao, K.S.M.S.; Kumar, S.; Tharanathan, R.N. Grinding characteristics and hydration properties of coconut residue: A source of dietary fiber. J. Food Eng. 2006, 72, 281–286. [Google Scholar] [CrossRef]
- Yi, J.Y.; Lyu, J.; Bi, J.F.; Zhou, L.Y.; Zhou, M. Hot air drying and freeze drying pre-treatments coupled to explosion puffing drying in terms of quality attributes of mango, pitaya, and papaya fruit chips. J. Food Process. Preserv. 2017, 41, e13300. [Google Scholar] [CrossRef]
- Owusu, J.; Haile, M.Y.; Zhen-bin, W.; Amissa, A. Effect of Drying Methods on Physicochemical Properties of Pretreated Tomato (lycopersicon esculentum mill.) Slices | Semantic Scholar. Croat. J. Food Technol. Biotechnol. Nutr. 2012, 7, 106–111. [Google Scholar]
- Bas-Bellver, C.; Andrés, C.; Seguí, L.; Barrera, C.; Jiménez-Hernández, N.; Artacho, A.; Betoret, N.; Gosalbes, M.J. Valorization of Persimmon and Blueberry Byproducts to Obtain Functional Powders: In Vitro Digestion and Fermentation by Gut Microbiota. J. Agric. Food Chem. 2020, 68, 8080–8090. [Google Scholar] [CrossRef] [PubMed]
- Macura, R.; Michalczyk, M.; Fiutak, G.; Maciejaszek, I. Effect of freeze-drying and air-drying on the content of carotenoids and anthocyanins in stored purple carrot. Acta Sci. Pol Technol. Aliment. 2019, 18, 135–142. [Google Scholar]
- Sablani, S.S.; Andrews, P.K.; Davies, N.M.; Walters, T.; Saez, H.; Bastarrachea, L. Effects of Air and Freeze Drying on Phytochemical Content of Conventional and Organic Berries. Dry Technol. 2011, 29, 205–216. [Google Scholar] [CrossRef]
- Barden, L.; Decker, E.A. Lipid Oxidation in Low-moisture Food: A Review. Crit. Rev. Food Sci. Nutr. 2016, 56, 2467–2482. [Google Scholar] [CrossRef]
- Bernaert, N.; de Clercq, H.; van Bockstaele, E.; de Loose, M.; van Droogenbroeck, B. Antioxidant changes during postharvest processing and storage of leek (Allium ampeloprasum var. porrum). Postharvest. Biol. Technol. 2013, 86, 8–16. [Google Scholar] [CrossRef]
- Gahler, S.; Otto, K.; Böhm, V. Alterations of vitamin C, total phenolics, and antioxidant capacity as affected by processing tomatoes to different products. J. Agric. Food Chem. 2003, 51, 7962–7968. [Google Scholar] [CrossRef] [PubMed]
- Liyana-Pathirana, C.; Shahidi, F. Antioxidant activity of commercial soft and hard wheat (Triticum aestivum L.) as affected by gastric pH conditions. J. Agric. Food Chem. 2005, 53, 2433–2440. [Google Scholar] [CrossRef] [PubMed]
- Miletic, N.; Mitrovic, O.; Popovic, B.; Nedovic, V.; Zlatkovic, B.; Kandic, M. Polyphenolic content and antioxidant capacity in fruits of plum (Prunus Domestica L.) cultivars ‘Valjevka’ and ‘Mildora’ as influenced by air drying. J. Food Qual. 2013, 36, 229–237. [Google Scholar] [CrossRef]
- Bas-Bellver, C.; Barrera, C.; Betoret, N.; Seguí, L. Impact of Disruption and Drying Conditions on Physicochemical, Functional and Antioxidant Properties of Powdered Ingredients Obtained from Brassica Vegetable By-Products. Foods. 2022, 11, 3663. [Google Scholar] [CrossRef]
- Albanese, D.; Adiletta, G.; D′Acunto, M.; Cinquanta, L.; di Matteo, M. Tomato peel drying and carotenoids stability of the extracts. Int. J. Food Sci. Technol. 2014, 49, 2458–2463. [Google Scholar] [CrossRef]
- Hinestroza-Córdoba, L.I.; Serna, S.D.; Seguí, L.; Barrera, C.; Betoret, N. Characterization of Powdered Lulo (Solanum quitoense) Bagasse as a Functional Food Ingredient. Foods 2020, 9, 723. [Google Scholar] [CrossRef]
- Zhang, Z.; Wei, Q.; Nie, M.; Jiang, N.; Liu, C.; Liu, C.; Li, D.; Xu, L. Microstructure and bioaccessibility of different carotenoid species as affected by hot air drying: Study on carrot, sweet potato, yellow bell pepper and broccoli. LWT 2018, 96, 357–363. [Google Scholar] [CrossRef]
- Leong, S.Y.; Oey, I. Effects of processing on anthocyanins, carotenoids and vitamin C in summer fruits and vegetables. Food Chem. 2012, 133, 1577–1587. [Google Scholar] [CrossRef]
- Pereira-Caro, G.; Ordóñez-Díaz, J.L.; de Santiago, E.; Moreno-Ortega, A.; Cáceres-Jiménez, S.; Sánchez-Parra, M.; Roldán-Guerra, F.J.; Ortiz-Somovilla, V.; Moreno-Rojas, J.M. Antioxidant Activity and Bio-Accessibility of Polyphenols in Black Carrot (Daucus carota L. ssp. sativus var. atrorubens Alef.) and Two Derived Products during Simulated Gastrointestinal Digestion and Colonic Fermentation. Foods 2021, 10, 457. [Google Scholar] [CrossRef]
- Gouw, V.P.; Jung, J.; Zhao, Y. Functional properties, bioactive compounds, and in vitro gastrointestinal digestion study of dried fruit pomace powders as functional food ingredients. LWT 2017, 80, 136–144. [Google Scholar] [CrossRef]
- Nayak, P.K.; Sundarsingh, A.; Kesavan, R. In vitro gastrointestinal digestion studies on total phenols, flavonoids, anti-oxidant activity and vitamin C in freeze-dried vegetable powders. J. Food Sci. Technol. 2022, 59, 4253–4261. [Google Scholar] [CrossRef]
- Nayak, P.K.; Chandrasekar, C.M.; Sundarsingh, A.; Kesavan, R.K. Effect of in-vitro digestion on the bio active compounds and biological activities of fruit pomaces. J. Food Sci. Technol. 2020, 57, 4707–4715. [Google Scholar] [CrossRef]
- Chen, G.L.; Chen, S.G.; Chen, F.; Xie, Y.Q.; Han, M.D.; Luo, C.X.; Zhao, Y.Y.; Gao, Y.Q. Nutraceutical potential and antioxidant benefits of selected fruit seeds subjected to an in vitro digestion. J. Funct. Foods 2016, 20, 317–331. [Google Scholar] [CrossRef]
- Martínez-Las Heras, R.; Pinazo, A.; Heredia, A.; Andrés, A. Evaluation studies of persimmon plant (Diospyros kaki) for physiological benefits and bioaccessibility of antioxidants by in vitro simulated gastrointestinal digestion. Food Chem. 2017, 214, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Vitaglione, P.; Napolitano, A.; Fogliano, V. Cereal dietary fibre: A natural functional ingredient to deliver phenolic compounds into the gut. Trends Food Sci. Technol. 2008, 19, 451–463. [Google Scholar] [CrossRef]
- Dong, R.; Liu, S.; Xie, J.; Chen, Y.; Zheng, Y.; Zhang, X.; Zhao, E.; Wang, Z.; Xu, H.; Yu, Q. The recovery, catabolism and potential bioactivity of polyphenols from carrot subjected to in vitro simulated digestion and colonic fermentation. Food Res. Int. 2021, 143, 110263. [Google Scholar] [CrossRef]
- Platzer, M.; Kiese, S.; Herfellner, T.; Schweiggert-Weisz, Y.; Miesbauer, O.; Eisner, P. Common Trends and Differences in Antioxidant Activity Analysis of Phenolic Substances Using Single Electron Transfer Based Assays. Molecules 2021, 26, 1244. [Google Scholar] [CrossRef]
- Panzella, L.; Moccia, F.; Nasti, R.; Marzorati, S.; Verotta, L.; Napolitano, A. Bioactive Phenolic Compounds From Agri-Food Wastes: An Update on Green and Sustainable Extraction Methodologies. Front. Nutr. 2020, 7, 60. [Google Scholar] [CrossRef]
- Ozgen, M.; Reese, R.N.; Tulio, A.Z.; Scheerens, J.C.; Miller, A.R. Modified 2,2-Azino-bis-3-ethylbenzothiazoline-6-sulfonic Acid (ABTS) Method to Measure Antioxidant Capacity of Selected Small Fruits and Comparison to Ferric Reducing Antioxidant Power (FRAP) and 2,2‘-Diphenyl-1-picrylhydrazyl (DPPH) Methods. J. Agric. Food Chem. 2006, 54, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Gullon, B.; Pintado, M.; Barber, X.; Fernández-López, J.; Pérez-Álvarez, J.; Viuda-Martos, M. Bioaccessibility, changes in the antioxidant potential and colonic fermentation of date pits and apple bagasse flours obtained from co-products during simulated in vitro gastrointestinal digestion. Food Res. Int. 2015, 78, 169–176. [Google Scholar] [CrossRef]
- Lyu, Y.; Bi, J.; Chen, Q.; Wu, X.; Qiao, Y.; Hou, H.; Zhang, X. Bioaccessibility of carotenoids and antioxidant capacity of seed-used pumpkin byproducts powders as affected by particle size and corn oil during in vitro digestion process. Food Chem. 2021, 343, 128541. [Google Scholar] [CrossRef]
- Rodríguez-Roque, M.; Rojas-Graü, M.; Elez-Martínez, P.; Martín-Belloso, O. Changes in vitamin C, phenolic, and carotenoid profiles throughout in vitro gastrointestinal digestion of a blended fruit juice. J. Agric. Food Chem. 2013, 61, 1859–1867. [Google Scholar] [CrossRef]
- Tydeman, E.A.; Parker, M.L.; Wickham, M.S.J.; Rich, G.T.; Faulks, R.M.; Gidley, M.J.; Fillery-Travis, A.; Waldron, K.W. Effect of Carrot (Daucus carota) Microstructure on Carotene Bioaccessibilty in the Upper Gastrointestinal Tract. 1. In Vitro Simulations of Carrot Digestion. J. Agric. Food Chem. 2010, 58, 9847–9854. [Google Scholar] [CrossRef]
- Eriksen, J.N.; Luu, A.Y.; Dragsted, L.O.; Arrigoni, E. Adaption of an in vitro digestion method to screen carotenoid liberation and in vitro accessibility from differently processed spinach preparations. Food Chem. 2017, 224, 407–413. [Google Scholar] [CrossRef]
- Moelants, K.R.M.; Lemmens, L.; Vandebroeck, M.; van Buggenhout, S.; van Loey, A.M.; Hendrickx, M.E. Relation between Particle Size and Carotenoid Bioaccessibility in Carrot- and Tomato-Derived Suspensions. J. Agric. Food Chem. 2012, 60, 11995–12003. [Google Scholar] [CrossRef] [PubMed]
- van het Hof, K.; Gärtner, C.; West, C.; Tijburg, L. Potential of vegetable processing to increase the delivery of carotenoids to man. Int. J. Vitam. Nutr. Res. 1998, 68, 366–370. [Google Scholar] [PubMed]
- Aguilar Espinosa, M.; Alcalde, M.J.; Alonso, G.L.; Álvarez, R. Carotenoides en Agroalimentación y Salud; Meléndez-Martínez, A.J., Gómez Gómez, L., Olmedilla Alonso, B., Pérez-Gálvez, A., Hornero-Méndez, D., Eds.; Editorial Terracota, SA: Mexico City, México, 2017. [Google Scholar]
- Hu, X.; Jandacek, R.J.; White, W.S. Intestinal absorption of β-carotene ingested with a meal rich in sunflower oil or beef tallow: Postprandial appearance in triacylglycerol-rich lipoproteins in women. Am. J. Clin. Nutr. 2000, 71, 1170–1180. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhang, Z.; Zou, L.; Xiao, H.; Zhang, G.; Decker, E.A.; McClements, D.J. Enhancing Nutraceutical Bioavailability from Raw and Cooked Vegetables Using Excipient Emulsions: Influence of Lipid Type on Carotenoid Bioaccessibility from Carrots. J. Agric. Food Chem. 2015, 63, 10508–10517. [Google Scholar] [CrossRef] [PubMed]


| Sample | aw | xw (gw/100 g) | Xss (gss/gdm) |
|---|---|---|---|
| G_HAD60 | 0.254 ± 0.008 b | 2.9 ± 0.4 b | 0.667 ± 0.017 a |
| C_HAD60 | 0.239 ± 0.010 ab | 2.96 ± 0.10 b | 0.659 ± 0.017 a |
| G_HAD70 | 0.236 ± 0.011 a | 1.6 ± 0.3 a | 0.685 ± 0.011 ab |
| C_HAD70 | 0.240 ± 0.005 ab | 3.26 ± 0.12 b | 0.685 ± 0.012 bc |
| FD | 0.236 ± 0.007 a | 2.80 ± 0.11 b | 0.724 ± 0.018 c |
| DRY PROCEDURE | |||||
| D[4,3] | D[3,2] | d10 | d50 | d90 | |
| G_HAD60 | 171 ± 6 c | 34.6 ± 1.3 b | 11.6 ± 0.3 b | 137 ± 7 c | 391 ± 10 c |
| C_HAD60 | 210 ± 6 e | 51.2 ± 1.5 c | 17.8 ± 0.5 d | 190 ± 7 e | 442 ± 12 e |
| G_HAD70 | 155 ± 3 b | 26.0 ± 0.6 a | 8.6 ± 0.2 a | 126 ± 5 b | 358 ± 5 b |
| C_HAD70 | 200 ± 6 d | 50 ± 3 c | 17.36 ± 0.7 d | 180 ± 7 d | 423 ± 9 d |
| FD | 124 ± 3 a | 33.1 ± 1.7 b | 12.5 ± 0.8 c | 107 ± 3 a | 258 ± 6 a |
| WET PROCEDURE | |||||
| D[4,3] | D[3,2] | d10 | d50 | d90 | |
| G_HAD60 | 245 ± 24 b | 53 ± 3 c | 20.6 ± 1.2 b | 209 ± 20 b | 530 ± 54 b |
| C_HAD60 | 348 ± 37 d | 78.8 ± 1.9 e | 35.4 ± 1.1 d | 292 ± 16 d | 723 ± 97 c |
| G_HAD70 | 228 ± 8 b | 48 ± 2 b | 18.8 ± 1.1 a | 194 ± 9 b | 500 ± 23 b |
| C_HAD70 | 273 ± 18 c | 67.1 ± 1.9 d | 28.8 ± 0.8 c | 249 ± 16 c | 562 ± 37 b |
| FD | 156 ± 2 a | 39.0 ± 0.4 a | 15.8 ± 0.3 a | 123.6 ± 1.4 a | 350 ± 6 a |
| Sample | Total Phenols (mg GAE/gdm) | Total Flavonoids (mg QE/gdm) | DPPH (mg TE/gdm) | ABTS (mg TE/gdm) |
|---|---|---|---|---|
| Carrot waste | 2.03 ± 0.09 c | 1.210 ± 0.012 a | 4.28 ± 0.04 e | 14.0 ± 0.6 a |
| G_HAD60 | 1.53 ± 0.12 b | 1.24 ± 0.06 ab | 1.90 ± 0.12 b | 55 ± 2 b |
| C_HAD60 | 2.06 ± 0.16 c | 1.464 ± 0.003 c | 2.1 ± 0.2 c | 57.5 ± 1.4 b |
| G_HAD70 | 2.004 ± 0.013 c | 1.27 ± 0.03 b | 1.69 ± 0.10 b | 62 ± 3 c |
| C_HAD70 | 2.42 ± 0.15 d | 1.45 ± 0.02 c | 2.65 ± 0.11 d | 64.8 ± 1.7 c |
| FD | 0.74 ± 0.14 a | 1.26 ± 0.03 ab | 1.01 ± 0.11 a | 16.9 ± 0.3 a |
| Sample | Lycopene (µg/gdm) |
Lutein (µg/gdm) |
β-Carotene (µg/gdm) | α-Carotene (µg/gdm) |
Total (µg/gdm) |
|---|---|---|---|---|---|
| G_HAD60 | 3.19 ± 0.02 c | 1.29 ± 0.04 c | 59 ± 15 c | 9.99 ± 0.10 b | 73 ± 15 c |
| C_HAD60 | 2.64 ± 0.07 b | 0.92 ± 0.15 a | 29.1 ± 0.2 a | 4.08 ± 0.04 a | 37.0 ± 0.6 a |
| G_HAD70 | 3.08 ± 0.03 c | 1.036 ± 0.011 ab | 42.2 ± 1.7 b | 9.4 ± 1.2 b | 56 ± 3 b |
| C_HAD70 | 2.517 ± 0.011 a | 1.142 ± 0.006 bc | 52.5 ± 0.8 bc | 12.04 ± 0.05 b | 67.8 ± 0.6 c |
| FD | 4.77 ± 0.09 d | 1.147 ± 0.005 bc | 221 ± 4 d | 59 ± 2 c | 286 ± 2 d |
| Sample | BD | Gastric Phase (GP) | Intestinal Phase (IP) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| S | P | TOTAL (%RI) | S | P | TOTAL (%RI) | %BI | |||
|
Total phenol content (mg GAE/g) | G_HAD60 | 1.48 ± 0.11 b | 0.24 ± 0.02 b | 0.68 ± 0.10 b | 0.93 ± 0.10 b (63% ± 7 c) | 1.01 ± 0.04 b | 0.35 ± 0.02 a | 1.37 ± 0.05 b (92% ± 3 b) | 100 ± 4 c |
| C_HAD60 | 2.00 ± 0.15 c | 0.259 ± 0.011 b | 0.7 ± 0.2 b | 0.96 ± 0.18 b (48% ± 9 b) | 1.22 ± 0.04 c | 0.42 ± 0.05 a | 1.64 ± 0.04 c (82% ± 2 ab) | 82 ± 3 b | |
| G_HAD70 | 1.972 ± 0.013 c | 0.49 ± 0.02 d | 1.41 ± 0.08 c | 1.89 ± 0.10 c (96% ± 5 d) | 2.05 ± 0.10 d | 0.85 ± 0.12 b | 2.9 ± 0.2 d (147% ± 10 c) | 140 ± 7 d | |
| C_HAD70 | 2.34 ± 0.14 d | 0.46 ± 0.02 c | 1.8 ± 0.2 d | 2.2 ± 0.2 d (95% ± 8 d) | 0.84 ± 0.05 a | 0.96 ± 0.15 b | 1.81 ± 0.10 c (77% ± 4 a) | 66 ± 4 a | |
| FD | 0.72 ± 0.13 a | 0.157 ± 0.005 a | 0.052 ± 0.014 a | 0.21 ± 0.02 a (29% ± 3 a) | 0.81 ± 0.03 a | 0.33 ± 0.03 a | 1.14 ± 0.6 a (158% ± 8 c) | 158 ± 5 e | |
|
Total flavonoid content (mg QE/g) | G_HAD60 | 1.20 ± 0.06 a | 0.44 ± 0.05 b | 0.245 ± 0.008 b | 0.69 ± 0.05 b (57% ± 4 c) | 0.210 ± 0.005 a | 1.22 ± 0.08 d | 1.43 ± 0.09 d (118% ± 7 e) | 25.5 ± 0.6 a |
| C_HAD60 | 1.421 ± 0.003 c | 0.24 ± 0.08 a | 0.272 ± 0.009 b | 0.51 ± 0.09 a (36% ± 6 a) | 0.279 ± 0.013 ab | 0.47 ± 0.04 b | 0.75 ± 0.04 b (53% ± 2 b) | 26.4 ± 1.2 a | |
| G_HAD70 | 1.25 ± 0.03 b | 0.39 ± 0.04 b | 0.73 ± 0.05 c | 1.13 ± 0.06 c (90% ± 5 d) | 0.83 ± 0.03 d | 0.462 ± 0.009 b | 1.30 ± 0.02 c (104% ± 2 d) | 90 ± 3 c | |
| C_HAD70 | 1.40 ± 0.02 c | 0.3813 ± 0.0011 b | 1.137 ± 0.011 d | 1.518 ± 0.010 d (108.6% ± 0.7 e) | 0.59 ± 0.04 c | 0.658 ± 0.012 c | 1.25 ± 0.04 c (89% ± 3 c) | 77 ± 5 b | |
| FD | 1.23 ± 0.03 ab | 0.44 ± 0.05 b | 0.090 ± 0.013 a | 0.53 ± 0.04 a (44% ± 3 b) | 0.28 ± 0.074 b | 0.27 ± 0.05 a | 0.55 ± 0.05 a (45% ± 4 a) | 33 ± 8 a | |
|
DPPH (mg TE/g) | G_HAD60 | 1.85 ± 0.12 b | 0.25 ± 0.03 c | 7.7 ± 0.9 b | 7.9 ± 0.9 b (428% ± 49 a) | 0.64 ± 0.07 a | 3.1 ± 0.4 b | 3.3 ± 0.7 b (181% ± 38 ab) | 51 ± 6 a |
| C_HAD60 | 2.1 ± 0.2 c | 0.180 ± 0.003 b | 8.5 ± 0.6 bc | 8.6 ± 0.6 bc (417% ± 28 a) | 0.79 ± 0.05 b | 2.9 ± 0.4 b | 3.7 ± 0.3 bc (179% ± 16 a) | 51 ± 3 a | |
| G_HAD70 | 1.67 ± 0.10 b | 0.319 ± 0.003 d | 8.3 ± 0.3 bc | 8.6 ± 0.4 bc (519% ± 21 b) | 1.37 ± 0.05 c | 2.90 ± 0.13 b | 4.27 ± 0.08 c (256% ± 5 c) | 111 ± 4 c | |
| C_HAD70 | 2.56 ± 0.10 d | 0.318 ± 0.004 d | 8.8 ± 0.5 c | 9.1 ± 0.5 c (356% ± 19 a) | 0.63 ± 0.06 a | 3.3 ± 0.8 b | 3.9 ± 0.8 bc (153% ± 31 a) | 45 ± 4 a | |
| FD | 0.99 ± 0.11 a | 0.100 ± 0.004 a | 6.1 ± 0.6 a | 6.3 ± 0.6 a (634% ± 64 c) | 0.53 ± 0.10 a | 1.61 ± 0.11 a | 2.1 ± 0.2 a (217% ± 20 b) | 76 ± 14 b | |
|
ABTS (mg TE/g) | G_HAD60 | 53 ± 2 b | 2.25 ± 0.03 d | 2.6 ± 0.9 a | 4.9 ± 0.9 a (9.2% ± 1.7 a) | 8.88 ± 0.09 c | 4.0 ± 0.5 a | 12.9 ± 0.4 b (24.4% ± 0.8 b) | 24.4 ± 0.2 b |
| C_HAD60 | 55.8 ± 1.3 b | 1.45 ± 0.02 b | 4.6 ± 1.3 b | 6.0 ± 1.3 a (11% ± 2 a) | 11.0 ± 0.2 d | 6.8 ± 0.6 bc | 17.8 ± 0.8 cd (31.9% ± 1.4 c) | 26.5 ± 0.5 c | |
| G_HAD70 | 61 ± 3 c | 2.06 ± 0.03 c | 3.6 ± 0.9 ab | 5.7 ± 0.9 a (9.3% ± 1.4 a) | 12.3 ± 0.3 e | 6.3 ± 1.4 b | 18.54 ± 1.13 d (30.3% ± 1.8 c) | 27.0 ± 0.6 c | |
| C_HAD70 | 62.6 ± 1.6 c | 2.01 ± 0.02 c | 3.4 ± 0.4 ab | 5.4 ± 0.4 a (8.6% ± 0.6 a) | 7.1 ± 0.3 a | 3.7 ± 1.2 a | 10.8 ± 1.6 a (17% ± 2 a) | 20.8 ± 0.9 a | |
| FD | 16.4 ± 0.3 a | 1.06 ± 0.05 a | 15.05 ± 1.12 c | 16.11 ± 1.09 b (98% ± 7 b) | 7.5 ± 0.2 b | 8.4 ± 0.9 c | 15.9 ± 0.9 c (97% ± 6 d) | 64.1 ± 1.4 d | |
| Carrot | G_HAD60 | C_HAD60 | G_HAD70 | C_HAD70 | FD | |||
|---|---|---|---|---|---|---|---|---|
| Lycopene | GP | µg/g | 12.96 ± 0.05 e | 11.2 ± 0.2 b | 10.54 ± 0.06 a | 12.53 ± 0.08 d | 13.50 ± 0.04 f | 11.76 ± 0.07 c |
| %RI | 241.2 ± 0.9 a | 362 ± 7 c | 411 ± 2 d | 414 ± 3 d | 554.3 ± 1.7 e | 253.3 ± 1.5 b | ||
| IP | µg/g | 22.22 ± 0.07 c | 21.02 ± 0.09 a | 21.43 ± 0.05 b | 23.13 ± 0.17 e | 22.6 ± 0.3 d | 22.8 ± 0.5 de | |
| %RI | 413.7 ± 1.3 a | 679 ± 3 c | 836.7 ± 1.9 e | 764 ± 6 d | 929 ± 12 f | 490 ± 10 b | ||
| Lutein | GP | µg/g | 4.93 ± 0.03 e | 3.2 ± 0.2 c | 2.66 ± 0.02 b | 3.09 ± 0.06 c | 3.68 ± 0.08 d | 2.48 ± 0.02 a |
| %RI | 40.5 ± 0.2 a | 258 ± 19 c | 297 ± 2 d | 303 ± 6 d | 333 ± 7 e | 222 ± 2 b | ||
| IP | µg/g | 5.91 ± 0.10 c | 4.83 ± 0.07 b | 4.84 ± 0.10 b | 4.78 ± 0.14 b | 4.72 ± 0.11 ab | 4.61 ± 0.09 a | |
| %RI | 48.5 ± 0.9 a | 385 ± 6 b | 540 ± 12 e | 469 ± 14 d | 428 ± 10 c | 414 ± 8 c | ||
| β-carotene | GP | µg/g | 611 ± 15 f | 182 ± 10 b | 138 ± 3 a | 415 ± 12 d | 443 ± 14 e | 331 ± 10 c |
| %RI | 109 ± 3 a | 318 ± 17 c | 490 ± 12 d | 1000 ± 30 f | 878 ± 28 e | 154 ± 5 b | ||
| IP | µg/g | 350 ± 40 b | 436 ± 14 c | 100 ± 4 a | 422 ± 5 c | 456 ± 10 c | 528 ± 55 d | |
| %RI | 62 ± 7 a | 764 ± 25 d | 355 ± 13 c | 1017 ± 11 f | 905 ±21 e | 246 ± 26 b | ||
| α-carotene | GP | µg/g | 157 ± 9 e | 37 ± 3 c | 17.5 ± 0.3 a | 27.2 ± 0.5 b | 14.2 ± 0.5 a | 92 ± 5 d |
| %RI | 98 ± 6 a | 383 ± 27 e | 441 ± 7 f | 294 ± 6 d | 122 ± 4 b | 160 ± 9 c | ||
| IP | µg/g | 128 ± 15 d | 52.2 ± 0.9 c | 30 ± 2 b | 37.6 ± 0.7 b | 16.2 ± 0.8 a | 166 ± 8 e | |
| %RI | 80 ± 9 a | 537 ± 10 e | 752 ± 62 f | 406 ± 7 d | 139 ± 7 b | 291 ± 13 c | ||
| Total | GP | µg/g | 787 ± 27 e | 223 ± 12 b | 169 ± 3 a | 458 ± 13 d | 474 ± 14 d | 437 ± 11 c |
| %RI | 106 ± 3 a | 328 ± 17 c | 470 ± 10 d | 835 ± 23 f | 723 ± 22 e | 157 ± 4 b | ||
| IP | µg/g | 505 ± 27 b | 514 ± 15 b | 156.1 ± 1.6 a | 488 ± 4 b | 500 ± 11 b | 722 ± 61 c | |
| %RI | 68 ± 4 a | 723 ± 22 d | 435 ± 4 c | 890 ± 8 f | 762 ± 17 e | 260 ± 22 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bas-Bellver, C.; Barrera, C.; Betoret, N.; Seguí, L. Effect of Processing and In Vitro Digestion on Bioactive Constituents of Powdered IV Range Carrot (Daucus carota, L.) Wastes. Foods 2023, 12, 731. https://doi.org/10.3390/foods12040731
Bas-Bellver C, Barrera C, Betoret N, Seguí L. Effect of Processing and In Vitro Digestion on Bioactive Constituents of Powdered IV Range Carrot (Daucus carota, L.) Wastes. Foods. 2023; 12(4):731. https://doi.org/10.3390/foods12040731
Chicago/Turabian StyleBas-Bellver, Claudia, Cristina Barrera, Noelia Betoret, and Lucía Seguí. 2023. "Effect of Processing and In Vitro Digestion on Bioactive Constituents of Powdered IV Range Carrot (Daucus carota, L.) Wastes" Foods 12, no. 4: 731. https://doi.org/10.3390/foods12040731