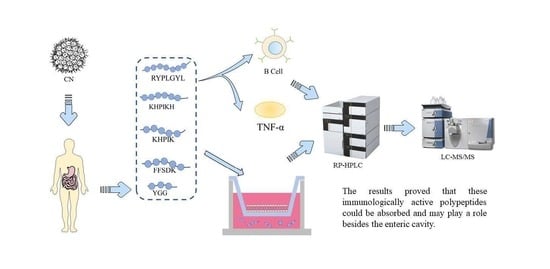
Identification of Immune-Active Peptides in Casein Hydrolysates and Its Transport Mechanism on a Caco-2 Monolayer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Simulated Gastrointestinal Digestion
2.3. Immune Activity Assay
2.3.1. MTT Assay to Evaluate B Lymphocyte Proliferation
2.3.2. Detection of TNF-α
2.4. RP-HPLC Detection of Immunologically Active Peptides
2.5. LC-MS/MS Detection of Immunologically Active Peptides
2.6. Transportation Assay
2.7. Transport Mechanism of the Immunologically Active Peptide
2.7.1. Study on the Absorption Mechanism of Peptides
2.7.2. Study on Peptide Efflux
2.8. Statistical Analysis
3. Results
3.1. Identification of Immune-Active Peptides in the Gastrointestinal Hydrolysate In Vitro
3.2. Immunoactivity Test of the Five Synthetic Peptides
3.3. Construction of the Caco-2 Cell Model
3.4. The Optimal Transport Condition of the KHPIK Peptide
3.5. RP-HPLC Analyzing of the Immune-Active Peptides after Transportation
3.6. Study on the Absorption Mechanism of the Immune-Active Peptides
3.7. The Efflux Mechanism of the Immune-Active Peptides
3.8. Integrity Analysis of the Peptide Fragment Passing through the Membrane
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Duanis-Assaf, D.; Kenan, E.; Sionov, R.; Steinberg, D.; Shemesh, M. Proteolytic Activity of Bacillus Subtilis upon κ-Casein Undermines Its “Caries-Safe” Effect. Microorganisms 2020, 8, 221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.E.; Lee, H.G. Amino Acids Supplementation for the Milk and Milk Protein Production of Dairy Cows. Animals 2021, 11, 2118. [Google Scholar] [CrossRef] [PubMed]
- Freund, M.A.; Chen, B.; Decker, E.A. The Inhibition of Advanced Glycation End Products by Carnosine and Other Natural Dipeptides to Reduce Diabetic and Age-Related Complications. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1367–1378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Q.; Hong, H.; Wu, J.; Yan, X. Bioavailability of Bioactive Peptides Derived from Food Proteins across the Intestinal Epithelial Membrane: A Review. Trends Food Sci. Technol. 2019, 86, 399–411. [Google Scholar] [CrossRef]
- Bohn, T.; Carriere, F.; Day, L.; Deglaire, A.; Egger, L.; Freitas, D.; Golding, M.; Le Feunteun, S.; Macierzanka, A.; Menard, O.; et al. Correlation between in Vitro and in Vivo Data on Food Digestion. What Can We Predict with Static in Vitro Digestion Models? Crit. Rev. Food Sci. Nutr. 2018, 58, 2239–2261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baptista, D.P.; Gigante, M.L. Bioactive Peptides in Ripened Cheeses: Release during Technological Processes and Resistance to the Gastrointestinal Tract. J. Sci. Food Agric. 2021, 101, 4010–4017. [Google Scholar] [CrossRef]
- Shivanna, S.K.; Nataraj, B.H. Revisiting Therapeutic and Toxicological Fingerprints of Milk-Derived Bioactive Peptides: An Overview. Food Biosci. 2020, 38, 100771. [Google Scholar] [CrossRef]
- Pihlanto-Leppälä, A. Bioactive Peptides Derived from Bovine Whey Proteins: Opioid and Ace-Inhibitory Peptides. Trends Food Sci. Technol. 2000, 11, 347–356. [Google Scholar] [CrossRef]
- Wieczorek, Z.; Zimecki, M.; Janusz, M.; Staroscik, K.; Lisowski, J. Proline-Rich Polypeptide from Ovine Colostrum—Its Effect on Skin Permeability and on the Immune-Response. Immunology 1979, 36, 875–881. [Google Scholar]
- Gill, H.S.; Doull, F.; Rutherfurd, K.J.; Cross, M.L. Immunoregulatory Peptides in Bovine Milk. Br. J. Nutr. 2000, 84, S111–S117. [Google Scholar] [CrossRef]
- Jaziri, M.; Migliore-Samour, D.; Casabianca-Pignède, M.R.; Keddad, K.; Morgat, J.L.; Jollès, P. Specific Binding Sites on Human Phagocytic Blood Cells for Gly-Leu-Phe and Val-Glu-Pro-Ile-Pro-Tyr, Immunostimulating Peptides from Human Milk Proteins. Biochim. Biophys. Acta (BBA)/Protein Struct. Mol. 1992, 1160, 251–261. [Google Scholar] [CrossRef]
- Parma, J.; Duprez, L.; Sande, J.V.; Cochaux, P.; Gervy, C.; Mockel, J.; Dumont, J.; Vassart, G. Somatic Mutations in the Thyrotropin Receptor Gene Cause Hyperfunctioning Thyroid Adenomas. Nature 1993, 365, 649–651. [Google Scholar] [CrossRef] [PubMed]
- Miguel, M.; Contreras, M.M.; Recio, I.; Aleixandre, A. ACE-Inhibitory and Antihypertensive Properties of a Bovine Casein Hydrolysate. Food Chem. 2009, 112, 211–214. [Google Scholar] [CrossRef]
- Sorrenti, V.; Ali, S.; Mancin, L.; Davinelli, S.; Paoli, A.; Scapagnini, G. Cocoa Polyphenols and Gut Microbiota Interplay: Bioavailability, Prebiotic Effect, and Impact on Human Health. Nutrients 2020, 12, 1908. [Google Scholar] [CrossRef] [PubMed]
- Darling, N.J.; Mobbs, C.L.; González-Hau, A.L.; Freer, M.; Przyborski, S. Bioengineering Novel in Vitro Co-Culture Models That Represent the Human Intestinal Mucosa with Improved Caco-2 Structure and Barrier Function. Front. Bioeng. Biotechnol. 2020, 8, 1–15. [Google Scholar] [CrossRef]
- Boim, A.G.F.; Wragg, J.; Canniatti-Brazaca, S.G.; Alleoni, L.R.F. Human Intestinal Caco-2 Cell Line in Vitro Assay to Evaluate the Absorption of Cd, Cu, Mn and Zn from Urban Environmental Matrices. Environ. Geochem. Health 2020, 42, 601–615. [Google Scholar] [CrossRef]
- Wang, B.; Xie, N.; Li, B. Influence of Peptide Characteristics on Their Stability, Intestinal Transport, and in Vitro Bioavailability: A Review. J. Food Biochem. 2019, 43, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Le Roux, L.; Chacon, R.; Dupont, D.; Jeantet, R.; Deglaire, A.; Nau, F. In Vitro Static Digestion Reveals How Plant Proteins Modulate Model Infant Formula Digestibility. Food Res. Int. 2020, 130, 108917. [Google Scholar] [CrossRef]
- Bagul, M.; Kakumanu, S.; Wilson, T.A. Crude Garlic Extract Inhibits Cell Proliferation and Induces Cell Cycle Arrest and Apoptosis of Cancer Cells in Vitro. J. Med. Food 2015, 18, 731–737. [Google Scholar] [CrossRef]
- Gallego, M.; Grootaert, C.; Mora, L.; Aristoy, M.C.; Van Camp, J.; Toldrá, F. Transepithelial Transport of Dry-Cured Ham Peptides with ACE Inhibitory Activity through a Caco-Cell Monolayer. J. Funct. Foods 2016, 21, 388–395. [Google Scholar] [CrossRef]
- Cieza, R.J.; Cao, A.T.; Cong, Y.; Torres, A.G. Immunomodulation for Gastrointestinal Infections. Expert Rev. Anti. Infect. Ther. 2012, 10, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Hadi, S.; Akbar, A.; Chobert, J.; Haertle, T. Kinetic Characterization of Hydrolysis of Camel and Bovine Milk Proteins by Pancreatic Enzymes. Int. Dairy J. 2008, 18, 1097–1102. [Google Scholar] [CrossRef]
- Potolicchio, I.; Santambrogio, L.; Strominger, J.L. Molecular Interaction and Enzymatic Activity of Macrophage Migration Inhibitory Factor with Immunorelevant Peptides. J. Biol. Chem. 2003, 278, 30889–30895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, Y.; Gallo, R.L. AMPed up Immunity: How Antimicrobial Peptides Have Multiple Roles in Immune Defense. Trends Immunol. 2009, 30, 131–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Xue, F.; Xia, G.; Zhao, Z.; Chen, C.; Li, Y.; Zhang, Y. Transepithelial Transport Mechanisms of 7,8-Dihydroxyflavone, a Small Molecular TrkB Receptor Agonist, in Human Intestinal Caco-2 Cells. Food Funct. 2019, 10, 5215–5227. [Google Scholar] [CrossRef] [PubMed]
- Boyer, J.; Brown, D.; Liu, R.H. In Vitro Digestion and Lactase Treatment Influence Uptake of Quercetin and Quercetin Glucoside by the Caco-2 Cell Monolayer. Nutr. J. 2005, 4, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Yin, N.; Cai, X.; Du, H.; Zhang, Z.; Li, Z.; Chen, X.; Sun, G.; Cui, Y. In Vitro Study of Soil Arsenic Release by Human Gut Microbiota and Its Intestinal Absorption by Caco-2 Cells. Chemosphere 2017, 168, 358–364. [Google Scholar] [CrossRef]
- Wang, Q.; Qiao, X.; Qian, Y.; Li, Z.-w.; Tzeng, Y.-m.; Zhou, D.-m.; Guo, D.-a.; Ye, M. Intestinal Absorption of Ergostane and Lanostane Triterpenoids from Antrodia Cinnamomea Using Caco-2 Cell Monolayer Model. Nat. Products Bioprospect. 2015, 5, 237–246. [Google Scholar] [CrossRef] [Green Version]
- Brandsch, M.; Knütter, I.; Bosse-Doenecke, E. Pharmaceutical and Pharmacological Importance of Peptide Transporters. J. Pharm. Pharmacol. 2010, 60, 543–585. [Google Scholar] [CrossRef]
- Stroever, S.; Hayes, K.; Hice, J.L.; Baldauf, G.; Woodard, C.; Martin, R. Construction and Implementation of an Operating Room Management Plan for the Prevention of Perioperative Hypothermia. Am. J. Infect. Control 2015, 43, S12–S13. [Google Scholar] [CrossRef]
- Pappenheimer, J.R.; Dahl, C.E.; Karnovsky, M.L.; Maggio, J.E. Intestinal Absorption and Excretion of Octapeptides Composed of D Amino Acids. Proc. Natl. Acad. Sci. USA 1994, 91, 1942–1945. [Google Scholar] [CrossRef] [PubMed]
- Adson, A.; Raub, T.J.; Burton, P.S.; Barsuhn, C.L.; Hilgers, A.R.; Audus, K.L.; Ho, N.F.H. Quantitative Approaches to Delineate Paracellular Diffusion in Cultured Epithelial-Cell Monolayers. J. Pharm. Sci. 1994, 83, 1529–1536. [Google Scholar] [CrossRef] [PubMed]









| Chromatographic Column C18. | Wavelength | Sample Size | Flow Rate | Mobile Phase A | Mobile Phase B |
|---|---|---|---|---|---|
| 4.6 mm 5 μm | 220 nm | 20 μm | 1.0 mL/min | 0.1%TFA acetonitrile | 0.1% TFA ultrapure water |
| Primary Mass Spectrometry Parameters | Resolution | AGC Target | Maximum IT | Scan Range | |
|---|---|---|---|---|---|
| 7000 | 3e6 | 40 ms | 50 To 750 m/z (YPFPGPI) 50 to 1800 m/z (FFSDK, KHPIK) | ||
| Two-stage mass spectrum parameters | Resolution | AGC target | Maximum IT | TopN | NCE/Stepped |
| 17500 | 1e5 | 60 ms | 20 | 27 | |
| Sequence Identified | Source | Activity |
|---|---|---|
| ALARPKHPIKHQ | αS1 18–23 | KHPIKH [21] |
| VAVALARPKHPIKHQGLPQEVLNENLL | ||
| RYPLGYLEQLLR | αS1 105–110 | RYPLGYL |
| KDDVPSERYLGYLEQLLR | ||
| RYLGYLEQLLR | ||
| KHIQKEDVPSERYLGYLEQLLR | ||
| PNSVEQKHIQKEDVPSERYLGYLEQL | ||
| LAYFYPEL | αS1 157–164 | LAYFYPEL |
| EPMIGVNQELAYFYPEL | ||
| EPMIGVNQELAYFYPELF | ||
| LAYFYPEL | ||
| NPWDQ | αS2 122–126 | NPWDQ |
| PIVLNPWDQVK | ||
| QGPIVLNPWDQVK | ||
| QGPIVLNPWDQVKR | ||
| YPFPGPI | β-casein 75–81 [8] | YPFPGPI |
| VYPFPGPIPN | β-casein 78–83 | |
| LVYPFPGPIPN | ||
| SLVYPFPGPIPN | ||
| QSLVYPFPGPIPN | ||
| VYPFPGPIPNSLPQ | ||
| SLVYPFPGPIPNSLPQ | ||
| AQTQSLVYPFPGPIPN | ||
| AQTQSLVYPFPGPIPNSLPQ | ||
| VYPFPGPIPNSLPQNIPPLTQT | ||
| YQEPVLGPVR | β-casein 209–213 | YQEPVLGPVR |
| YQEPVLGPVR | ||
| LYQEPVLGPVR | ||
| LLYQEPVLGPVR | ||
| QEPVLGPVRGPFPIIV | ||
| YQEPVLGPVRGPFPIIV | ||
| LYQEPVLGPVRGPFPII | ||
| YQEPVLGPVRGPFPIIV | β-casein 208–224 [22] | YQEPVLGPVRGPFPIIV |
| LYQEPVLGPVRGPFPIILYQEPVLGPVRGPFPIIV | ||
| PGPIPN | ||
| FFSDKIAK | κ-Casein 38–42 | FFSDK |
| Molecular Weight | −H20 | −NH3 | Spectral Value | Seq | Credibility | Concentration (ppm) |
|---|---|---|---|---|---|---|
| 391.54 | 370.75 | 374.29 | 337.18 | YPL | * | 6.33 |
| 434.54 | 412.52 | 417.84 | 395.09 | RYP | * | 4.79 |
| 448.66 | 430.76 | 431.95 | 423.2 | PLGY | * | 4.10 |
| 561.88 | 543.9 | 545.18 | 488.2 | PLGYL | * | 3.59 |
| 725.1 | 707.12 | 708.4 | 580.2 | YPLGYL | * | 9.21 |
| 881.32 | 863.34 | 864.62 | 738.4 | RYPLGYL | * | 10.74 |
| 380.54 | 362.56 | 363.83 | 358.20 | KHP | * | 16.62 |
| 356.54 | 338.56 | 338.83 | 311.14 | PIK | * | 12.47 |
| 493.76 | 475.78 | 477.05 | 390.17 | KHPI | * | 4.01 |
| 493.77 | 475.79 | 477.06 | 429.94 | HPIK | * | 3.36 |
| 621.98 | 604 | 605.27 | 648.73 | KHPIK | * | 2.59 |
| 399.54 | 381.56 | 382.84 | 365.23 | FFS | * | 8.62 |
| 348.44 | 330.48 | 331.73 | 308.69 | SDK | * | 5.47 |
| 495.66 | 477.68 | 478.95 | 450.27 | FSDK | * | 3.01 |
| 514.66 | 496.68 | 497.95 | 491.32 | FFSD | * | 5.36 |
| 642.88 | 624.90 | 626.17 | 646.37 | FFSDK | * | 2.59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, H.; Han, J.; Ma, J.; Song, H.; He, B.; Liu, X.; Yi, M.; Zhang, L. Identification of Immune-Active Peptides in Casein Hydrolysates and Its Transport Mechanism on a Caco-2 Monolayer. Foods 2023, 12, 373. https://doi.org/10.3390/foods12020373
Xue H, Han J, Ma J, Song H, He B, Liu X, Yi M, Zhang L. Identification of Immune-Active Peptides in Casein Hydrolysates and Its Transport Mechanism on a Caco-2 Monolayer. Foods. 2023; 12(2):373. https://doi.org/10.3390/foods12020373
Chicago/Turabian StyleXue, Haiyan, Jingjing Han, Jun Ma, Hongxin Song, Baoyuan He, Xiaofeng Liu, Meixia Yi, and Lei Zhang. 2023. "Identification of Immune-Active Peptides in Casein Hydrolysates and Its Transport Mechanism on a Caco-2 Monolayer" Foods 12, no. 2: 373. https://doi.org/10.3390/foods12020373