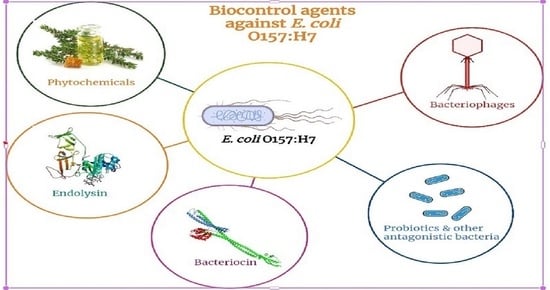
Biocontrol Approaches against Escherichia coli O157:H7 in Foods
Abstract
:1. Introduction
2. Bacteriophages
2.1. Phages for E. coli O157:H7 Control in Foods
2.2. Phage Stability
2.3. Stability Improvement Strategies
2.4. Sources of E. coli O157:H7-Specific Phages
3. Plant-Derived Natural Compounds
4. Probiotics
5. Other Antagonistic Bacteria
6. Endolysins
7. Bacteriocins
8. Bio-Enzymes
9. Hurdle Technology
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nawrocki, E.M.; Mosso, H.M.; Dudley, E.G. A toxic environment: A growing understanding of how microbial communities affect Escherichia coli O157:H7 Shiga toxin expression. Appl. Environ. Microbiol. 2020, 86, e00509-20. [Google Scholar] [CrossRef] [PubMed]
- O’flynn, G.; Ross, R.P.; Fitzgerald, G.F.; Coffey, A. Evaluation of a cocktail of three bacteriophages for biocontrol of Escherichia coli O157:H7. Appl. Environ. Microbiol. 2004, 70, 3417–3424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lennon, M.; Liao, Y.-T.; Salvador, A.; Lauzon, C.R.; Wu, V.C.H. Bacteriophages specific to Shiga toxin-producing Escherichia coli exist in goat feces and associated environments on an organic produce farm in Northern California, USA. PLoS ONE 2020, 15, e0234438. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.K.; Chen, H.; Shu, M.; Zhong, C.; Bi, Y.; Yang, H.H.; Wu, G.P. Isolation, characterization and application of an alkaline resistant virulent bacteriophage JN01 against Escherichia coli O157:H7 in milk and beef. LWT Food Sci. Technol. 2021, 144, 111266. [Google Scholar] [CrossRef]
- Doyle, M.P. Escherichia coli O157:H7 and its significance in foods. Int. J. Food Microbiol. 1991, 12, 289–301. [Google Scholar] [CrossRef]
- Harris, L.J.; Farber, J.N.; Beuchat, L.R.; Parish, M.E.; Suslow, T.V.; Garrett, E.H.; Busta, F.F. Outbreaks associated with fresh produce: Incidence, growth, and survival of pathogens in fresh and fresh-cut produce. Compr. Rev. Food Sci. Food Saf. 2003, 2, 78–141. [Google Scholar] [CrossRef]
- Al-kuzayi, A.K.; Al-Sahlany, S.T. Detecting for E. Coli O157:H7 in dairy products which were locally processed and found in Basra city markets. Basrah J. Agric. Sci. 2011, 24, 290–299. [Google Scholar]
- Rangel, J.M.; Sparling, P.H.; Crowe, C.; Griffin, P.M.; Swerdlow, D.L. Epidemiology of Escherichia coli O157:H7 outbreaks, United States, 1982–2002. Emerg. Infect. Dis. 2005, 11, 603–609. [Google Scholar] [CrossRef]
- Chauret, C. Survival and control of Escherichia coli O157:H7 in foods, beverages, soil and water. Virulence 2011, 2, 593–601. [Google Scholar] [CrossRef] [Green Version]
- Abadias, M.; Alegre, I.; Oliveira, M.; Altisent, R.; Viñas, I. Growth potential of Escherichia coli O157:H7 on fresh-cut fruits (melon and pineapple) and vegetables (carrot and escarole) stored under different conditions. Food Control 2012, 27, 37–44. [Google Scholar] [CrossRef]
- Oliveira, M.; Usall, J.; Solsona, C.; Alegre, I.; Viñas, I.; Abadias, M. Effects of packaging type and storage temperature on the growth of foodborne pathogens on shredded ‘Romaine’ lettuce. Food Microbiol. 2010, 27, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Posada-Izquierdo, G.D.; Pérez-Rodríguez, F.; López-Gálvez, F.; Allende, A.; Selma, M.V.; Gil, M.I.; Zurera, G. Modelling growth of Escherichia coli O157:H7 in fresh-cut lettuce submitted to commercial process conditions: Chlorine washing and modified atmosphere packaging. Food Microbiol. 2013, 33, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dong, L.; Zhang, J.; Shi, J.; Wang, Y.; Wang, S. Adverse effects of thermal food processing on the structural, nutritional, and biological properties of proteins. Annu. Rev. Food Sci. Technol. 2021, 12, 259–286. [Google Scholar] [CrossRef] [PubMed]
- Schilling, M.W.; Yoon, Y.; Tokarsky, O.; Pham, A.J.; Williams, R.C.; Marshall, D.L. Effects of ionizing irradiation and hydrostatic pressure on Escherichia coli O157:H7 inactivation, chemical composition and sensory acceptability of ground beef patties. Meat Sci. 2009, 81, 705–710. [Google Scholar] [CrossRef]
- Jeong, S.; Marks, B.P.; Ryser, E.T.; Moosekian, S.R. Inactivation of Escherichia coli O157:H7 on lettuce, using low-energy X-ray irradiation. J. Food Prot. 2010, 73, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Sauer, A.; Moraru, C.I. Inactivation of Escherichia coli ATCC 25922 and Escherichia coli O157:H7 in apple juice and apple cider, using pulsed light treatment. J. Food Prot. 2009, 72, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Saldaña, G.; Monfort, S.; Condón, S.; Raso, J.; Álvarez, I. Effect of temperature, pH and presence of nisin on inactivation of Salmonella Typhimurium and Escherichia coli O157: H7 by pulsed electric fields. Food Res. Int. 2012, 45, 1080–1086. [Google Scholar] [CrossRef]
- Yoder, S.F.; Henning, W.R.; Mills, E.W.; Doores, S.; Ostiguy, N.; Cutter, C.N. Investigation of chemical rinses suitable for very small meat plants to reduce pathogens on beef surfaces. J. Food Protect. 2012, 75, 14–21. [Google Scholar] [CrossRef]
- Gonzalez, R.J.; Luo, Y.; Ruiz-Cruz, S.; McEvoy, J.L. Efficacy of sanitizers to inactivate Escherichia coli O157:H7 on fresh-cut carrot shreds under simulated process water conditions. J. Food Protect. 2004, 67, 2375–2380. [Google Scholar] [CrossRef]
- Lee, C.H.; Kang, J.H.; Woo, H.J.; Song, K.B. Inactivation of Listeria monocytogenes and Escherichia coli O157:H7 inoculated on fresh-cut romaine lettuce by peanut skin extract/benzethonium chloride emulsion washing. Food Control 2021, 119, 107479. [Google Scholar] [CrossRef]
- Dock, L.L.; Floros, J.D.; Linton, R.H. Heat inactivation of Escherichia coli O157:H7in apple cider containing malic acid, sodium benzoate, and potassium sorbate. J. Food Prot. 2000, 63, 1026–1031. [Google Scholar] [CrossRef] [PubMed]
- Steenstrup, L.L.; Floros, J.D. Statistical modeling of D- and z-value of Escherichia coli O157:H7 and pH in apple cider containing preservatives. J. Food Sci. 2002, 67, 793–796. [Google Scholar] [CrossRef]
- Piper, J.D.; Piper, P.W. Benzoate and sorbate salts: A systematic review of the potential hazards of these invaluable preservatives and the expanding spectrum of clinical uses for sodium benzoate. Compr. Rev. Food Sci. Food Saf. 2017, 16, 868–880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chauhan, R.; Singh, N.; Pal, G.K.; Goel, G. Trending biocontrol strategies against Cronobacter sakazakii: A recent updated review. Food Res. Int. 2020, 137, 109385. [Google Scholar] [CrossRef]
- Oliveira, M.; Ferreira, V.; Magalhães, R.; Teixeira, P. Biocontrol strategies for Mediterranean-style fermented sausages. Food Res. Int. 2018, 103, 438–449. [Google Scholar] [CrossRef]
- Kazi, M.; Annapure, U.S. Bacteriophage biocontrol of foodborne pathogens. J. Food Sci. Technol. 2016, 53, 1355–1362. [Google Scholar] [CrossRef] [Green Version]
- Abedon, S.T. Kinetics of phage-mediated biocontrol of bacteria. Foodborne Pathog. Dis. 2009, 6, 807–815. [Google Scholar] [CrossRef]
- Lu, Z.; Breidt, F. Escherichia coli O157:H7 bacteriophage Φ241 isolated from an industrial cucumber fermentation at high acidity and salinity. Front. Microbiol. 2015, 6, 67. [Google Scholar] [CrossRef] [Green Version]
- Kudva, I.T.; Jelacic, S.; Tarr, P.I.; Youderian, P.; Hovde, C.J. Biocontrol of Escherichia coli O157 with O157-specific bacteriophages. Appl. Environ. Microbiol. 1999, 65, 3767–3773. [Google Scholar] [CrossRef] [Green Version]
- Niu, Y.D.; Stanford, K.; Kropinski, A.M.; Ackermann, H.W.; Johnson, R.P.; She, Y.M.; Ahmed, R.; Villegas, A.; McAllister, T.A. Genomic, proteomic and physiological characterization of a T5-like bacteriophage for control of Shiga toxin-producing Escherichia coli O157:H7. PLoS ONE 2012, 7, e34585. [Google Scholar] [CrossRef] [Green Version]
- Greer, G.G. Bacteriophage control of foodborne bacteria. J. Food Prot. 2005, 68, 1102–1111. [Google Scholar] [CrossRef] [PubMed]
- Hudson, J.A.; Billington, C.; Cornelius, A.J.; Wilson, T.; On, S.L.W.; Premaratne, A.; King, N.J. Use of a bacteriophage to inactivate Escherichia coli O157:H7 on beef. Food Microbiol. 2013, 36, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Hudson, J.A.; Billington, C.; Wilson, T.; On, S.L. Effect of phage and host concentration on the inactivation of Escherichia coli O157:H7 on cooked and raw beef. Food Sci. Technol. Int. 2015, 21, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Gencay, Y.E.; Ayaz, N.D.; Copuroglu, G.; Erol, I. Biocontrol of shiga toxigenic Escherichia coli O157:H7 in Turkish raw meatball by bacteriophage. J. Food Saf. 2016, 36, 120–131. [Google Scholar] [CrossRef]
- Cufaoglu, G.; Onaran, B.; Ayaz, N.D.; Goncuoglu, M.; Ormanci, F.S. Biocontrol of Escherichia coli O157:H7 in ready-to-eat salad using a lytic bacteriophage. Med. Weter. 2017, 73, 422–424. [Google Scholar] [CrossRef] [Green Version]
- Coffey, B.; Rivas, L.; Duffy, G.; Coffey, A.; Ross, R.P.; McAuliffe, O. Assessment of Escherichia coli O157:H7-specific bacteriophages e11/2 and e4/1c in model broth and hide environments. Int. J. Food Microbiol. 2011, 147, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.D.; Kim, J.Y.; Park, J.H. Characteristics of coliphage ECP4 and potential use as a sanitizing agent for biocontrol of Escherichia coli O157:H7. Food Control 2013, 34, 255–260. [Google Scholar] [CrossRef]
- Liu, H.; Niu, Y.D.; Meng, R.; Wang, J.; Li, J.; Johnson, R.P.; McAllister, T.A.; Stanford, K. Control of Escherichia coli O157 on beef at 37, 22 and 4 °C by T5-, T1-, T4-and O1-like bacteriophages. Food Microbiol. 2015, 51, 69–73. [Google Scholar] [CrossRef]
- Abuladze, T.; Li, M.; Menetrez, M.Y.; Dean, T.; Senecal, A.; Sulakvelidze, A. Bacteriophages reduce experimental contamination of hard surfaces, tomato, spinach, broccoli, and ground beef by Escherichia coli O157:H7. Appl. Environ. Microbiol. 2008, 74, 6230. [Google Scholar] [CrossRef] [Green Version]
- Tomat, D.; Mercanti, D.; Balagué, C.; Quiberoni, A. Phage biocontrol of enteropathogenic and Shiga toxin-producing Escherichia coli during milk fermentation. Lett. Appl. Microbiol. 2013, 57, 3–10. [Google Scholar] [CrossRef]
- Tomat, D.; Quiberoni, A.; Mercanti, D.; Balagué, C. Hard surfaces decontamination of enteropathogenic and Shiga toxin-producing Escherichia coli using bacteriophages. Food Res. Int. 2014, 57, 123–129. [Google Scholar] [CrossRef]
- Tomat, D.; Casabonne, C.; Aquili, V.; Balagué, C.; Quiberoni, A. Evaluation of a novel cocktail of six lytic bacteriophages against Shiga toxin-producing Escherichia coli in broth, milk and meat. Food Microbiol. 2018, 76, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Çufaoğlu, G.; Derinöz, A.N.; Ayaz, N.D. An investigation on biocontrol of Escherichia coli O157:H7 by a bacteriophage cocktail in pastirma. Ank. Üniversitesi Vet. Fakültesi Derg. 2019, 66, 7–11. [Google Scholar]
- Vengarai Jagannathan, B.; Kitchens, S.; Priyesh Vijayakumar, P.; Price, S.; Morgan, M. Efficacy of bacteriophage cocktail to control E. coli O157:H7 contamination on baby spinach leaves in the presence or absence of organic load. Microorganisms 2021, 9, 544. [Google Scholar] [CrossRef] [PubMed]
- Tomat, D.; Soazo, M.; Verdini, R.; Casabonne, C.; Aquili, V.; Balagué, C.; Quiberoni, A. Evaluation of an WPC edible film added with a cocktail of six lytic phages against foodborne pathogens such as enteropathogenic and Shigatoxigenic Escherichia coli. LWT Food Sci. Technol. 2019, 113, 108316. [Google Scholar] [CrossRef]
- Korf, I.H.; Kittler, S.; Bierbrodt, A.; Mengden, R.; Rohde, C.; Rohde, M.; Kroj, A.; Lehnherr, T.; Fruth, A.; Flieger, A.; et al. In vitro evaluation of a phage cocktail controlling infections with Escherichia coli. Viruses 2020, 12, 1470. [Google Scholar] [CrossRef]
- Duc, H.M.; Son, H.M.; Yi, H.P.S.; Sato, J.; Ngan, P.H.; Masuda, Y.; Honjoh, K.; Miyamoto, T. Isolation, characterization and application of a polyvalent phage capable of controlling Salmonella and Escherichia coli O157:H7 in different food matrices. Food Res. Int. 2020, 131, 108977. [Google Scholar] [CrossRef]
- Sváb, D.; Falgenhauer, L.; Rohde, M.; Chakraborty, T.; Tóth, I. Identification and characterization of new broad host-range rV5-like coliphages C203 and P206 directed against enterobacteria. Infect. Genet. Evol. 2018, 64, 254–261. [Google Scholar] [CrossRef] [Green Version]
- Niu, Y.D.; Liu, H.; Johnson, R.P.; McAllister, T.A.; Stanford, K. Effect of a bacteriophage T5virus on growth of Shiga toxigenic Escherichia coli and Salmonella strains in individual and mixed cultures. Virol. J. 2020, 17, 3. [Google Scholar] [CrossRef] [Green Version]
- Hudson, J.A.; Bigwood, T.; Premaratne, A.; Billington, C.; Horn, B.; McIntyre, L. Potential to use ultraviolet-treated bacteriophages to control foodborne pathogens. Foodborne Pathog. Dis. 2010, 7, 687–693. [Google Scholar] [CrossRef]
- Hudson, J.A.; Billington, C.; Premaratne, A.; On, S.L.W. Inactivation of Escherichia coli O157:H7 using ultraviolet light-treated bacteriophages. Food Sci. Technol. Int. 2016, 22, 3–9. [Google Scholar] [CrossRef] [PubMed]
- López-Cuevas, O.; Castro-Del Campo, N.; León-Félix, J.; Valdez-Torres, B.; Chaidez, C. Evaluation of bacteriophage av-08 for simultaneous biocontrol of Salmonella montevideo and Escherichia coli O157:H7 in experimentally contaminated chicken. J. Food Saf. 2012, 32, 305–310. [Google Scholar] [CrossRef]
- Amarillas, L.; Chaidez, C.; Lugo, Y.; León-Félix, J. Complete genome sequence of Escherichia coli O157:H7 bacteriophage phiJLA23 isolated in Mexico. Genome Announc. 2013, 1, e00219-12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomat, D.D.; Migliore, L.; Aquili, V.; Quiberoni, A.D.L.; Balagué, C. Phage biocontrol of enteropathogenic and shiga toxin-producing Escherichia coli in meat products. Front. Cell. Infect. Microbiol. 2013, 3, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snyder, A.B.; Perry, J.J.; Yousef, A.E. Developing and optimizing bacteriophage treatment to control enterohemorrhagic Escherichia coli on fresh produce. Int. J. Food Microbiol. 2016, 236, 90–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.; Ku, H.-J.; Lee, D.-H.; Kim, Y.-T.; Shin, H.; Ryu, S.; Lee, J.-H. Characterization and genomic study of the novel bacteriophage HY01 infecting both Escherichia coli O157:H7 and Shigella flexneri: Potential as a biocontrol agent in food. PLoS ONE 2016, 11, e0168985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amarillas, L.; Chaidez, C.; González-Robles, A.; León-Félix, J. Complete genome sequence of new bacteriophage phiE142, which causes simultaneously lysis of multidrug-resistant Escherichia coli O157:H7 and Salmonella enterica. Stand. Genom. Sci. 2016, 11, 89. [Google Scholar] [CrossRef] [Green Version]
- Lin, L.; Zhu, Y.; Cui, H. Inactivation of Escherichia coli O157:H7 treated by poly-L-lysine-coated bacteriophages liposomes in pork. J. Food Saf. 2018, 38, e12535. [Google Scholar] [CrossRef]
- Son, H.M.; Duc, H.M.; Masuda, Y.; Honjoh, K.I.; Miyamoto, T. Application of bacteriophages in simultaneously controlling Escherichia coli O157:H7 and extended-spectrum beta-lactamase producing Escherichia coli. Appl. Microbiol. Biotechnol. 2018, 102, 10259–10271. [Google Scholar] [CrossRef]
- Grygorcewicz, B.; Chajęcka-Wierzchowska, W.; Augustyniak, A.; Wasak, A.; Stachurska, X.; Nawrotek, P.; Dołęgowska, B. In-milk inactivation of Escherichia coli O157:H7 by the environmental lytic bacteriophage ECPS-6. J. Food Saf. 2019, 40, e12747. [Google Scholar] [CrossRef]
- Vikram, A.; Tokman, J.I.; Woolston, J.; Sulakvelidze, A. Phage biocontrol improves food safety by significantly reducing the level and prevalence of Escherichia coli O157:H7 in various foods. J. Food Prot. 2020, 83, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Park, D.W.; Lim, G.Y.; Lee, Y.D.; Park, J.H. Characteristics of lytic phage vB_EcoM-ECP26 and reduction of shiga-toxin producing Escherichia coli on produce romaine. Appl. Biol. Chem. 2020, 63, 19. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Shigemura, K.; Duc, H.M.; Shen, C.; Huang, H.H.; Sato, J.; Masuda, Y.; Honjoh, K.; Miyamoto, T. Effects of bacteriophage on inhibition and removal of mixed biofilm of enterohemorrhagic Escherichia coli O157:H7 and O91:H-. LWT Food Sci. Technol. 2020, 134, 109945. [Google Scholar] [CrossRef]
- Guenther, S.; Huwyler, D.; Richard, S.; Loessner, M.J. Virulent bacteriophage for efficient biocontrol of Listeria monocytogenes in ready-to-eat foods. Appl. Environ. Microbiol. 2009, 75, 93–100. [Google Scholar] [CrossRef] [Green Version]
- García-Anaya, M.C.; Sepúlveda, D.R.; Rios-Velasco, C.; Zamudio-Flores, P.B.; Sáenz-Mendoza, A.I.; Acosta-Muñiz, C.H. The role of food compounds and emerging technologies on phage stability. Innov. Food Sci. Emerg. Technol. 2020, 64, 102436. [Google Scholar] [CrossRef]
- Jończyk-Matysiak, E.; Łodej, N.; Kula, D.; Owczarek, B.; Orwat, F.; Międzybrodzki, R.; Neuberg, J.; Bagińska, N.; Weber-Dąbrowska, B.; Górski, A. Factors determining phage stability/activity: Challenges in practical phage application. Expert Rev. Anti Infect. Ther. 2019, 17, 583–606. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Yuan, L.; Lin, L. Novel chitosan film embedded with liposome-encapsulated phage for biocontrol of Escherichia coli O157:H7 in beef. Carbohydr. Polym. 2017, 177, 156–164. [Google Scholar] [CrossRef]
- Ramirez, K.; Cazarez-Montoya, C.; Lopez-Moreno, H.S.; Castro-del Campo, N. Bacteriophage cocktail for biocontrol of Escherichia coli O157:H7: Stability and potential allergenicity study. PLoS ONE 2018, 13, e0195023. [Google Scholar] [CrossRef] [Green Version]
- Amarillas, L.; Lightbourn-Rojas, L.; Angulo-Gaxiola, A.K.; Basilio Heredia, J.; González-Robles, A.; León-Félix, J. The antibacterial effect of chitosan-based edible coating incorporated with a lytic bacteriophage against Escherichia coli O157:H7 on the surface of tomatoes. J. Food Saf. 2018, 38, e12571. [Google Scholar] [CrossRef]
- Anany, H.; Chen, W.; Pelton, R.; Griffiths, M.W. Biocontrol of Listeria monocytogenes and Escherichia coli O157:H7 in meat by using phages immobilized on modified cellulose membranes. Appl. Environ. Microbiol. 2011, 77, 6379–6387. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.D.; Park, J.H. Characterization and application of phages isolated from sewage for reduction of Escherichia coli O157:H7 in biofilm. LWT Food Sci. Technol. 2015, 60, 571–577. [Google Scholar] [CrossRef]
- Fan, C.; Tie, D.; Sun, Y.; Jiang, J.; Huang, H.; Gong, Y.; Zhao, C. Characterization and genomic analysis of Escherichia coli O157:H7 bacteriophage FEC14, a new member of genus Kuttervirus. Curr. Microbiol. 2021, 78, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Amarillas, L.; Villicaña, C.; Lightbourn-Rojas, L.; González-Robles, A.; León-Félix, J. The complete genome and comparative analysis of the phage phiC120 infecting multidrug-resistant Escherichia coli and Salmonella strains. G3 Genes Genomes Genet. 2021, 11, jkab014. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Choi, I.Y.; Park, D.H.; Park, M.K. Isolation and characterization of a novel Escherichia coli O157:H7-specific phage as a biocontrol agent. J. Environ. Health Sci. Eng. 2020, 18, 189–199. [Google Scholar] [CrossRef]
- Yıldirim, Z.; Sakin, T.; Akçelik, M.; Akçelik, N. Identification and characterization of lytic bacteriophages specific to foodborne pathogenic Escherichia coli O157:H7. Food Sci. Technol. Int. 2021, 27, 56–72. [Google Scholar] [CrossRef]
- Safwat Mohamed, D.; Farouk Ahmed, E.; Mohamed Mahmoud, A.; Abd El-Baky, R.M.; John, J. Isolation and evaluation of cocktail phages for the control of multidrug-resistant Escherichia coli serotype O104:H4 and E. coli O157:H7 isolates causing diarrhea. FEMS Microbiol. Lett. 2018, 365, fnx275. [Google Scholar] [CrossRef]
- Wintola, O.A.; Afolayan, A.J. The antibacterial, phytochemicals and antioxidants evaluation of the root extracts of Hydnora africana Thunb. used as antidysenteric in Eastern Cape Province, South Africa. BMC Complement Altern. Med. 2015, 15, 307. [Google Scholar] [CrossRef] [Green Version]
- Sagdic, O.; Silici, S.; Yetim, H. Fate of Escherichia coli and E. coli O157:H7 in apple juice treated with propolis extract. Ann. Microbiol. 2007, 57, 345–348. [Google Scholar] [CrossRef]
- Sewlikar, S.; D’Souza, D.H. Antimicrobial effects of Quillaja saponaria extract against Escherichia coli O157:H7 and the emerging non-O157 Shiga toxin-producing E. coli. J. Food Sci. 2017, 82, 1171–1177. [Google Scholar] [CrossRef]
- Javadian, S.R.; Shahosseini, S.R.; Ariaii, P. The effects of liposomal encapsulated thyme extract on the quality of fish mince and Escherichia coli O157:H7 inhibition during refrigerated storage. J. Aquat. Food Prod. Technol. 2017, 26, 115–123. [Google Scholar] [CrossRef]
- Sakagami, Y. Inhibitory effect of the extract of Prunus mume Sieb. et Zucc. on vero-toxin production by enterohemorrhagic Escherichia coli O157:H7. Biocontrol Sci. 2001, 6, 53–56. [Google Scholar] [CrossRef]
- Hara-Kudo, Y.; Okubo, T.; Tanaka, S.; Chu, D.C.; Juneja, L.R.; Saito, N.; Sugita-Konishi, Y. Bactericidal action of green tea extract and damage to the membrane of Escherichia coli O157:H7. Biocontrol Sci. 2001, 6, 57–61. [Google Scholar] [CrossRef]
- Moreira, M.R.; Ponce, A.G.; Del Valle, C.E.; Roura, S.I. Effects of clove and tea tree oils on Escherichia coli O157:H7 in blanched spinach and minced cooked beef. J. Food Process. Preserv. 2007, 31, 379–391. [Google Scholar] [CrossRef]
- Singh, N.; Singh, R.K.; Bhunia, A.K.; Stroshine, R.L. Efficacy of chlorine dioxide, ozone, and thyme essential oil or a sequential washing in killing Escherichia coli O157: H7 on lettuce and baby carrots. LWT Food Sci. Technol. 2002, 35, 720–729. [Google Scholar] [CrossRef]
- Harich, M.; Maherani, B.; Salmieri, S.; Lacroix, M. Evaluation of antibacterial activity of two natural bio-preservatives formulations on freshness and sensory quality of ready to eat (RTE) foods. Food Control 2018, 85, 29–41. [Google Scholar] [CrossRef]
- Shekarforoush, S.S.; Nazer, A.H.K.; Firouzi, R.; Rostami, M. Effects of storage temperatures and essential oils of oregano and nutmeg on the growth and survival of Escherichia coli O157:H7 in barbecued chicken used in Iran. Food Control 2006, 18, 1428–1433. [Google Scholar] [CrossRef]
- Munekata, P.E.S.; Pateiro, M.; Rodríguez-Lázaro, D.; Domínguez, R.; Zhong, J.; Lorenzo, J.M. The role of essential oils against pathogenic Escherichia coli in food products. Microorganisms 2020, 8, 924. [Google Scholar] [CrossRef]
- Ponce, A.; Avalo, R.; Roura, S.I.; Moreira, M.D.R. Biocontrol of Escherichia coli O157:H7 exerted by endogenous microflora and oleoresins in minimally processed lettuce and carrot. Int. J. Postharvest Technol. Innov. 2011, 2, 243–259. [Google Scholar]
- Barbieri, R.; Coppo, E.; Marchese, A.; Daglia, M.; Sobarzo-Sánchez, E.; Nabavi, S.F.; Nabavi, S.M. Phytochemicals for human disease: An update on plant-derived compounds antibacterial activity. Microbiol. Res. 2017, 196, 44–68. [Google Scholar] [CrossRef]
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef] [Green Version]
- Ullah, F.; Ayaz, M.; Sadiq, A.; Ullah, F.; Hussain, I.; Shahid, M.; Yessimbekov, Z.; Adhikari-Devkota, A.; Devkota, H.P. Potential role of plant extracts and phytochemicals against foodborne pathogens. Appl. Sci. 2020, 10, 4597. [Google Scholar] [CrossRef]
- Devlieghere, F.; Vermeiren, L.; Debevere, J. New preservation technologies: Possibilities and limitations. Int. Dairy J. 2004, 14, 273–285. [Google Scholar] [CrossRef]
- Salminen, S.; Bouley, C.; Boutron-Ruault, M.-C.; Cummings, J.H.; Franck, A.; Gibson, G.F.R.; Isolauri, E.; Moreau, M.-C.; Roberfroid, M.; Rowland, I. Functional food science and gastrointestinal physiology and function. Brit. J. Nutr. 1998, 80, S147–S171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russo, P.; de Chiara, M.L.V.; Vernile, A.; Amodio, M.L.; Arena, M.P.; Capozzi, V.; Massa, S.; Spano, G. Fresh-cut pineapple as a new carrier of probiotic lactic acid bacteria. BioMed Res. Int. 2014, 2014, 309183. [Google Scholar] [CrossRef] [Green Version]
- Gómez, N.C.; Ramiro, J.M.; Quecan, B.X.; de Melo Franco, B.D. Use of potential probiotic lactic acid bacteria (LAB) biofilms for the control of Listeria monocytogenes, Salmonella Typhimurium, and Escherichia coli O157:H7 biofilms formation. Front. Microbiol. 2016, 7, 863. [Google Scholar] [CrossRef] [Green Version]
- Goktepe, I. Probiotics as biopreservatives for enhancing food safety. In Probiotics in Food Safety and Human Health; Goktepe, I., Juneja, V.K., Ahmedna, M., Eds.; Taylor & Francis Group: Boca Raton, FL, USA, 2006; pp. 285–307. [Google Scholar]
- Ayala, D.I.; Cook, P.W.; Franco, J.G.; Bugarel, M.; Kottapalli, K.R.; Loneragan, G.H.; Brashears, M.M.; Nightingale, K.K. A systematic approach to identify and characterize the effectiveness and safety of novel probiotic strains to control foodborne pathogens. Front. Microbiol. 2019, 10, 1108. [Google Scholar] [CrossRef] [Green Version]
- Speranza, B.; Liso, A.; Russo, V.; Corbo, M.R. Evaluation of the potential of biofilm formation of Bifidobacterium longum subsp. infantis and Lactobacillus reuteri as competitive biocontrol agents against pathogenic and food spoilage bacteria. Microorganisms 2020, 8, 177. [Google Scholar]
- Alvarez, M.V.; Bambace, M.F.; Quintana, G.; Gomez-Zavaglia, A.; del Rosario Moreira, M. Prebiotic-alginate edible coating on fresh-cut apple as a new carrier for probiotic lactobacilli and bifidobacteria. LWT Food Sci. Technol. 2021, 137, 110483. [Google Scholar] [CrossRef]
- Bambace, M.F.; Alvarez, M.V.; Moreira, M.R. Ready-to-eat blueberries as fruit-based alternative to deliver probiotic microorganisms and prebiotic compounds. LWT Food Sci. Technol. 2021, 142, 111009. [Google Scholar] [CrossRef]
- Bambace, M.F.; Alvarez, M.V.; Moreira, M.D.R. Novel functional blueberries: Fructo-oligosaccharides and probiotic lactobacilli incorporated into alginate edible coatings. Food Res. Int. 2019, 122, 653–660. [Google Scholar] [CrossRef]
- Janisiewicz, W.J.; Conway, W.S.; Leverentz, B. Biological control of postharvest decays of apple can prevent growth of Escherichia coli O157:H7 in apple wounds. J. Food Prot. 1999, 62, 1372–1375. [Google Scholar] [CrossRef]
- Alegre, I.; Vinas, I.; Usall, J.; Teixido, N.; Figge, M.J.; Abadias, M. Control of foodborne pathogens on fresh-cut fruit by a novel strain of Pseudomonas graminis. Food Microbiol. 2013, 34, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Abadias, M.; Colás-Medà, P.; Usall, J.; Viñas, I. Biopreservative methods to control the growth of foodborne pathogens on fresh-cut lettuce. Int. J. Food Microbiol. 2015, 214, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Deering, A.J.; Pruitt, R.E.; Mauer, L.J.; Reuhs, B.L. Identification of the cellular locationof internalized Escherichia coli O157:H7 in mung bean, Vigna radiata, byimmunocy-tochemical techniques. J. Food Prot. 2011, 74, 1224–1230. [Google Scholar] [CrossRef]
- Shen, Z.; Mustapha, A.; Lin, M.; Zheng, G. Biocontrol of the internalization of Salmonella enterica and Enterohaemorrhagic Escherichia coli in mung bean sprouts with an endophytic Bacillus subtilis. Int. J. Food Microbiol. 2017, 250, 37–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, C.H. Control of foodborne pathogens and soft-rot bacteria on bell pepper by three strains of bacterial antagonists. J. Food Prot. 2009, 72, 85–92. [Google Scholar] [CrossRef] [Green Version]
- Olanya, O.M.; Ukuku, D.O.; Annous, B.A.; Niemira, B.A.; Sommers, C.H. Efficacy of Pseudomonas fluorescens for biocontrol of Escherichia coli O157:H7 on spinach. J. Food Agric. Environ. 2013, 11, 86–91. [Google Scholar]
- Olanya, O.M.; Ukuku, D.O.; Niemira, B.A. Effects of temperatures and storage time on resting populations of Escherichia coli O157:H7 and Pseudomonas fluorescens in vitro. Food Control 2014, 39, 128–134. [Google Scholar] [CrossRef]
- Lim, J.A.; Lee, D.H.; Kim, B.Y.; Heu, S. Draft genome sequence of Pantoea agglomerans R190, a producer of antibiotics against phytopathogens and foodborne pathogens. J. Biotechnol. 2014, 188, 7–8. [Google Scholar] [CrossRef]
- Richards, G.P.; Fay, J.P.; Uknalis, J.; Olanya, O.M.; Watson, M.A. Purification and host specificity of predatory Halobacteriovorax isolates from seawater. Appl. Environ. Microbiol. 2016, 82, 922–927. [Google Scholar] [CrossRef] [Green Version]
- Olanya, O.M.; Niemira, B.A.; Cassidy, J.M.; Boyd, G.; Uknalis, J. Pathogen reduction by predatory bacteria and survival of Bdellovibrio bacteriovorus and Escherichia coli on produce and buffer treated with low-dose gamma radiation. LWT Food Sci. Technol. 2020, 130, 109630. [Google Scholar] [CrossRef]
- Cisneros, L.; Cattelan, N.; Villalba, M.I.; Rodriguez, C.; Serra, D.O.; Yantorno, O.; Fadda, S. Lactic acid bacteria biofilms and their ability to mitigate Escherichia coli O157:H7 surface colonization. Lett. Appl. Microbiol. 2021, 73, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Bang, J.; Kim, H.; Beuchat, L.R.; Ryu, J.H. Inactivation of Escherichia coli O157:H7 on stainless steel upon exposure to Paenibacillus polymyxa biofilms. Int. J. Food Microbiol. 2013, 167, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Baker, C.A.; De, J.; Schneider, K.R. Influence of soil microbes on Escherichia coli O157:H7 survival in soil rinse and artificial soil. J. Appl. Microbiol. 2021, 131, 1531–1583. [Google Scholar] [CrossRef] [PubMed]
- Nachimuthu, R.; Madurantakam Royam, M.; Manohar, P.; Leptihn, S. Application of bacteriophages and endolysins in aquaculture as a biocontrol measure. Biological Control 2021, 160, 104678. [Google Scholar] [CrossRef]
- Nelson, D.C.; Schmelcher, M.; Rodriguez-Rubio, L.; Klumpp, J.; Pritchard, D.G.; Dong, S.; Donovan, D.M. Endolysins as antimicrobials. Adv. Virus Res. 2012, 83, 299–365. [Google Scholar] [PubMed] [Green Version]
- Park, D.W.; Park, J.H. Characterization of endolysin LysECP26 derived from rV5-like phage vB_EcoM-ECP26 for inactivation of Escherichia coli O157:H7. J. Microbiol. Biotechnol. 2020, 30, 1552–1558. [Google Scholar] [CrossRef]
- Love, M.J.; Coombes, D.; Manners, S.H.; Abeysekera, G.S.; Billington, C.; Dobson, R.C. The molecular basis for Escherichia coli O157:H7 phage FAHEc1 endolysin function and protein engineering to increase thermal stability. Viruses 2021, 13, 1101. [Google Scholar] [CrossRef]
- Briers, Y.; Walmagh, M.; Van Puyenbroeck, V.; Cornelissen, A.; Cenens, W.; Aertsen, A.; Oliveira, H.; Azeredo, J.; Verween, G.; Pirnay, J.-P.; et al. Engineered endolysin-based “Artilysins” to combat multidrug-resistant Gram-negative pathogens. MBio 2014, 5, e01379-14. [Google Scholar] [CrossRef] [Green Version]
- Xu, S.; Campisi, E.; Li, J.; Fischetti, V.A. Decontamination of Escherichia coli O157:H7 on fresh Romaine lettuce using a novel bacteriophage lysin. Int. J. Food Microbiol. 2021, 341, 109068. [Google Scholar] [CrossRef]
- Xu, Y. Phage and phage lysins: New era of bio-preservatives and food safety agents. J. Food Sci. 2021, 86, 3349–3373. [Google Scholar] [CrossRef]
- Simons, A.; Alhanout, K.; Duval, R.E. Bacteriocins, antimicrobial peptides from bacterial origin: Overview of their biology and their impact against multidrug-resistant bacteria. Microorganisms 2020, 8, 639. [Google Scholar] [CrossRef] [PubMed]
- Öncül, N.; Yıldırım, Z. Inhibitory effect of bacteriocins against Escherichia coli O157: H7. Food Sci. Technol. Int. 2019, 25, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Goni, M.G.; Tomadoni, B.M.; Audisio, M.C.; Ibarguren, C.; Roura, S.I.; Moreira, M.D.R.; Ponce, A.G. Application of bacteriocins from Enterococcus hirae on butterhead lettuce seeds inoculated with Escherichia coli O157:H7. Int. Food Res. J. 2016, 23, 2653–2660. [Google Scholar]
- Nandiwada, L.S.; Schamberger, G.P.; Schafer, H.W.; Diez-Gonzalez, F. Characterization of an E2-type colicin and its application to treat alfalfa seeds to reduce Escherichia coli O157:H7. Int. J. Food Microbiol. 2004, 93, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Zeng, P.; Liu, J.; Wong, K.Y.; Chan, E.W.C.; Lin, Y.; Chan, K.F.; Chen, S. Antimicrobial peptide zp37 inhibits Escherichia coli O157:H7 in alfalfa sprouts by inflicting damage in cell membrane and binding to DNA. LWT Food Sci. Technol. 2021, 146, 111392. [Google Scholar] [CrossRef]
- Corbalán, N.; Quiroga, M.; Masias, E.; Peralta, D.; Velázquez, J.B.; Acuña, L.; Vincent, P. Antimicrobial activity of MccJ25 (G12Y) against Gram-negative foodborne pathogens in vitro and in food models. Int. J. Food Microbiol. 2021, 352, 109267. [Google Scholar] [CrossRef]
- van der Linden, D.S.; Short, D.; Dittmann, A.; Yu, P.L. Synergistic effects of ovine-derived cathelicidins and other antimicrobials against Escherichia coli O157:H7 and Staphylococcus aureus 1056 MRSA. Biotechnol. Lett. 2009, 31, 1265–1267. [Google Scholar] [CrossRef]
- Lim, E.S.; Koo, O.K.; Kim, M.J.; Kim, J.S. Bio-enzymes for inhibition and elimination of Escherichia coli O157:H7 biofilm and their synergistic effect with sodium hypochlorite. Sci. Rep. 2019, 9, 9920. [Google Scholar] [CrossRef]
- Aaliya, B.; Sunooj, K.V.; Navaf, M.; Akhila, P.P.; Sudheesh, C.; Mir, S.A.; Sabu, S.; Sasidharan, A.; Hlaing, M.T.; George, J. Recent trends in bacterial decontamination of food products by hurdle technology: A synergistic approach using thermal and non-thermal processing techniques. Food Res. Int. 2021, 147, 110514. [Google Scholar] [CrossRef]
- Cui, H.; Bai, M.; Yuan, L.; Surendhiran, D.; Lin, L. Sequential effect of phages and cold nitrogen plasma against Escherichia coli O157:H7 biofilms on different vegetables. Int. J. Food Microbiol. 2018, 268, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Ma, C.; Lin, L. Synergetic antibacterial efficacy of cold nitrogen plasma and clove oil against Escherichia coli O157:H7 biofilms on lettuce. Food Control 2016, 66, 8–16. [Google Scholar] [CrossRef]
- Viazis, S.; Akhtar, M.; Feirtag, J.; Diez-Gonzalez, F. Reduction of Escherichia coli O157:H7 viability on leafy green vegetables by treatment with a bacteriophage mixture and trans-cinnamaldehyde. Food Microbiol. 2011, 28, 149–157. [Google Scholar] [CrossRef]
- Matan, N.; Puangjinda, K.; Phothisuwan, S.; Nisoa, M. Combined antibacterial activity of green tea extract with atmospheric radio-frequency plasma against pathogens on fresh-cut dragon fruit. Food Control 2015, 50, 291–296. [Google Scholar] [CrossRef]
- Ait-Ouazzou, A.; Espina, L.; García-Gonzalo, D.; Pagán, R. Synergistic combination of physical treatments and carvacrol for Escherichia coli O157: H7 inactivation in apple, mango, orange, and tomato juices. Food Control 2013, 32, 159–167. [Google Scholar] [CrossRef]
- Bortolotto, F.C.K.; Ceccoti, S.P.C.; Orso, P.B.; Wolupeck, H.L.; Holley, R.A.; Luciano, F.B.; de Macedo, R.E.F. Combined effects of garlic essential oil and allyl isothiocyanate against Escherichia coli O157:H7 in vitro and in pork sausage. Cienc. Rural. 2018, 48, e20180233. [Google Scholar] [CrossRef]
- Meira, N.V.; Holley, R.A.; Bordin, K.; de Macedo, R.E.; Luciano, F.B. Combination of essential oil compounds and phenolic acids against Escherichia coli O157: H7 in vitro and in dry-fermented sausage production. Int. J. Food Microbiol. 2017, 260, 59–64. [Google Scholar] [CrossRef]
- Moradpour, Z.; Sepehrizadeh, Z.; Rahbarizadeh, F.; Ghasemian, A.; Yazdi, M.T.; Shahverdi, A.R. Genetically engineered phage harbouring the lethal catabolite gene activator protein gene with an inducer-independent promoter for biocontrol of Escherichia coli. FEMS Microbiol. Lett. 2009, 296, 67–71. [Google Scholar] [CrossRef] [Green Version]
| Bacteriophage | Target Strain | Result | Reference |
|---|---|---|---|
| Lytic bacteriophage Av-08 | E. coli O157:H7 on chicken skin surface | 4.9 log10 CFU/4 cm2 reduction | [52] |
| Phage phiJLA23 | E. coli O157:H7 | High lytic activity | [53] |
| Phages DT1 and DT6, either alone or mixed in a cocktail (107–108 PFU/mL) | E. coli O157:H7 STEC464 (final concentration approx. 5 × 102–5 × 103 CFU/mL) | The cocktail rapidly and completely inactivated the pathogen | [40] |
| Phage DT6 (1.4 × 1010 PFU/mL) Phage cocktail (DT1 and DT6 in equal proportions) | E. coli O157:H7 STEC 464 | Viable cell reduction of 1.15 log after 6 h The phage cocktail reduced viable counts by up to 2.58 log at 6 h and 2.20 log at 24 h | [54] |
| Phage FAHEc1 at 107 PFU/mL | E. coli O157:H7 in broth in vitro at 5 °C | A reduction of 4 log10 | [32] |
| Phage FAHEc1 at 3.2 × 107 PFU/4 cm2 | E. coli O157:H7 on pieces of sliced beef at 37 °C | A reduction of >2.7 log10 | [32] |
| Phage FAHEc1 (107 PFU/cm2 UV-treated phages (pre-UV treatment titer)) | E. coli O157:H7 on raw meat (106 CFU/cm2) | Viable counts were reduced by 1–2 log10 CFU/cm2 | [51] |
| Coliphage ECP4 (with 8 log PFU/mL) | E. coli O157:H7 on cabbage (7–8 log CFU/g) | Complete reduction at 3 h after treatment with the phage | [37] |
| Phage M8AEC16 | E. coli O157:H7 in raw meatballs | Reduction of viable counts by 0.69–2.09 log CFU/g in the first 5 h of replica trials | [34] |
| E. coli phage OSY-SP (108 PFU/mL) | E. coli O157:H7 EDL933 on green peppers E. coli O157:H7 B6–914 on baby spinach | Reduced by 2.4–3.0 log CFU/g (5 min rinse) Reduced by 3.4–3.5 log CFU/g (2 min rinse) | [55] |
| Bacteriophage HY01 | E. coli O157:H7 strains (ATCC 43890 and ATCC 43895) | >2 log reductions during the first 2 h of incubation | [56] |
| Bacteriophage phiE142 | Multidrug-resistant E. coli O157:H7 strains | Lysis and wide host range | [57] |
| Phage M8AEC16 (at 5.0 log initial MOI) | E. coli O157:H7 strain ATCC 43895 on RTE Italian salads (4.3 log CFU/g initial count) | 2.7 log CFU/g reduction in 5 h incubation at 22 °C | [35] |
| E. coli O157:H7 phage (1.5 × 109 PFU/mL) | E. coli EHEC O157:H7 CICC 21530 on nutrient agar plates (approx. 105–106 CFU/mL) | ~3.88 log CFU/mL reductions after 8 h of treatment and no visible colonies observed when reacting time extended to 12 h | [58] |
| Phage PE37 | STEC O157:H7 in broth Raw beef artificially contaminated with STEC O157:H7 | 2.6 and 4.9 log CFU/mL reductions following incubation for 6 h at 8 and 25 °C, respectively 0.9 and 2.3 log CFU/piece reductions following storage for 24 h at 8 and 25 °C, respectively | [59] |
| Alkali-resistant phage JN01 (109 PFU/mL) | E. coli O157:H7 in tryptic soy broth (103 CFU/mL) E. coli O157:H7 in UHT milk (2.3 log10 CFU/mL) E. coli O157:H7 on beef samples (4.5 log10 CFU/cm2) | A complete inhibition within 48 h Undetectable within 1–3 days of storage at 4 °C A reduction of >2 log10 CFU/cm2 during storage at 4 °C | [4] |
| Bacteriophage ECPS-6 (MOI: 5 at 25 °C) (MOI: 50 at 25 °C) | E. coli O157:H7 A-1 and A-2 in filtered raw milk (1 × 105 CFU × mL−1) E. coli O157:H7 A-2 in unfiltered raw milk (1 × 105 CFU × mL−1) | 2.97 log10 CFU × mL−1 and 4.1 log10 CFU × mL−1 reductions after 24 h The contamination level was below the detection limit after 6 h of incubation | [60] |
| EcoShield PX™, a cocktail of lytic bacteriophages (5 × 106 and 1 × 107 PFU/g) (ca. 1 × 106 PFU/g) | E. coli O157:H7 (ca. 3.0 log CFU/g) in beef chuck roast, ground beef, cooked chicken, chicken breast, cheese, salmon, romaine lettuce, and cantaloupe E. coli O157:H7 (ca. 1 to 10 CFU/10 g) on beef chuck roast samples | Significant reductions (p < 0.05), up to 97% in all foods Significantly reduced (p < 0.05) by ≥80% | [61] |
| Phage vB_EcoM-ECP26 (106 PFU/mL) | E. coli O157:H7 NCCP 13930 on romaine lettuce at 4 °C | Viable counts reduced to undetectable levels in 5 days | [62] |
| Phage FP43 | A mixed biofilm of EHEC O157:H7 and E. coli O91:H− | Decreased the formation of biofilm by 82.4% Following 6 h of incubation, viable counts of total cells in the biofilm and E. coli O157:H7 were reduced by 2.85 and 2.76 log, respectively | [63] |
| Phytochemical | Target Strain | Result | Reference |
|---|---|---|---|
| Propolis extract (2% and 5% concentrations) | E. coli O157:H7 in apple juice | Viable counts were reduced to undetectable levels within 24 h | [78] |
| Aqueous bark extracts of Quillaja saponaria | E. coli O157:H7 | >6.0 log CFU reductions at 37 °C within 1 h | [79] |
| Liposomal encapsulated (0.5% w/w) thyme extract | Inoculated E. coli O157:H7 in silver carp mince | Viable counts of the pathogen were reduced below the acceptable level (<2 log CFU/g) from day 9 to day 15 of storage | [80] |
| Target Strain | Synergistic Method | Result of the Study | Reference |
|---|---|---|---|
| E. coli EHEC O157:H7 CICC 21530 | The sequential treatment of cold nitrogen plasma (400 W, 2 min) and E. coli O157:H7 phages (5%, 30 min) | Viable population of biofilms of the pathogen was decreased by 2 log CFU/cm2 following individual treatment, while the sequential treatment decreased viable count by 5.71 log CFU/cm2 | [132] |
| E. coli EHEC O157:H7 CICC 21530 biofilms on lettuce | The combined treatment of clove oil (4 mg/mL, 30 min) and cold nitrogen plasma (400 W, 3 min) | 5.48 log CFU/cm2 reduction with no effect on the appearance quality of lettuce | [133] |
| E. coli O157:H7 on leafy green vegetables (lettuce and spinach) | A cocktail of phages (BEC8, approx. 106 PFU/leaf) and the essential oil trans-cinnameldehyde (TC, 0.5% v/v) | Upon treatment of both leaves with BEC8 or TC individually, no survivors were detected after 24 h at 23 and 37 °C at low levels of inoculum; when the two treatments were combined, complete inactivation (5 log CFU/leaf reduction) occurred within 10 min at all inoculum levels and temperatures on both leaves | [134] |
| E. coli on the surface of inoculated fresh-cut dragon fruit | The combined treatment of green tea extract (at tea: water; g/mL, ratio of 5%) and radio frequency (RF) cold plasma (40 W) | A complete inhibition (~5 log CFU/g) | [135] |
| E. coli O157:H7 in juices | Simultaneous application of pulsed electric fields (PEFs, 30 kV/cm) and 1.3 mM of carvacrol (a major component of certain essential oils) | 5 log10 cycles of inactivation of E. coli O157:H7 in less than 50 pulses | [136] |
| E. coli O157:H7 in pork sausage | Garlic essential oil (GO) and allyl isothiocyanate (AITC) 125 ppm GO + 250 ppm AITC 250 ppm GO + 250 ppm AITC | 1.01–1.87 log CFU/g reduction of the initial count after 20 days at 6 °C storage | [137] |
| E. coli O157:H7 in dry-cured sausage | AITC + o-coumaric acid (CA) at 20 × FIC (fractional inhibitory concentration) | Reduced by ≥5 log CFU/g after 21 days | [138] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puligundla, P.; Lim, S. Biocontrol Approaches against Escherichia coli O157:H7 in Foods. Foods 2022, 11, 756. https://doi.org/10.3390/foods11050756
Puligundla P, Lim S. Biocontrol Approaches against Escherichia coli O157:H7 in Foods. Foods. 2022; 11(5):756. https://doi.org/10.3390/foods11050756
Chicago/Turabian StylePuligundla, Pradeep, and Seokwon Lim. 2022. "Biocontrol Approaches against Escherichia coli O157:H7 in Foods" Foods 11, no. 5: 756. https://doi.org/10.3390/foods11050756