Influence of Drought Stress on Physiological Responses and Bioactive Compounds in Chicory (Cichorium intybus L.): Opportunity for a Sustainable Agriculture
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cultural Practices and Experimental Treatments
2.2. Leaf Traits and Gas-Exchange Measurements
2.3. Chemicals and Reagents
2.4. Nutritional Analysis
2.5. Statistical Analysis
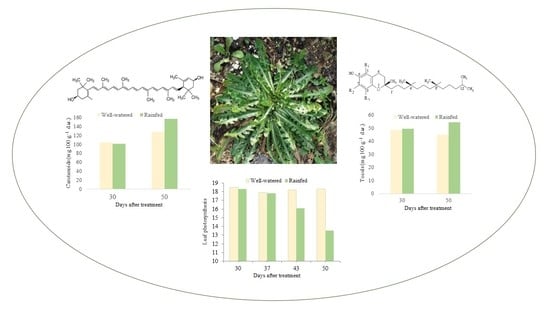
3. Results and Discussion
3.1. Weather Data
3.2. Fresh Biomass and Dry Matter Accumulation
3.3. Relative Water Content
3.4. Photosynthesis, Stomatal (gs) and Mesophyll (gm) Conductance
3.5. Nutritional Composition
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Garcia-Herrera, P.; Sanchez-Mata, M.C.; Camara, M.; Fernandez-Ruiz, V.; Diez-Marques, C.; Molina, M.; Tardio, J. Nutrient composition of six wild edible Mediterranean Asteraceae plants of dietary interest. J. Food Comp. Anal. 2014, 34, 163–170. [Google Scholar] [CrossRef]
- Wang, Q.; Cui, J. Perspectives and utilization technologies of chicory (Cichorium intybus L.): A review. Afr. J. Biotechnol. 2011, 10, 1966–1977. [Google Scholar]
- Fratianni, A.; D’Agostino, A.; Niro, S.; Bufano, A.; Paura, B.; Panfili, G. Loss or gain of lipophilic bioactive compounds in vegetables after domestic cooking? Effect of steaming and boiling. Foods 2021, 10, 960. [Google Scholar] [CrossRef] [PubMed]
- Montefusco, A.; Semitaio, G.; Marrese, P.P.; Iurlaro, A.; De Caroli, M.; Piro, G.; Dalessandro, G.; Lenucci, M.S. Antioxidants in varieties of chicory (Cichorium intybus L.) and wild poppy (Papaver rhoeas L.) of southern Italy. J. Chem. 2015, 923142, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Mata, M.C.; Cabrera Loera, R.D.; Morales, P.; Fernández-Ruiz, V.; Cámara, M.; Díez Marqués, C.; Pardo-de-Santayana, M.; Tardío, J. Wild vegetables of the Mediterranean area as valuable sources of bioactive compounds. Genet. Resour. Crop Evol. 2012, 59, 431–443. [Google Scholar] [CrossRef]
- FAO. The State of Food and Agriculture—Women in Agriculture, Closing the Gender Gap for Development. 2011. Available online: https://www.fao.org/3/i2050e/i2050e00.htm (accessed on 16 June 2022).
- Lovelli, S.; Perniola, M.; Scalcione, E.; Troccoli, A.; Ziska, L.H. Future climate change in the Mediterranean area: Implications for water use and weed management. Ital. J. Agron. 2012, 7, 45–49. [Google Scholar] [CrossRef] [Green Version]
- Bu, L.D.; Liu, J.L.; Zhu, L.; Luo, S.S.; Chen, X.P.; Li, S.Q.; Hill, R.L.; Zhao, Y. The effects of mulching on maize growth, yield and water use in a semi-arid region. Agric. Water Manag. 2013, 123, 71–78. [Google Scholar] [CrossRef]
- Zurita, M.L.; Thomsen, D.C.; Holbrook, N.J.; Smith, T.F.; Lyth, A.; Munro, P.G.; de Bruin, A.; Seddaiu, G.; Roggero, P.P.; Baird, J.; et al. Global water governance and climate change: Identifying innovative arrangements for adaptive transformation. Water 2018, 10, 29. [Google Scholar] [CrossRef] [Green Version]
- Sarabi, V.; Arjmand-Ghajur, E. Exogenous plant growth regulators/plant growth promoting bacteria roles in mitigating water-deficit stress on chicory (Cichorium pumilum Jacq.) at a physiological level. Agric. Water Manag. 2021, 245, 106439. [Google Scholar] [CrossRef]
- Rasmussen, C.R.; Thorup-Kristensen, K.; Dresbøll, D.B. Uptake of subsoil water below 2 m fails to alleviate drought response in deep-rooted Chicory (Cichorium intybus L.). Plant Soil 2020, 446, 275–290. [Google Scholar] [CrossRef] [Green Version]
- Langeroodi, A.R.S.; Osipitan, A.O.; Radicetti, E.; Mancinelli, R. To what extent arbuscular mycorrhiza can protect chicory (Cichorium intybus L.) against drought stress. Sci. Hortic. 2020, 263, 109109. [Google Scholar] [CrossRef]
- Monti, A.; Amaducci, M.T.; Pritoni, G.; Venturi, G. Growth, fructan yield, and quality of chicory (Cichorium intybus L.) as related to photosynthetic capacity, harvest time, and water regime. J. Exp. Bot. 2005, 56, 1389–1395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cranston, L.M.; Kenyon, P.R.; Morris, S.T.; Lopez-Villalobos, N.; Kemp, P.D. Morphological and physiological responses of plantain (Plantago lanceolata) and chicory (Cichorium intybus) to water stress and defoliation frequency. J. Agron. Crop Sci. 2016, 202, 13–24. [Google Scholar] [CrossRef]
- Delfine, S.; Marrelli, M.; Conforti, F.; Formisano, C.; Rigano, D.; Menichini, F.; Senatore, F. Variation of Malva sylvestris essential oil yield, chemical composition and biological activity in response to different environments across Southern Italy. Ind. Crop Prod. 2017, 98, 29–37. [Google Scholar] [CrossRef]
- Delfine, S.; Loreto, F.; Alvino, A. Drought-stress effects on physiology, growth and biomass production of rainfed and irrigated bell pepper plants in the Mediterranean region. J. Am. Soc. Hort. Sci. 2001, 126, 297–304. [Google Scholar] [CrossRef] [Green Version]
- Smirnoff, N. Antioxidant systems and plant response to the environment. In Environment and Plant Metabolism: Flexibility and Acclimation; Smirnoff, N., Ed.; Bios Scientific Publishers: Oxford, UK, 1995; pp. 217–243. [Google Scholar]
- Huchzermeyer, B.; Menghani, E.; Khardia, P.; Shilu, A. Metabolic pathway of natural antioxidants, antioxidant enzymes and ROS providence. Antioxidants 2022, 11, 761. [Google Scholar] [CrossRef]
- Dumanović, J.; Nepovimova, E.; Natić, M.; Kuča, K.; Jaćević, V. The significance of reactive oxygen species and antioxidant defense system in plants: A concise overview. Front. Plant Sci. 2021, 11, 11–19. [Google Scholar] [CrossRef]
- Toscano, S.; Trivellini, A.; Cocetta, G.; Bulgari, R.; Francini, A.; Romano, D.; Ferrante, A. Effect of preharvest abiotic stresses on the accumulation of bioactive compounds in horticultural produce. Front. Plant. Sci. 2019, 10, 1212. [Google Scholar] [CrossRef] [Green Version]
- Fratianni, A.; Giuzio, L.; Di Criscio, T.; Flagella, Z.; Panfili, G. Response of carotenoids and tocols of durum wheat in relation to water stress and sulfur fertilization. J. Agric. Food Chem. 2013, 61, 2583–2590. [Google Scholar] [CrossRef]
- Medyouni, I.; Zouaoui, R.; Rubio, E.; Serino, S.; Ahmed, H.B.; Bertin, N. Effects of water deficit on leaves and fruit quality during the development period in tomato plant. Food Sci. Nutr. 2021, 9, 1949–1960. [Google Scholar] [CrossRef]
- Casadesús, A.; Arabia, A.; Pujolriu, R.; Munné-Bosch, S. Differential accumulation of tocochromanols in photosynthetic and non-photosynthetic tissues of strawberry plants subjected to reiterated water deficit. Plant Physiol. Biochem. 2020, 155, 868–876. [Google Scholar] [CrossRef] [PubMed]
- Al Hassan, M.; Martínez Fuertes, M.; Ramos Sánchez, F.J.; Vicente, O.; Boscaiu, M. Effects of salt and water stress on plant growth and on accumulation of osmolytes and antioxidant compounds in cherry tomato. Not. Bot. Horti. Agrobo. 2015, 43, 1–11. [Google Scholar] [CrossRef]
- Eichholz, I.; Förster, N.; Ulrichs, C.; Schreiner, M.; Huyskens-Keil, S. Survey of bioactive metabolites in selected cultivars and varieties of Lactuca sativa L. under water stress. J. Appl. Bot. Food Qual. 2014, 87, 265–273. [Google Scholar] [CrossRef]
- Jaleel, C.A.; Manivannan, P.; Wahid, A.; Farooq, M.; Somasundaram, R.; Panneerselvam, R. Drought stress in plants: A review on morphological characteristics and pigments composition. Int. J. Agric. Biol. 2009, 11, 100–105. [Google Scholar]
- Eggersdorfer, M.; Wyss, A. Carotenoids in human nutrition and health. Arch. Biochem. Biophys. 2018, 15, 18–26. [Google Scholar] [CrossRef]
- Swapnil, P.; Meena, M.; Singh, S.K.; Dhuldhaj, U.P.; Harish; Marwal, A. Vital roles of carotenoids in plants and humans to deteriorate stress with its structure, biosynthesis, metabolic engineering and functional aspects. Curr. Plant Biol. 2021, 26, 100203. [Google Scholar] [CrossRef]
- Saini, R.K.; Nile, S.H.; Park, S. Carotenoids from fruits and vegetables: Chemistry, analysis, occurrence, bioavailability and biological activities. Food Res. Int. 2015, 76, 735–750. [Google Scholar] [CrossRef]
- Hong-bo, S.; Li-ye, C.; Ming-an, S.; Jaleel, C.A.; Hong-mei, M. Higher plant antioxidants and redox signaling under environmental stresses. C. R. Biol. 2008, 331, 433–441. [Google Scholar] [CrossRef]
- Munné-Bosch, S. The role of α-tocopherol in plant stress tolerance. J. Plant Physiol. 2005, 162, 743–748. [Google Scholar] [CrossRef]
- Munné-Bosch, S.; Alegre, L. The function of tocopherols and tocotrienols in plants. Crit. Rev. Plant Sci. 2002, 21, 31–57. [Google Scholar] [CrossRef]
- Sadiq, M.; Akram, N.A.; Ashraf, M.; Al-Qurainy, F.; Ahmad, P. Alpha-Tocopherol-induced regulation of growth and metabolism in plants under non-stress and stress conditions. J. Plant Growth Reg. 2019, 38, 1325–1340. [Google Scholar] [CrossRef]
- Shahidi, F.; de Camargo, A.C. Tocopherols and tocotrienols in common and emerging dietary sources: Occurrence, applications, and health benefits. Int. J. Mol. Sci. 2016, 17, 1745. [Google Scholar] [CrossRef] [PubMed]
- Mignogna, R.; Fratianni, A.; Niro, S.; Panfili, G. Tocopherol and tocotrienol analysis as a tool to discriminate different fat ingredients in bakery products. Food Control 2015, 54, 31–38. [Google Scholar] [CrossRef]
- Delfine, S.; Loreto, F.; Pinelli, P.; Tognetti, R.; Alvito, A. Isoprenoids content and photosynthetic limitations in rosemary and spearmint plants under water stress. Agric. Ecosyst. Environ. 2005, 106, 243–252. [Google Scholar] [CrossRef]
- Panfili, G.; Fratianni, A.; Irano, M. Normal phase high-performance liquid chromatography method for the determination of tocopherols and tocotrienols in cereals. J. Agric. Food Chem. 2003, 51, 3940–3944. [Google Scholar] [CrossRef] [PubMed]
- AOAC International. Official Methods of Analysis, 17th ed.; AOAC International: Washington, DC, USA, 2000. [Google Scholar]
- Baldini, M.; Fabietti, F.; Giammarioli, S.; Onori, R.; Orefice, L.; Stacchini, A. Rapporti ISTISAN 96/34. Metodi di Analisi Utilizzati per il Controllo Chimico degli Alimenti. Istituto Superiore di Sanità, Ed.; Istituto Superiore di Sanità: Rome, Italy, 1996; pp. 41–43. [Google Scholar]
- Panfili, G.; Fratianni, A.; Irano, M. Improved normal-phase high-performance liquid chromatography procedure for the determination of carotenoids in cereals. J. Agric. Food Chem. 2004, 52, 6373–6377. [Google Scholar] [CrossRef]
- Fernandes, R.D.M.; Frizzone, J.A.; José, J.V. Chicory (Cichorium intybus L.) yield under water stress and estimation of leaf area using allometric relations. Aust. J. Crop Sci. 2017, 11, 1547–1552. [Google Scholar] [CrossRef]
- Delfine, S.; Alvino, A.; Villani, M.C.; Loreto, F. Restriction to carbon dioxide conductance and photosynthesis in spinach leaves recovering from salt stress. Plant Physiol. 1999, 119, 1101–1106. [Google Scholar] [CrossRef] [Green Version]
- Loreto, F.; Harley, P.C.; Di Marco, G.; Sharkey, T.D. Estimation of mesophyll conductance to CO2 flux by three different methods. Plant Physiol. 1992, 98, 1437–1443. [Google Scholar] [CrossRef] [Green Version]
- Delfine, S.; Alvino, A.; Zacchini, M.; Loreto, F. Resistances to CO2 diffusion in salt stressed leaves. Austral. J. Plant Physiol. 1998, 25, 395–402. [Google Scholar]
- Flexas, J.; Bota, J.; Loreto, F.; Cornic, G.; Sharkey, T.D. Diffusive and metabolic limitations to photosynthesis under drought and salinity in C3 plants. Plant Biol. 2004, 6, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Nwafor, I.C.; Shale, K.; Achilonu, M.C. Chemical composition and nutritive benefits of chicory (Cichorium intybus) as an ideal complementary and/or alternative livestock feed supplement. Sci. World J. 2017, 2017, 7343928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panfili, G.; Niro, S.; Bufano, A.; D’Agostino, A.; Fratianni, A.; Paura, B.; Falasca, L.; Cinquanta, L. Bioactive compounds in wild Asteraceae edible plants consumed in the Mediterranean diet. Plant Foods Hum. Nutr. 2020, 75, 540–546. [Google Scholar] [CrossRef]
- Fratianni, A.; Mignogna, R.; Niro, S.; Panfili, G. Determination of lutein from fruit and vegetables through an alkaline hydrolysis extraction method and HPLC analysis. J. Food Sci. 2015, 80, 2686–2691. [Google Scholar] [CrossRef] [PubMed]
- Knecht, K.; Sandfuchs, K.; Kulling, S.E.; Bunzel, D. Tocopherol and tocotrienol analysis in raw and cooked vegetables: A validated method with emphasis on sample preparation. Food Chem. 2015, 169, 20–27. [Google Scholar] [CrossRef]
- Xu, C.; He, C.G.; Wang, Y.J.; Bi, Y.F.; Jiang, H. Effect of drought and heat stresses on photosynthesis, pigments, and xanthophyll cycle in alfalfa (Medicago sativa L.). Photosynthetica 2020, 58, 1226–1236. [Google Scholar] [CrossRef]
- Song, X.S.; Shang, Z.W.; Yin, Z.P.; Ren, J.; Sun, M.C.; Ma, X.L. Mechanism of xanthophyll-cycle-mediated photoprotection in Cerasus humilis seedlings under water stress and subsequent recovery. Photosynthetica 2011, 49, 523–530. [Google Scholar] [CrossRef]
- Buezo, J.; Sanz-Saez, Á.; Moran, J.F.; Soba, D.; Aranjuelo, I.; Esteban, R. Drought tolerance response of high-yielding soybean varieties to mild drought: Physiological and photochemical adjustments. Physiol. Plant. 2019, 166, 88–104. [Google Scholar] [CrossRef]
- Huang, H.Y.; Zhang, Q.; Zhao, L.P.; Feng, J.N.; Peng, C.L. Does lutein play a key role in the protection of photosynthetic apparatus in arabidopsis under severe oxidative stress? Pak. J. Bot. 2010, 42, 2765–2774. [Google Scholar]




| Month | Average Max Temperature (°C) | Average Min Temperature (°C) | Rainfall (mm) |
|---|---|---|---|
| January | 14.9 | −2.9 | 102.6 |
| February | 20.9 | −4.6 | 22.2 |
| March | 20.1 | −1.4 | 17.2 |
| April | 26.8 | −0.7 | 10.6 |
| May | 28.7 | 5.8 | 0.4 |
| June | 29.9 | 7.9 | 0.0 |
| DAT | Samples | Protein | Fat | Ash | Carbohydrates b |
|---|---|---|---|---|---|
| 30 | W | 19.5 (0.1) | 1.8 (0.1) | 14.5 (0.6) | 64.2 (0.1) |
| R | 18.8 (0.1) | 1.8 (0.1) | 15.1 (0.1) | 64.3 (0.1) | |
| 50 | W | 16.1 (0.4) | 2.4 (0.1) | 15.2 (0.4) | 66.3 (0.9) |
| R | 15.8 (1.2) | 2.7 (0.7) | 15.1 (0.1) | 66.4 (1.8) |
| DAT | Samples | Violaxanthin | Neoxanthin | Lutein | Zeaxanthin | α-Carotene | 13-Cis-β-carotene | β-Carotene | 9-Cis-β-carotene | Totals |
|---|---|---|---|---|---|---|---|---|---|---|
| 30 | W | 10.7 (0.8) | 9.9 (1.0) | 57.2 (5.5) | 5.2 (1.2) | 2.6 (0.4) | 2.8 (0.4) | 13.3 (3.5) | 2.4 (0.3) | 104.1 (10.6) |
| R | 11.3 (2.2) | 10.5 (1.3) | 53.8 (2.1) | 5.5 (0.1) | 2.4 (0.8) | 2.7 (0.5) | 13.3 (1.6) | 2.1 (0.4) | 101.7 (2.5) | |
| 50 | W | 11.3 (1.5) * | 12.6 (0.4) * | 72.3 (9.3) * | 4.1 (0.7) * | 2.7 (0.1) | 3.4 (0.3) | 18.9 (0.9) | 3.0 (0.3) | 128.4 (0.7) * |
| R | 15.4 (0.5) * | 16.0 (1.1) * | 96.4 (4.6) * | 5.9 (0.5) * | 2.4 (0.6) | 3.0 (0.2) | 15.1 (2.4) | 2.5 (0.3) | 156.7 (2.5) * |
| DAT | Samples | α-T | γ-T | Totals |
|---|---|---|---|---|
| 30 | W | 30.9 (2.6) | 17.6 (1.1) | 48.5 (3.7) |
| R | 31.9 (0.4) | 17.5 (0.2) | 49.4 (0.5) | |
| 50 | W | 33.4 (1.7) * | 11.4 (0.7) * | 44.9 (2.4) * |
| R | 38.5 (0.1) * | 15.8 (0.6) * | 54.3 (0.5) * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delfine, S.; Fratianni, A.; D'Agostino, A.; Panfili, G. Influence of Drought Stress on Physiological Responses and Bioactive Compounds in Chicory (Cichorium intybus L.): Opportunity for a Sustainable Agriculture. Foods 2022, 11, 3725. https://doi.org/10.3390/foods11223725
Delfine S, Fratianni A, D'Agostino A, Panfili G. Influence of Drought Stress on Physiological Responses and Bioactive Compounds in Chicory (Cichorium intybus L.): Opportunity for a Sustainable Agriculture. Foods. 2022; 11(22):3725. https://doi.org/10.3390/foods11223725
Chicago/Turabian StyleDelfine, Sebastiano, Alessandra Fratianni, Annacristina D'Agostino, and Gianfranco Panfili. 2022. "Influence of Drought Stress on Physiological Responses and Bioactive Compounds in Chicory (Cichorium intybus L.): Opportunity for a Sustainable Agriculture" Foods 11, no. 22: 3725. https://doi.org/10.3390/foods11223725