Exploring the Potential of Grape Pomace Extract to Inhibit Thermo-Oxidative Degradation of Sunflower Oil: From Routine Tests to ATR-FTIR Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
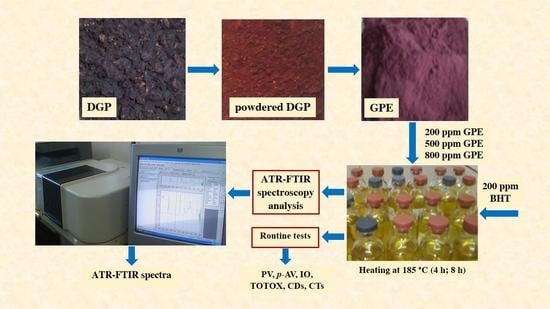
2.1. Processing of Grape Pomace Extract (GPE)
2.2. Sunflower Oil Sample Preparation and Heating Protocol
2.3. Analysis of Raw Grape Pomace, Dried Grape Pomace and Grape Pomace Extract
2.3.1. Moisture Content
2.3.2. Assessment of Total Antioxidant Capacity by the Ferric-Reducing Antioxidant Power Assay
2.3.3. Assessment of Radical Scavenging Activity by 1,1-Diphenyl-2-picrylhydrazyl (DPPH) Assay
2.3.4. Evaluation of Total Phenolic Content (TPC)
2.4. Routine Tests to Evaluate the Progress of Sunflower Oil Thermo-Oxidation
2.5. ATR-FTIR Spectra Acquisition
2.6. Statistical Analysis
3. Results and Discussion
3.1. Antioxidant Properties of Grape Pomace and Grape Pomace Extract
3.2. Assessing Thermo-Oxidative Degradation of Sunflower Oil Samples by Routine Chemical Indices
3.2.1. Peroxide Value (PV)
3.2.2. The Inhibition of Oil Oxidation (IO)
3.2.3. Para-Anisidine Value (p-AV)
3.2.4. Total Oxidation Value (TOTOX)
3.2.5. Specific Extinction Coefficients Measurement
3.3. Assessing the Thermo-Oxidative Degradation of Sunflower Oil Samples by ATR-FTIR Spectroscopy
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saeed, R.; Naz, S. Effect of heating on the oxidative stability of corn oil and soybean oil. Grasas Aceites 2019, 70, e303. [Google Scholar] [CrossRef]
- Zhang, Q.; Saleh, A.S.; Chen, J.; Shen, Q. Chemical alterations taken place during deep-fat frying based on certain reaction products: A review. Chem. Phys. Lipids 2012, 165, 662–681. [Google Scholar] [CrossRef] [PubMed]
- Aladedunye, F.A.; Przybylski, R. Degradation and nutritional quality changes of oil during frying. J. Am. Oil Chem. Soc. 2009, 86, 149–156. [Google Scholar] [CrossRef]
- Leong, X.F.; Ng, C.Y.; Jaarin, K.; Mustafa, M.R. Effects of repeated heating of cooking oils on antioxidant content and endothelial function. Austin J. Pharmacol. Ther. 2015, 3, 1–7. [Google Scholar]
- Iqbal, S.; Bhanger, M.I. Stabilization of sunflower oil by garlic extract during accelerated storage. Food Chem. 2007, 100, 246–254. [Google Scholar] [CrossRef]
- Fadda, A.; Sanna, D.; Sakar, E.H.; Gharby, S.; Mulas, M.; Medda, S.; Yesilcubuk, N.S.; Karaca, A.C.; Gozukirmizi, C.K.; Lucarini, M.; et al. Innovative and Sustainable Technologies to Enhance the Oxidative Stability of Vegetable Oils. Sustainability 2022, 14, 849. [Google Scholar] [CrossRef]
- Bustamante, M.A.; Moral, R.; Paredes, C.; Perez-Espinosa, A.; Moreno-Caselles, J.; Perez-Murcia, M.D. Agrochemical characterization of the solid by-products and residues from the winery and distillery industry. J. Waste Manag. 2008, 28, 372–380. [Google Scholar] [CrossRef]
- Louli, V.; Ragoussis, N.; Magoulas, K. Recovery of phenolic antioxidants from wine industry by-products. Bioresour. Technol. 2004, 92, 201–208. [Google Scholar] [CrossRef]
- Lafka, T.I.; Sinanoglou, V.; Lazos, E.S. On the extraction and antioxidant activity of phenolic compounds from winery wastes. Food Chem. 2007, 104, 1206–1214. [Google Scholar] [CrossRef]
- Wang, X.; Tong, H.; Chen, F.; Gangemi, J.D. Chemical characterization and antioxidant evaluation of muscadine grape pomace extract. Food Chem. 2010, 123, 1156–1162. [Google Scholar] [CrossRef]
- Garrido, T.; Gizdavic-Nikolaidis, M.; Leceta, I.; Urdanpilleta, M.; Guerrero, P.; de la Caba, K.; Kilmartin, P.A. Optimizing the extraction process of natural antioxidants from chardonnay grape marc using microwave-assisted extraction. Waste Manag. 2019, 88, 110–117. [Google Scholar] [CrossRef]
- Tournour, H.M.; Segundoc, M.A.; Magalhaes, L.M.; Barreiros, L.; Queiroz, J.; Cunha, L.M. Valorization of grape pomace: Extraction of bioactive phenolics with antioxidant properties. Ind. Crops Prod. 2015, 74, 397–406. [Google Scholar] [CrossRef]
- Negro, C.; Tommasi, L.; Miceli, A. Phenolic compounds and antioxidative activity from red grape marc extracts. Bioresour. Technol. 2003, 87, 431–444. [Google Scholar] [CrossRef]
- Peixoto, C.M.; Dias, M.I.; Alves, M.J.; Calhelha, R.C.; Barros, L.; Pinho, S.P.; Ferreira, I.C. Grape pomace as a source of phenolic compounds and diverse bioactive properties. Food Chem. 2018, 253, 132–138. [Google Scholar] [CrossRef] [Green Version]
- Panzella, L.; Moccia, F.; Nasti, R.; Marzorati, S.; Verotta, L.; Napolitano, A. Bioactive phenolic compounds from agri-food wastes: An update on green and sustainable extraction methodologies. Front. Nutr. 2020, 7, 60. [Google Scholar] [CrossRef]
- Casagrande, M.; Zanela, J.; Pereira, D.; de Lima, V.A.; Oldoni, T.L.C.; Carpes, S.T. Optimization of the extraction of antioxidant phenolic compounds from grape pomace using response surface methodology. J. Food Meas. Charact. 2019, 13, 1120–1129. [Google Scholar] [CrossRef]
- Chidambara, M.K.N.; Singh, R.P.M.; Jayaprakasha, G.K. Antioxidant activities of grape (Vitis vinifera) pomace extracts. J. Agric. Food Chem. 2002, 50, 5909–5914. [Google Scholar] [CrossRef]
- Mielnik, M.B.; Olsen, E.; Vogt, G.; Adeline, D.; Skrede, G. Grape seed extract as antioxidant in cooked, cold stored turkey meat. LWT-Food Sci. Technol. 2006, 39, 191–198. [Google Scholar] [CrossRef]
- Brannan, R.G.; Mah, E. Grape seed extract inhibits lipid oxidation in muscle from different species during refrigerated and frozen storage and oxidation catalyzed by peroxynitrite and iron/ascorbate in a pyrogallol red model system. Meat Sci. 2007, 77, 540–546. [Google Scholar] [CrossRef]
- Rababah, T.M.; Yucel, S.; Ereifej, K.I.; Alhamad, M.N.; Al-Mahasneh, M.A.; Yang, W.; Al-Muhammad, A.H.; Ismaeal, K. Effect of grape seed extracts on the physicochemical and sensory properties of corn chips during storage. J. Am. Oil Chem. Soc. 2011, 88, 631–637. [Google Scholar] [CrossRef]
- El-Hadary, A.E.; Tahoon, N.A. Effect of grape seed extract on lipid oxidation and hydroperoxide formation in soybean oil. J. Biol. Chem. Environ. Sci. 2013, 8, 99–110. [Google Scholar]
- Poiana, M.A. Enhancing oxidative stability of sunflower oil during convective and microwave heating using grape seed extract. Int. J. Mol. Sci. 2012, 13, 9240–9259. [Google Scholar] [CrossRef] [PubMed]
- Shaker, E.S. Antioxidative effect of extracts from red grape seed and peel on lipid oxidation in oils of sunflower. LWT-Food Sci. Technol. 2006, 39, 883–892. [Google Scholar] [CrossRef]
- Guillen, M.D.; Cabo, N. Fourier transform infrared spectra data versus peroxide and anisidine values to determine oxidative stability of edible oils. Food Chem. 2002, 77, 503–510. [Google Scholar] [CrossRef]
- Guillen, M.D.; Cabo, N.; Ibargoitia, M.L.; Ruiz, A. Study of both sunflower oil and its headspace throughout the oxidation process. Occurrence in the headspace of toxic oxygenated aldehydes. J. Agric. Food Chem. 2005, 53, 1093–1101. [Google Scholar] [CrossRef]
- Kozlowska, M.; Zbikowska, A.; Marciniak-Lukasiak, K.; Kowalska, M. Herbal Extracts Incorporated into Shortbread Cookies: Impact on Color and Fat Quality of the Cookies. Biomolecules 2019, 9, 858. [Google Scholar] [CrossRef] [Green Version]
- Rohman, A.; Che Man, Y.B. Application of FTIR spectroscopy for monitoring the stabilities of selected vegetable oils during thermal oxidation. Int. J. Food Prop. 2013, 16, 1594–1603. [Google Scholar] [CrossRef]
- Rexhepia, F.; Surlevab, A.; Hysenia, A.; Brucia, M.; Kodraliua, B. Comprehensive investigation of thermal degradation characteristics and properties changes of plant edible oils by FTIR spectroscopy. Acta Chem. Iasi 2019, 27, 263–286. [Google Scholar] [CrossRef] [Green Version]
- Cakmak-Arslan, G. Monitoring of hazelnut oil quality during thermal processing in comparison with extra virgin olive oil by using ATR-FTIR spectroscopy combined with chemometrics. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 266, 120461. [Google Scholar] [CrossRef]
- Vlachos, N.; Skopelitis, Y.; Psaroudaki, M.; Konstantinidou, V.; Chatzilazarou, A.; Tegou, E. Applications of Fourier transform—Infrared spectroscopy to edible oils. Anal. Chim. Acta. 2006, 573–574, 459–465. [Google Scholar] [CrossRef]
- Guillen, M.; Cabo, N. Relationships between the composition of edible oils and lard and the ratio of the absorbance of specific bands of their Fourier transform infrared spectra. Role of some bands of the fingerprint region. J. Agric. Food Chem. 1998, 46, 1788–1793. [Google Scholar] [CrossRef]
- Moharam, M.A.; Abbas, L.M. A study on the effect of microwave heating on the properties of edible oils using FTIR spectroscopy. Afr. J. of Microbiol. Res. 2010, 4, 1921–1927. [Google Scholar]
- Spigno, G.; Faveri, D.M. Antioxidants from grape stalks and marc: Influence of extraction procedure on yield, purity and antioxidant power of the extracts. J. Food Eng. 2007, 78, 793–801. [Google Scholar] [CrossRef]
- Alexa, E.; Poiana, M.A.; Sumalan, R.M. Mycoflora and ochratoxin A control in wheat grain using natural extracts obtained from wine industry by-products. Int. J. Mol. Sci. 2012, 13, 4949–4967. [Google Scholar] [CrossRef]
- Mariod, A.A.; Matthäus, B.; Eichner, K.; Hussein, H.I. Antioxidant activity of extracts from Sclerocarya birrea kernel oil cake. Grasas Aceites 2006, 57, 361–366. [Google Scholar] [CrossRef]
- Official Methods of Analysis of the AOAC. Anal. Chem. 1980, 52, 148A. [CrossRef]
- Benzie, I.F.F.; Strain, L. Ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Hue, H.T.; Tinh, H.T.; Van Bao, N.; Dao, P.T.A. Screening for antioxidant activity of vegetable and fruit by-products and evaluating the ability of coffee sediment to preserve fish meal. SN Appl. Sci. 2020, 2, 1282. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Meth. Enzymol. 1999, 299, 152–178. [Google Scholar]
- ISO 3960:2009; Animal and Vegetable Fats and Oils—Determination of Peroxide Value—Iodometric Visual Endpoint Determination. International Organization for Standardization (ISO): Geneva, Switzerland, 2009.
- ISO 6885:2008; Animal and Vegetable Fats and Oils—Determination of Anisidine Value. International Organization for Standardization (ISO): Geneva, Switzerland, 2008.
- ISO 3656:2011; Animal and Vegetable Fats and Oils—Determination of Ultraviolet Absorbance Expressed as Specific UV Extinction. International Organization for Standardization (ISO): Geneva, Switzerland, 2011.
- Raba, D.N.; Poiana, M.A.; Borozan, A.B.; Stef, M.; Radu, F.; Popa, M.V. Investigation on crude and high-temperature heated coffee oil by ATR-FTIR spectroscopy along with antioxidant and antimicrobial properties. PLoS ONE 2015, 10, e0138080. [Google Scholar] [CrossRef] [Green Version]
- Iora, S.R.; Maciel, G.M.; Zielinski, A.A.; da Silva, M.V.; Pontes, P.V.D.A.; Haminiuk, C.W.; Granato, D. Evaluation of the bioactive compounds and the antioxidant capacity of grape pomace. Int. J. Food Sci. Technol. 2015, 50, 62–69. [Google Scholar] [CrossRef]
- Negro, C.; Aprile, A.; Luvisi, A.; De Bellis, L.; Miceli, A. Antioxidant activity and polyphenols characterization of four monovarietal grape pomaces from Salento (Apulia, Italy). Antioxidants 2021, 10, 1406. [Google Scholar] [CrossRef] [PubMed]
- Rockenbach, I.I.; Rodrigues, E.; Valdemiro Gonzaga, L.; Caliari, V.; Genovese, M.I.; de Souza Schmidt Gonçalves, A.E.; Fett, R. Phenolic compounds content and antioxidant activity in pomace from selected red grapes (Vitis vinifera L. and Vitis labrusca L.) widely produced in Brazil. Food Chem. 2011, 127, 174–179. [Google Scholar] [CrossRef]
- Teles, A.S.C.; Chavez, D.W.H.; Gomes, F.S.; Cabral, L.M.C.; Tonon, R.V. Effect of temperature on the degradation of bioactive compounds of Pinot Noir grape pomace during drying. Braz. J. Food Technol. 2018, 21, e2017059. [Google Scholar] [CrossRef]
- Beuchat, L.; Komitopoulou, E.; Beckers, H.; Betts, R.P.; Bourdichon, F.; Fanning, S.; Joosten, H.M.; Ter Kuile, B.H. Low–water activity foods: Increased concern as vehicles of foodborne pathogens. J. Food Prot. 2013, 76, 150–172. [Google Scholar] [CrossRef]
- Fontana, A.R.; Antoniolli, A.; Bottini, R. Grape pomace as a sustainable source of bioactive compounds: Extraction, characterization, and biotechnological applications of phenolics. J. Agric. Food Chem. 2013, 61, 8987–9003. [Google Scholar] [CrossRef]
- Yeler, H.B.; Nas, S. Optimization of extraction time and temperature for natural antioxidants of öküzgözü grape pomace using various solvent ratios. Food Sci. Technol. 2020, 41, 127–135. [Google Scholar] [CrossRef]
- Karasu, S.; Başlar, M.; Karaman, S.; Kılıçlı, M.; Us, A.A.; Yaman, H.; Sağdıç, O. Characterization of some bioactive compounds and physicochemical properties of grape varieties grown in Turkey: Thermal degradation kinetics of anthocyanin. Turk. J. Agric. For. 2016, 40, 177–185. [Google Scholar] [CrossRef]
- Veskoukis, A.S.; Kyparos, A.; Nikolaidis, M.G.; Stagos, D.; Aligiannis, N.; Halabalaki, M.; Kouretas, D. The antioxidant effects of a polyphenol-rich grape pomace extract in vitro do not correspond in vivo using exercise as an oxidant stimulus. Oxid. Med. Cell. Longev. 2012, 2012, 185867. [Google Scholar] [CrossRef] [Green Version]
- Saratale, G.D.; Saratale, R.G.; Kim, D.S.; Kim, D.Y.; Shin, H.S. Exploiting fruit waste grape pomace for silver nanoparticles synthesis, assessing their antioxidant, antidiabetic potential and antibacterial activity against human pathogens: A novel approach. J. Nanomater. 2020, 10, 1457. [Google Scholar] [CrossRef]
- Andrade, L.B.; Hollas, F.F.; Locatelli, C. Antioxidant and Photoprotector effect from grape marc hydroalcoholic extract: In vitro assays. Braz. J. Dev. 2021, 7, 10433–10443. [Google Scholar] [CrossRef]
- Ravi Kiran, C.; Sasidharan, I.; Soban Kumar, D.R.; Sundaresan, A. Influence of natural and synthetic antioxidants on the degradation of Soybean oil at frying temperature. J. Food Sci. Technol. 2015, 52, 5370–5375. [Google Scholar] [CrossRef] [Green Version]
- Kalantzakis, G.; Blekas, G. Effect of Greek sage and summer savory extracts on vegetable oil thermal stability. Eur. J. Lipid Sci. Technol. 2006, 108, 842–847. [Google Scholar] [CrossRef]
- De Abreu, D.A.P.; Paseiro Losada, P.; Maroto, J.; Cruz, J.M. Evaluation of the effectiveness of a new active packaging film containing natural antioxidants (from barley husks) that retard lipid damage in frozen Atlantic salmon (Salmo salar L.). Food Res. Int. 2010, 43, 1277–1282. [Google Scholar] [CrossRef]
- Farhoosh, R.; Moosavi, S.M.R. Evaluating the performance of peroxide and conjugated diene values in monitoring quality of used frying oils. J. Agric. Sci. Technol. 2009, 11, 173–179. [Google Scholar]
- Metzner Ungureanu, C.R.; Poiana, M.A.; Cocan, I.; Lupitu, A.I.; Alexa, E.; Moigradean, D. Strategies to improve the thermo-oxidative stability of sunflower oil by exploiting the antioxidant potential of blueberries processing by products. Molecules 2020, 25, 5688. [Google Scholar] [CrossRef]
- Ugarte-Espinoza, P.P.; Delgado-Soriano, V.; Estivi, L.; Hidalgo, A.; Pascual-Chagman, G. Goldenberry (Physalis peruviana L.) seed oil: Press extraction, optimization, characterization, and oxidative stability. Ital. J. Food Sci. 2021, 33, 107–117. [Google Scholar] [CrossRef]
- Abdelazim, A.A.; Mahmoud, A.; Ramadan-Hassanien, M.F. Oxidative stability of vegetable oils as affected by sesame extracts during accelerated oxidative storage. J. Food Sci. Technol. 2013, 50, 868–878. [Google Scholar] [CrossRef] [Green Version]
- Dostalova, J.; Hanzlik, P.; Reblova, Z.; Pokorny, J. Oxidative changes of vegetable oils during microwave heating. Czech J. Food Sci. 2005, 23, 230–239. [Google Scholar] [CrossRef] [Green Version]
- Poiana, M.A.; Alexa, E.; Munteanu, M.F.; Gligor, R.; Moigradean, D.; Mateescu, C. Use of ATR-FTIR spectroscopy to detect the changes in extra virgin olive oil by adulteration with soybean oil and high temperature heat treatment. Open Chem. J. 2015, 13, 689–698. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of infrared spectra, a practical approach. Encycl. Anal. Chem. 2000, 12, 10815–10837. [Google Scholar]
- Jović, O.; Smolić, T.; Jurišić, Z.; Meić, Z.; Hrenar, T. Chemometric analysis of croatian extra virgin olive oils from central Dalmatia region. Croat. Chem. 2013, 86, 335–344. [Google Scholar] [CrossRef]
- Zahir, E.; Saeed, R.; Hameed, M.A.; Yousuf, A. Study of physicochemical properties of edible oil and evaluation of frying oil quality by Fourier Transform-Infrared (FT-IR) Spectroscopy. Arab. J. Chem. 2017, 10, S3870–S3876. [Google Scholar] [CrossRef] [Green Version]
- Shi, L.; Liu, Z.; Li, J.; Qin, Z. Analysis of edible vegetable oils by infrared absorption spectrometry. Adv. Eng. Res. 2017, 86, 286–289. [Google Scholar]
- Gubitosa, J.; Rizzi, V.; Laurenzana, A.; Scavone, F.; Frediani, E.; Fibbi, G.; Fanelli, F.; Sibillano, T.; Giannini, C.; Fini, P.; et al. The “End Life” of the Grape Pomace Waste Become the New Beginning: The Development of a Virtuous Cycle for the Green Synthesis of Gold Nanoparticles and Removal of Emerging Contaminants from Water. Antioxidants 2022, 11, 994. [Google Scholar] [CrossRef]
- Peighambardoust, S.H.; Fasihnia, S.H.; Peighambardoust, S.J.; Pateiro, M.; Domínguez, R.; Lorenzo, J.M. Active Polypropylene-Based Films Incorporating Combined Antioxidants and Antimicrobials: Preparation and Characterization. Foods 2021, 10, 722. [Google Scholar] [CrossRef]
- Van de Voort, F.R.; Ismail, A.A.; Sedman, J.; Emo, G. Monitoring the oxidation of edible oils by Fourier transform infrared spectroscopy. J. Am. Oil Chem. Soc. 1994, 71, 243–253. [Google Scholar] [CrossRef]
- Vieira, T.M.; Regitano-d’Arce, M.A. Ultraviolet spectrophotometric evaluation of corn oil oxidative stability during microwave heating and oven test. J. Agric. Food Chem. 1999, 47, 2203–2206. [Google Scholar] [CrossRef]
- Moreno, M.M.; Olivares, D.M.; Lopez, F.A.; Adelantado, J.G.; Reig, F.B. Analytical evaluation of polyunsaturated fatty acids degradation during thermal oxidation of edible oils by Fourier transform infrared spectroscopy. Talanta 1999, 50, 269–275. [Google Scholar] [CrossRef]






| Sample | TPC (mg GAE/g d.s.) | FRAP Value (µM Fe2+/g d.s.) |
|---|---|---|
| RGP | 71.207 ± 1.816 a | 637.541 ± 3.461 a |
| DGP | 56.135 ± 1.542 b | 482.692 ± 3.047 b |
| GPE | 283.514 ± 2.131 c | 2611.291 ± 4.173 c |
| BHT | - | 3421.377 ± 4.306 d |
| AA | - | 4512.131 ± 4.531 e |
| Sample | DPPH Radical Inhibition (%) | ||||
|---|---|---|---|---|---|
| Concentration (μg/mL) | |||||
| 200 | 300 | 400 | 500 | 600 | |
| RGP | 36.863 ± 0.375 | 47.769 ± 0.558 | 57.600 ± 0.574 | 66.557 ± 0.512 | 72.260 ± 0.752 |
| DGP | 29.863 ± 0.452 | 38.785 ± 0.542 | 45.159 ± 0.523 | 57.953 ± 0.637 | 66.890 ± 0.711 |
| 10 | 20 | 30 | 40 | 50 | |
| GPE | 20.015 ± 0.347 | 33.520 ± 0.476 | 43.441 ± 0.591 | 56.798 ± 0.477 | 73.502 ± 0.528 |
| BHT | 28.056 ± 0.419 | 37.149 ± 0.548 | 43.647 ± 0.667 | 49.011 ± 0.702 | 57.488 ± 0.724 |
| AA | 37.136 ± 0.588 | 54.347 ± 0.543 | 65.234 ± 0.655 | 85.540 ± 0.759 | 96.479 ± 0.789 |
| Heating Time (h) | PV (meq/kg Oil) | ||||
|---|---|---|---|---|---|
| C | SFO + 200 ppm BHT | SFO + 200 ppm GPE | SFO + 500 ppm GPE | SFO + 800 ppm GPE | |
| 0 | 1.477 ± 0.057 a | 1.477 ± 0.057 a | 1.477 ± 0.057 a | 1.477 ± 0.057 a | 1.477 ± 0.057 a |
| 4 | 12.370 ± 0.501 a | 9.047 ± 0.415 c | 10.867 ± 0.484 b | 9.170 ± 0.442 c | 6.507 ± 0.306 d |
| 8 | 19.753 ± 0.756 a | 15.177 ± 0.551 c | 16.943 ± 0.586 b | 14.867 ± 0.396 c | 11.430 ± 0.536 d |
| Heating Time (h) | p-AV | ||||
|---|---|---|---|---|---|
| C | SFO + 200 ppm BHT | SFO + 200 ppm GPE | SFO + 500 ppm GPE | SFO + 800 ppm GPE | |
| 0 | 3.073 ± 0.140 a | 3.073 ± 0.140 a | 3.073 ± 0.140 a | 3.073 ± 0.140 a | 3.073 ± 0.140 a |
| 4 | 40.777 ± 1.948 a | 32.163 ± 1.092 b | 35.167 ± 1.390 b | 31.897 ± 1.408 b | 25.410 ± 1.218 c |
| 8 | 59.367 ± 2.066 a | 49.673 ± 0.983 c | 53.617 ± 0.929 b | 48.413 ± 1.010 c | 39.527 ± 1.164 d |
| Heating Time (h) | K232 | ||||
| C | SFO + 200 ppm BHT | SFO + 200 ppm GPE | SFO + 500 ppm GPE | SFO + 800 ppm GPE | |
| 0 | 0.223 ± 0.008 a | 0.223 ± 0.008 a | 0.223 ± 0.008 a | 0.223 ± 0.008 a | 0.223 ± 0.008 a |
| 4 | 2.011 ± 0.092 a | 1.505 ± 0.081 c | 1.792 ± 0.072 b | 1.424 ± 0.070 c | 1.080 ± 0.051 d |
| 8 | 2.437 ± 0.124 a | 1.812 ± 0.102 c | 2.104 ± 0.111 b | 1.730 ± 0.083 c | 1.489 ± 0.084 d |
| Heating Time (h) | K268 | ||||
| C | SFO + 200 ppm BHT | SFO + 200 ppm GPE | SFO + 500 ppm GPE | SFO + 800 ppm GPE | |
| 0 | 0.071 ± 0.004 a | 0.071 ± 0.004 a | 0.071 ± 0.004 a | 0.071 ± 0.004 a | 0.071 ± 0.004 a |
| 4 | 0.263 ± 0.012 a | 0.201 ± 0.008 c | 0.235 ± 0.010 b | 0.186 ± 0.011 c | 0.148 ± 0.007 d |
| 8 | 0.334 ± 0.015 a | 0.267 ± 0.013 b | 0.290 ± 0.014 b | 0.254 ± 0.013 b | 0.237 ± 0.012 c |
| Sample | Absorbance Ratio | |||
|---|---|---|---|---|
| RI | RII | RIII | RIV | |
| Heating time: 0 h | ||||
| C | 0.164 ± 0.002 a | 0.211 ± 0.002 a | 0.137 ± 0.002 a | 1.188 ± 0.002 a |
| SFO + 200 ppm BHT | 0.162 ± 0.001 a | 0.208 ± 0.001 a | 0.133 ± 0.002 a | 1.185 ± 0.003 a |
| SFO + 200 ppm GPE | 0.163 ± 0.002 a | 0.210 ± 0.002 a | 0.135 ± 0.001 a | 1.186 ± 0.002 a |
| SFO + 500 ppm GPE | 0.162 ± 0.003 a | 0.209 ± 0.003 a | 0.133 ± 0.003 a | 1.184 ± 0.003 a |
| SFO + 800 ppm GPE | 0.161 ± 0.002 a | 0.208 ± 0.003 a | 0.132 ± 0.001 a | 1.183 ± 0.002 a |
| Heating time: 4 h | ||||
| C | 0.191 ± 0.002 b | 0.270 ± 0.004 c | 0.170 ± 0.003 d | 1.211 ± 0.001 d |
| SFO + 200 ppm BHT | 0.187 ± 0.001 b | 0.250 ± 0.003 b | 0.158 ± 0.002 b | 1.204 ± 0.002 c |
| SFO + 200 ppm GPE | 0.185 ± 0.002 b | 0.260 ± 0.005 b | 0.160 ± 0.001 b | 1.200 ± 0.003 b |
| SFO + 500 ppm GPE | 0.187 ± 0.003 b | 0.264 ± 0.004 c | 0.163 ± 0.002 b | 1.197 ± 0.002 b |
| SFO + 800 ppm GPE | 0.190 ± 0.002 b | 0.266 ± 0.002 c | 0.167 ± 0.001 c | 1.189 ± 0.001 a |
| Heating time: 8 h | ||||
| C | 0.215 ± 0.004 d | 0.296 ± 0.004 e | 0.174 ± 0.004 c | 1.236 ± 0.006 d |
| SFO + 200 ppm BHT | 0.199 ± 0.003 b | 0.272 ± 0.005 b | 0.164 ± 0.003 b | 1.210 ± 0.005 c |
| SFO + 200 ppm GPE | 0.200 ± 0.003 b | 0.275 ± 0.003 b | 0.166 ± 0.005 b | 1.206 ± 0.005 c |
| SFO + 500 ppm GPE | 0.207 ± 0.004 b | 0.283 ± 0.003 c | 0.172 ± 0.004 b | 1.201 ± 0.004 b |
| SFO + 800 ppm GPE | 0.213 ± 0.003 c | 0.287 ±0.004 d | 0.179 ± 0.005 d | 1.191 ± 0.004 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poiana, M.-A.; Moigradean, D.; Dumbrava, D.-G.; Radulov, I.; Raba, D.N.; Rivis, A. Exploring the Potential of Grape Pomace Extract to Inhibit Thermo-Oxidative Degradation of Sunflower Oil: From Routine Tests to ATR-FTIR Spectroscopy. Foods 2022, 11, 3674. https://doi.org/10.3390/foods11223674
Poiana M-A, Moigradean D, Dumbrava D-G, Radulov I, Raba DN, Rivis A. Exploring the Potential of Grape Pomace Extract to Inhibit Thermo-Oxidative Degradation of Sunflower Oil: From Routine Tests to ATR-FTIR Spectroscopy. Foods. 2022; 11(22):3674. https://doi.org/10.3390/foods11223674
Chicago/Turabian StylePoiana, Mariana-Atena, Diana Moigradean, Delia-Gabriela Dumbrava, Isidora Radulov, Diana Nicoleta Raba, and Adrian Rivis. 2022. "Exploring the Potential of Grape Pomace Extract to Inhibit Thermo-Oxidative Degradation of Sunflower Oil: From Routine Tests to ATR-FTIR Spectroscopy" Foods 11, no. 22: 3674. https://doi.org/10.3390/foods11223674