Tenebrio molitor Proteins-Derived DPP-4 Inhibitory Peptides: Preparation, Identification, and Molecular Binding Mechanism
Abstract
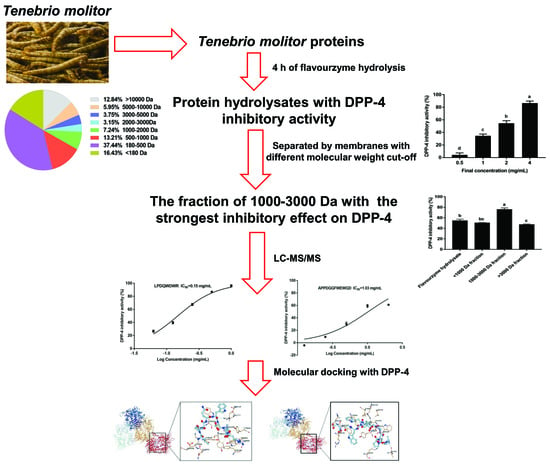
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. The Enzymatic Hydrolysis of T. molitor Protein
2.3. Degree of Hydrolysis (DH) Assay
2.4. Analysis of DPP-4 Inhibition Activity
2.5. Structural Characterization
2.5.1. Ultraviolet (UV) Absorption Spectroscopy
2.5.2. Relative Fluorescence Intensity
2.5.3. Circular Dichroism (CD) Spectroscopy
2.5.4. Molecular Weight Distribution
2.6. Peptide Identification
2.7. The Synthesis of Peptide
2.8. Molecular Docking
2.9. Statistical Analysis
3. Results
3.1. DH of the T. molitor Protein Hydrolysates
3.2. Inhibition Effect of the T. molitor Protein Hydrolysates on DPP-4
3.3. Structural Characteristics of the T. molitor Protein Hydrolysates
3.4. Identification of Peptides of the T. molitor Protein Hydrolysates
3.5. The Inhibition of the Synthesized Peptides on DPP-4
3.6. Interaction between the Synthesized Peptides and DPP-4 Active Site
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Makrilakis, K. The role of DPP-4 inhibitors in the treatment algorithm of type 2 diabetes mellitus: When to select, what to expect. Int. J. Environ. Res. Public Health 2019, 16, 2720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prahalad, P.; Tanenbaum, M.; Hood, K.; Maahs, D. Diabetes technology: Improving care, improving patient-reported outcomes and preventing complications in young people with Type 1 diabetes. Diabet. Med. 2018, 35, 419–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomha, S.M.; Eldebss, T.M.; Badrey, M.G.; Abdulla, M.M.; Mayhoub, A.S. Novel 4-heteroaryl-antipyrines as DPP-IV inhibitors. Chem. Biol. Drug Des. 2015, 86, 1292–1303. [Google Scholar] [CrossRef] [PubMed]
- Abbas, G.; Al Harrasi, A.; Hussain, H.; Hamaed, A.; Supuran, C.T. The management of diabetes mellitus-imperative role of natural products against dipeptidyl peptidase-4, α-glucosidase and sodium-dependent glucose co-transporter 2 (SGLT2). Bioorg. Chem. 2019, 86, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Casanova-Martí, À.; Bravo, F.I.; Serrano, J.; Ardévol, A.; Pinent, M.; Muguerza, B. Antihyperglycemic effect of a chicken feet hydrolysate via the incretin system: DPP-IV-inhibitory activity and GLP-1 release stimulation. Food Funct. 2019, 10, 4062–4070. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; Fitzgerald, R.J. Features of dipeptidyl peptidase IV (DPP-IV) inhibitory peptides from dietary proteins. J. Food Biochem. 2019, 43, e12451. [Google Scholar] [CrossRef] [Green Version]
- Singla, R.K.; Kumar, R.; Khan, S.; Kumari, K.; Garg, A. Natural products: Potential source of DPP-IV inhibitors. Curr. Protein Pept. Sci. 2019, 20, 1218–1225. [Google Scholar] [CrossRef]
- Majid, A.; Lakshmikanth, M.; Lokanath, N.; Priyadarshini, C.P. Generation, characterization and molecular binding mechanism of novel dipeptidyl peptidase-4 inhibitory peptides from sorghum bicolor seed protein. Food Chem. 2022, 369, 130888. [Google Scholar] [CrossRef]
- Chalamaiah, M.; Ulug, S.K.; Hong, H.; Wu, J. Regulatory requirements of bioactive peptides (protein hydrolysates) from food proteins. J. Funct. Foods 2019, 58, 123–129. [Google Scholar] [CrossRef]
- Li, T.; Zhang, X.; Ren, Y.; Zeng, Y.; Huang, Q.; Wang, C. Antihypertensive effect of soybean bioactive peptides: A review. Curr. Opin. Pharm. 2022, 62, 74–81. [Google Scholar] [CrossRef]
- Acquah, C.; Dzuvor, C.K.; Tosh, S.; Agyei, D. Anti-diabetic effects of bioactive peptides: Recent advances and clinical implications. Crit. Rev. Food Sci. Nutr. 2022, 62, 2158–2171. [Google Scholar] [CrossRef] [PubMed]
- Akbarian, M.; Khani, A.; Eghbalpour, S.; Uversky, V.N. Bioactive peptides: Synthesis, sources, applications, and proposed mechanisms of action. Int. J. Mol. Sci. 2022, 23, 1445. [Google Scholar] [CrossRef]
- Pei, J.; Liu, Z.; Pan, D.; Zhao, Y.; Dang, Y.; Gao, X. Transport, stability, and in vivo hypoglycemic effect of a broccoli-derived DPP-IV inhibitory peptide VPLVM. J. Agric. Food Chem. 2022, 70, 4934–4941. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Lu, P.; Wang, Y.; Fang, X.; Wu, J.; Wang, X. A novel dipeptidyl peptidase IV inhibitory tea peptide improves pancreatic β-cell function and reduces α-cell proliferation in streptozotocin-induced diabetic mice. Int. J. Mol. Sci. 2019, 20, 322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villaseñor, V.M.; Enriquez-Vara, J.N.; Urías-Silva, J.E.; Mojica, L. Edible insects: Techno-functional properties food and feed applications and biological potential. Food Rev. Int. 2021, 1–27. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, T.-K.; Jeong, C.H.; Yong, H.I.; Cha, J.Y.; Kim, B.-K.; Choi, Y.-S. Biological activity and processing technologies of edible insects: A review. Food Sci. Biotechnol. 2021, 30, 1003–1023. [Google Scholar] [CrossRef]
- Jeon, Y.-H.; Son, Y.-J.; Kim, S.-H.; Yun, E.-Y.; Kang, H.-J.; Hwang, I.-K. Physicochemical properties and oxidative stabilities of mealworm (Tenebrio molitor) oils under different roasting conditions. Food Sci. Biotechnol. 2016, 25, 105–110. [Google Scholar] [CrossRef]
- Zhang, F.; Cao, C.; Kong, B.; Sun, F.; Shen, X.; Yao, X.; Liu, Q. Pre-dried mealworm larvae flour could partially replace lean meat in frankfurters: Effect of pre-drying methods and replacement ratios. Meat Sci. 2022, 188, 108802. [Google Scholar] [CrossRef]
- Chen, F.; Jiang, H.; Lu, Y.; Chen, W.; Huang, G. Identification and in silico analysis of antithrombotic peptides from the enzymatic hydrolysates of Tenebrio molitor larvae. Eur. Food Res. Technol. 2019, 245, 2687–2695. [Google Scholar] [CrossRef]
- Cho, H.-R.; Lee, S.-O. Novel hepatoprotective peptides derived from protein hydrolysates of mealworm (Tenebrio molitor). Food Res. Int. 2020, 133, 109194. [Google Scholar] [CrossRef]
- Dai, C.; Ma, H.; Luo, L.; Yin, X. Angiotensin I-converting enzyme (ACE) inhibitory peptide derived from Tenebrio molitor (L.) larva protein hydrolysate. Eur. Food Res. Technol. 2013, 236, 681–689. [Google Scholar] [CrossRef]
- Yoon, S.; Wong, N.A.; Chae, M.; Auh, J.-H. Comparative characterization of protein hydrolysates from three edible insects: Mealworm larvae, adult crickets, and silkworm pupae. Foods 2019, 8, 563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dávalos Terán, I.; Imai, K.; Lacroix, I.M.; Fogliano, V.; Udenigwe, C.C. Bioinformatics of edible yellow mealworm (Tenebrio molitor) proteome reveal the cuticular proteins as promising precursors of dipeptidyl peptidase-IV inhibitors. J. Food Biochem. 2020, 44, e13121. [Google Scholar] [CrossRef] [PubMed]
- Rivero-Pino, F.; Guadix, A.; Guadix, E.M. Identification of novel dipeptidyl peptidase IV and α-glucosidase inhibitory peptides from Tenebrio molitor. Food Funct. 2021, 12, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhao, S.; Xin, X.; Zhang, B.; Thomas, A.; Charles, A.; Lee, K.S.; Jin, B.R.; Gui, Z. Purification, identification and functional analysis of a novel immunomodulatory peptide from silkworm pupa protein. Int. J. Pept. Res. Ther. 2020, 26, 243–249. [Google Scholar] [CrossRef]
- Nielsen, P.; Petersen, D.; Dambmann, C. Improved method for determining food protein degree of hydrolysis. J. Food Sci. 2001, 66, 642–646. [Google Scholar] [CrossRef]
- Song, J.; Wang, Q.; Du, M.; Ji, X.; Mao, X. Identification of dipeptidyl peptidase-IV inhibitory peptides from mare whey protein hydrolysates. J. Dairy Sci. 2017, 100, 6885–6894. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Z.; Li, J.; Li, J.; Sun, H.; Liu, Y. Physicochemical and antioxidative characteristics of black bean protein hydrolysates obtained from different enzymes. Food Hydrocolloid. 2019, 97, 105222. [Google Scholar] [CrossRef]
- Sun, R.; Liu, X.; Yu, Y.; Miao, J.; Leng, K.; Gao, H. Preparation process optimization, structural characterization and in vitro digestion stability analysis of Antarctic krill (Euphausia superba) peptides-zinc chelate. Food Chem. 2021, 340, 128056. [Google Scholar] [CrossRef]
- Zhang, Q.; Tong, X.; Qi, B.; Wang, Z.; Li, Y.; Sui, X.; Jiang, L. Changes in antioxidant activity of Alcalase-hydrolyzed soybean hydrolysate under simulated gastrointestinal digestion and transepithelial transport. J. Funct. Foods 2018, 42, 298–305. [Google Scholar] [CrossRef]
- Kothari, S.; Dhami-Shah, H.; Shah, S.R. Antidiabetic drugs and statins in nonalcoholic fatty liver disease. J. Clin. Exp. Hepatol. 2019, 9, 723–730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.; Lee, H.; Kim, Y.; Kim, E. Effect of DPP-IV inhibitors on glycemic variability in patients with T2DM: A systematic review and meta-analysis. Sci. Rep. 2019, 9, 13296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, R.; Cheng, J.; Wu, H. Discovery of food-derived dipeptidyl peptidase IV inhibitory peptides: A review. Int. J. Mol. Sci. 2019, 20, 463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, F.; Fu, Y.; Ma, L.; Dai, H.; Wang, H.; Chen, H.; Zhu, H.; Yu, Y.; Hou, Y.; Zhang, Y. Exploration of dipeptidyl Peptidase-IV (DPP-IV) inhibitory peptides from silkworm pupae (Bombyx mori) proteins based on in silico and in vitro assessments. J. Agric. Food Chem. 2022, 70, 3862–3871. [Google Scholar] [CrossRef]
- Vogelsang-O’dwyer, M.; Sahin, A.W.; Arendt, E.K.; Zannini, E. Enzymatic hydrolysis of pulse proteins as a tool to improve techno-functional properties. Foods 2022, 11, 1307. [Google Scholar] [CrossRef]
- Purschke, B.; Meinlschmidt, P.; Horn, C.; Rieder, O.; Jäger, H. Improvement of techno-functional properties of edible insect protein from migratory locust by enzymatic hydrolysis. Eur. Food Res. Technol. 2018, 244, 999–1013. [Google Scholar] [CrossRef] [Green Version]
- Grossmann, K.K.; Merz, M.; Appel, D.; De Araujo, M.M.; Fischer, L. New insights into the flavoring potential of cricket (Acheta domesticus) and mealworm (Tenebrio molitor) protein hydrolysates and their Maillard products. Food Chem. 2021, 364, 130336. [Google Scholar] [CrossRef]
- Jin, R.; Teng, X.; Shang, J.; Wang, D.; Liu, N. Identification of novel DPP–IV inhibitory peptides from Atlantic salmon (Salmo salar) skin. Food Res. Int. 2020, 133, 109161. [Google Scholar] [CrossRef]
- Lang, M.; Song, Y.; Li, Y.; Xiang, X.; Ni, L.; Miao, J. Purification, identification, and molecular mechanism of DPP-IV inhibitory peptides from defatted Antarctic krill powder. J. Food Biochem. 2021, 45, e13872. [Google Scholar] [CrossRef]
- Mudgil, P.; Kilari, B.P.; Kamal, H.; Olalere, O.A.; Fitzgerald, R.J.; Gan, C.-Y.; Maqsood, S. Multifunctional bioactive peptides derived from quinoa protein hydrolysates: Inhibition of α-glucosidase, dipeptidyl peptidase-IV and angiotensin I converting enzymes. J. Cereal Sci. 2020, 96, 103130. [Google Scholar] [CrossRef]
- Dai, L.; Kong, L.; Cai, X.; Jiang, P.; Liu, N.; Zhang, D.; Li, Z. Analysis of the structure and activity of dipeptidyl peptidase IV (DPP-IV) inhibitory oligopeptides from sorghum kafirin. J. Agric. Food Chem. 2022, 70, 2010–2017. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, R.; Cheng, C.; Zhang, Y.; Ma, Y.; Lu, W. Identification of two novel dipeptidyl peptidase-IV inhibitory peptides from sheep whey protein and inhibition mechanism revealed by molecular docking. Food Biosci. 2022, 48, 101733. [Google Scholar] [CrossRef]
- Gui, M.; Gao, L.; Rao, L.; Li, P.; Zhang, Y.; Han, J.W.; Li, J. Bioactive peptides identified from enzymatic hydrolysates of sturgeon skin. J. Sci. Food Agric. 2022, 102, 1948–1957. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, Z.; Wallace, M.B.; Stafford, J.A.; Kaldor, S.W.; Kassel, D.B.; Navre, M.; Shi, L.; Skene, R.J.; Asakawa, T. Discovery of alogliptin: A potent, selective, bioavailable, and efficacious inhibitor of dipeptidyl peptidase IV. J. Med. Chem. 2007, 50, 2297–2300. [Google Scholar] [CrossRef] [PubMed]





| Peptides | Molecular Weight (kDa) | Origin | PeptideRanker | Toxicity |
|---|---|---|---|---|
| APPDGGFWEWGD | 1333.54 | Beta-1, 3-glucanase | 0.93 | Non-Toxin |
| LPDQWDWR | 1115.32 | C1 family cathepsin L11 | 0.88 | Non-Toxin |
| NEAYFGFPR | 1100.31 | Hexamerin 1 | 0.87 | Non-Toxin |
| SNGLPFPTRP | 1085.36 | Hexamerin 1 | 0.84 | Non-Toxin |
| GLQFPTRPNF | 1176.48 | 86 kDa early-staged encapsulation inducing protein | 0.81 | Non-Toxin |
| FPDDVTNPGGKPW | 1429.73 | Beta-1,3-glucanase | 0.76 | Non-Toxin |
| IDDHFLFKEGDRF | 1638.98 | Arginine kinase | 0.70 | Non-Toxin |
| GDYDPDAFNNDIGLIKL | 1880.29 | Putative serine proteinase | 0.69 | Non-Toxin |
| APVIEKPSPGAF | 1212.57 | Acetylcholinesterase | 0.66 | Non-Toxin |
| NGLQFPTRP | 1029.29 | 86 kDa early-staged encapsulation inducing protein | 0.66 | Non-Toxin |
| IGGGDANAGEFPF | 1251.50 | Putative serine proteinase | 0.64 | Non-Toxin |
| YPFWMSGEEFNLK | 1648.05 | Hexamerin 2 | 0.61 | Non-Toxin |
| APDYEEANGKGVIIF | 1623.01 | Glutathione S-transferase delta | 0.60 | Non-Toxin |
| Peptides | Salt Bridges | π-Cation Interactions | π-π Stacking | Hydrogen Bonding | Hydrophobic Interactions |
|---|---|---|---|---|---|
| LPDQWDWR | Arg125, Asp556, Lys554 | Arg125 | Tyr662 | Arg125, Gln553, Tyr547 | Val711, Val656, Tyr666, Trp659, Phe357, Tyr631, Trp629 |
| APPDGGFWEWGD | Arg125, Arg356, Arg358, Lys554 | / | Tyr662 | Leu561, Ser630 | Val711, Val656, Tyr666, Arg358, Phe357, Tyr631, Trp629, Lys554 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, J.; Yang, J.; Zhou, X.; Hamdy, A.M.; Zhang, X.; Suo, H.; Zhang, Y.; Li, N.; Song, J. Tenebrio molitor Proteins-Derived DPP-4 Inhibitory Peptides: Preparation, Identification, and Molecular Binding Mechanism. Foods 2022, 11, 3626. https://doi.org/10.3390/foods11223626
Tan J, Yang J, Zhou X, Hamdy AM, Zhang X, Suo H, Zhang Y, Li N, Song J. Tenebrio molitor Proteins-Derived DPP-4 Inhibitory Peptides: Preparation, Identification, and Molecular Binding Mechanism. Foods. 2022; 11(22):3626. https://doi.org/10.3390/foods11223626
Chicago/Turabian StyleTan, Jiao, Jing Yang, Xinyi Zhou, Ahmed Mahmoud Hamdy, Xilu Zhang, Huayi Suo, Yu Zhang, Ning Li, and Jiajia Song. 2022. "Tenebrio molitor Proteins-Derived DPP-4 Inhibitory Peptides: Preparation, Identification, and Molecular Binding Mechanism" Foods 11, no. 22: 3626. https://doi.org/10.3390/foods11223626