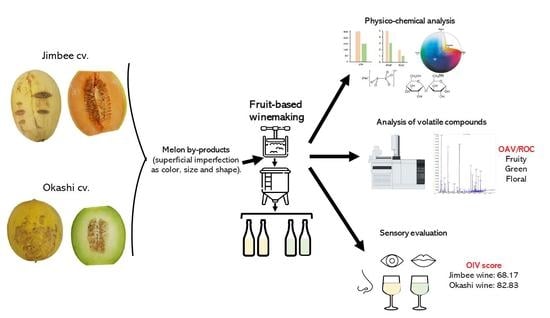
Fruit Wine Obtained from Melon by-Products: Physico-Chemical and Sensory Analysis, and Characterization of Key Aromas by GC-MS
Abstract
:1. Introduction
2. Materials and Methods
2.1. Small-Scale Melon Wine
2.2. Physicochemical Analysis
2.3. Individual Soluble Sugar by Ionic Chromatography (IC)
2.4. Determination of Total Polyphenol Content (TPC) and Antioxidant Capacities (FRAP and TEAC)
2.5. Analysis of Volatile Compounds by GC-MS
2.6. Odor Activity Value (OAV) and Relative Odor Contribution (ROC)
2.7. Sensory Evaluation
3. Results and Discussions
3.1. Must Composition
3.2. Melon Wines Characterization
3.3. Total Polyphenol Content (TPC) and Antioxidant Capacities (FRAP and TEAC)
3.4. Volatile Compounds in Melon Wines
3.5. Odor Activity Value (OAV) and Relative Odor Contribution (ROC)
3.6. Sensory Evaluation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hingston, S.T.; Noseworthy, T.J. On the Epidemic of Food Waste: Idealized Prototypes and the Aversion to Misshapen Fruits and Vegetables. Food Qual. Prefer. 2020, 86, 103999. [Google Scholar] [CrossRef]
- Garnett, T. Where Are the Best Opportunities for Reducing Greenhouse Gas Emissions in the Food System (Including the Food Chain)? Food Policy 2011, 36, S23–S32. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, Y.; Lv, J.; Ma, Y.; Guan, X. Changes in the Physicochemical Components, Polyphenol Profile, and Flavor of Persimmon Wine during Spontaneous and Inoculated Fermentation. Food Sci. Nutr. 2020, 8, 2728–2738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, N.; Gao, Z.; Li, S.; Chen, X.; Guo, J. Assessment of Chemical Constitution and Aroma Properties of Kiwi Wines Obtained from Pure and Mixed Fermentation with Wickerhamomyces Anomalus and Saccharomyces Cerevisiae. J. Sci. Food Agric. 2022, 102, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Eder, M.; Sanchez, I.; Brice, C.; Camarasa, C.; Legras, J.L.; Dequin, S. QTL Mapping of Volatile Compound Production in Saccharomyces Cerevisiae during Alcoholic Fermentation. BMC Genom. 2018, 19, 166. [Google Scholar] [CrossRef] [Green Version]
- Joshi, V.K.; Panesar, P.S.; Rana, V.S.; Kaur, S. Science and Technology of Fruit Wines: An Overview. In Science and Technology of Fruit Wine Production; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 1–72. ISBN 9780128010341. [Google Scholar]
- Matei, F. Technical Guide for Fruit Wine Production. In Science and Technology of Fruit Wine Production; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 663–703. ISBN 9780128010341. [Google Scholar]
- Rolim, P.M.; Seabra, L.M.J.; de Macedo, G.R. Melon By-Products: Biopotential in Human Health and Food Processing. Food Rev. Int. 2020, 36, 15–38. [Google Scholar] [CrossRef]
- FAOSTAT. Available online: https://www.fao.org/faostat/es/#data/TCL (accessed on 12 April 2022).
- Minkova, S.; Vlaeva, I.; Nikolova, K.; Petkova, N.; Gentscheva, G.; Tzvetkova, C. Assessment of the Elemental Composition, Antioxidant Activity, and Optical Properties of Non-Traditional Bulgarian Fruit Wines. Bulg. Chem. Commun. 2022, 54, 26–30. [Google Scholar] [CrossRef]
- Gómez, L.F.H.; Úbeda, J.; Briones, A. Characterization of Wines and Distilled Spirits from Melon (Cucumis Melo L.). Int. J. Food Sci. Technol. 2008, 43, 644–650. [Google Scholar] [CrossRef]
- OIV International Organisation of Vine and Wine. Compendium of International Methods of Analysis -OIV Chromatic Characteristics Method OIV-MA-AS2-11. 2009. Determination of Chromatic Characteristics According to CIELab (Resolution Oeno 1/2006). Available online: https://www.oiv.int/public/medias/7907/oiv-vol1-compendium-of-international-methods-of-analysis.pdf (accessed on 2 June 2022).
- Hu, X.; Fang, C.; Lu, L.; Hu, Z.; Shao, Y.; Zhu, Z. Determination of Soluble Sugar Profile in Rice. J. Chromatogr. B 2017, 1058, 19–23. [Google Scholar] [CrossRef]
- Martínez-Sánchez, A.; Guirao-Martínez, J.; Martínez, J.A.; Lozano-Pastor, P.; Aguayo, E. Inducing Fungal Resistance of Spinach Treated with Preharvest Hormetic Doses of UV-C. LWT Food Sci. Technol. 2019, 113, 108302. [Google Scholar] [CrossRef]
- Al-Duais, M.; Müller, L.; Böhm, V.; Jetschke, G. Antioxidant Capacity and Total Phenolics of Cyphostemma Digitatum before and after Processing: Use of Different Assays. Eur. Food Res. Technol. 2009, 228, 813–821. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Sun, Y.; Peng, W.; Zeng, L.; Xue, Y.; Lin, W.; Ye, X.; Guan, R.; Sun, P. Using Power Ultrasound to Release Glycosidically Bound Volatiles from Orange Juice: A New Method. Food Chem. 2021, 344, 128580. [Google Scholar] [CrossRef] [PubMed]
- Korenika, A.M.J.; Biloš, J.; Kozina, B.; Tomaz, I.; Preiner, D.; Jeromel, A. Effect of Different Reducing Agents on Aromatic Compounds, Antioxidant and Chromatic Properties of Sauvignon Blanc Wine. Foods 2020, 9, 996. [Google Scholar] [CrossRef] [PubMed]
- Cordente, A.G.; Solomon, M.; Schulkin, A.; Leigh Francis, I.; Barker, A.; Borneman, A.R.; Curtin, C.D. Novel Wine Yeast with ARO4 and TYR1 Mutations That Overproduce ‘Floral’ Aroma Compounds 2-Phenylethanol and 2-Phenylethyl Acetate. Appl. Microbiol. Biotechnol. 2018, 102, 5977–5988. [Google Scholar] [CrossRef]
- Welke, J.E.; Zanus, M.; Lazzarotto, M.; Alcaraz Zini, C. Quantitative Analysis of Headspace Volatile Compounds Using Comprehensive Two-Dimensional Gas Chromatography and Their Contribution to the Aroma of Chardonnay Wine. Food Res. Int. 2014, 59, 85–99. [Google Scholar] [CrossRef] [Green Version]
- Manchali, S.; Murthy, K.N.C.; Vishnuvardana; Patil, B.C. Nutritional Composition and Health Benefits of Various Botanical Types of Melon (Cucumis Melo L.). Plants 2021, 10, 1755. [Google Scholar] [CrossRef]
- Jackson, R.S. Wine Science: Principles and Application; Academic Press: Cambridge, MA, USA, 2008; ISBN 9780333227794. [Google Scholar]
- Giménez-Gómez, P.; Gutiérrez-Capitán, M.; Puig-Pujol, A.; Capdevila, F.; Muñoz, S.; Tobeña, A.; Miró, A.; Jiménez-Jorquera, C. Analysis of Free and Total Sulfur Dioxide in Wine by Using a Gas-Diffusion Analytical System with PH Detection. Food Chem. 2017, 228, 518–525. [Google Scholar] [CrossRef]
- Zhang, A.; Zeng, L.; Bo, H.; Hardie, W.J. Sulphite-Corrected, Non-Phenolic and Phenolic Antioxidant Capacities of Fruit Wines Profiled by Differential Folin-Ciocalteu Assay. Int. J. Food Sci. Technol. 2022, 57, 1259–1272. [Google Scholar] [CrossRef]
- Raposo, R.; Ruiz-Moreno, M.J.; Garde-Cerdán, T.; Puertas, B.; Moreno-Rojas, J.M.; Zafrilla, P.; Gonzalo-Diago, A.; Guerrero, R.F.; Cantos-Villar, E. Replacement of Sulfur Dioxide by Hydroxytyrosol in White Wine: Influence on Both Quality Parameters and Sensory. LWT Food Sci. Technol. 2016, 65, 214–221. [Google Scholar] [CrossRef]
- Santos, M.C.; Nunes, C.; Rocha, M.A.M.; Rodrigues, A.; Rocha, S.M.; Saraiva, J.A.; Coimbra, M.A. Impact of High Pressure Treatments on the Physicochemical Properties of a Sulphur Dioxide-Free White Wine during Bottle Storage: Evidence for Maillard Reaction Acceleration. Innov. Food Sci. Emerg. Technol. 2013, 20, 51–58. [Google Scholar] [CrossRef]
- Gordillo, B.; Ciaccheri, L.; Mignani, A.G.; Gonzalez-Miret, M.L.; Heredia, F.J. Influence of Turbidity Grade on Color and Appearance of Virgin Olive Oil. J. Am. Oil Chem. Soc. 2011, 88, 1317–1327. [Google Scholar] [CrossRef]
- Fundo, J.F.; Miller, F.A.; Garcia, E.; Santos, J.R.; Silva, C.L.M.; Brandão, T.R.S. Physicochemical Characteristics, Bioactive Compounds and Antioxidant Activity in Juice, Pulp, Peel and Seeds of Cantaloupe Melon. J. Food Meas. Charact. 2018, 12, 292–300. [Google Scholar] [CrossRef]
- Hodges, D.M.; Lester, G.E. Comparisons between Orange- and Green-Fleshed Non-Netted and Orange-Fleshed Netted Muskmelons: Antioxidant Changes Following Different Harvest and Storage Periods. J. Am. Soc. Hortic. Sci. 2006, 131, 110–117. [Google Scholar] [CrossRef] [Green Version]
- Benzie, I.F.F.; Strain, J.J. Ferric Reducing/Antioxidant Power Assay: Direct Measure of Total Antioxidant Activity of Biological Fluids and Modified Version for Simultaneous Measurement of Total Antioxidant Power and Ascorbic Acid Concentration. Methods Enzym. 1999, 299, 15–27. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Hawkins Byrne, D. Comparison of ABTS, DPPH, FRAP, and ORAC Assays for Estimating Antioxidant Activity from Guava Fruit Extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Lingua, M.S.; Fabani, M.P.; Wunderlin, D.A.; Baroni, M.V. From Grape to Wine: Changes in Phenolic Composition and Its Influence on Antioxidant Activity. Food Chem. 2016, 208, 228–238. [Google Scholar] [CrossRef]
- Tarko, T.; Duda-Chodak, A.; Soszka, A. Changes in Phenolic Compounds and Antioxidant Activity of Fruit Musts and Fruit Wines during Simulated Digestion. Molecules 2020, 25, 5574. [Google Scholar] [CrossRef]
- Saftner, R.; Abbott, J.A.; Lester, G.; Vinyard, B. Sensory and Analytical Comparison of Orange-Fleshed Honeydew to Cantaloupe and Green-Fleshed Honeydew for Fresh-Cut Chunks. Postharvest Biol. Technol. 2006, 42, 150–160. [Google Scholar] [CrossRef]
- Kourkoutas, D.; Elmore, J.S.; Mottram, D.S. Comparison of the Volatile Compositions and Flavour Properties of Cantaloupe, Galia and Honeydew Muskmelons. Food Chem. 2006, 97, 95–102. [Google Scholar] [CrossRef]
- Chen, L.; Li, D.; Ren, L.; Song, S.; Ma, X.; Rong, Y. Effects of Simultaneous and Sequential Cofermentation of Wickerhamomyces Anomalus and Saccharomyces Cerevisiae on Physicochemical and Flavor Properties of Rice Wine. Food Sci. Nutr. 2021, 9, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Cameleyre, M.; Lytra, G.; Tempere, S.; Barbe, J.C. 2-Methylbutyl Acetate in Wines: Enantiomeric Distribution and Sensory Impact on Red Wine Fruity Aroma. Food Chem. 2017, 237, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Lignou, S.; Parker, J.K.; Oruna-Concha, M.J.; Mottram, D.S. Flavour Profiles of Three Novel Acidic Varieties of Muskmelon (Cucumis Melo L.). Food Chem. 2013, 139, 1152–1160. [Google Scholar] [CrossRef] [PubMed]
- Baiano, A.; Mentana, A.; Varva, G.; Quinto, M. Effects of Different Vinification Procedures and Aging Containers on Phenolic and Volatile Composition of Greco White Wines. Eur. Food Res. Technol. 2017, 243, 1667–1680. [Google Scholar] [CrossRef]
- Hu, K.; Jin, G.J.; Mei, W.C.; Li, T.; Tao, Y.S. Increase of Medium-Chain Fatty Acid Ethyl Ester Content in Mixed H. Uvarum/S. Cerevisiae Fermentation Leads to Wine Fruity Aroma Enhancement. Food Chem. 2018, 239, 495–501. [Google Scholar] [CrossRef] [PubMed]

| Organoleptical Characteristic | Definition | Range (Excellent to Inadequate) |
|---|---|---|
| Visual: Discrimination of differences in outside world with sensory impressions from visible light rays. | ||
| Limpidity | Measure of cloudiness. | (5–1) |
| Aspect other than limpidity | Determine the full spectrum of visible properties of a product | (10–2) |
| Nose: Sensations perceived by the olfactory organ when stimulated by certain volatile substances. | ||
| Genuineness | Measure degree of sensation perceived (magnitude) by the nose, of a viticulture, oenological defect of product | (6–2) |
| Positive intensity | Degree (magnitude) of full spectrum of qualitative odors perceived by nose. | (8–2) |
| Quality | Spectrum of properties and characteristics of a wine that gives an aptitude to satisfy nose, implicit or expressed needs | (16–8) |
| Taste: Full spectrum of sensations perceived with wine mouthfeel. | ||
| Genuineness | Measure degree of sensation perceived (magnitude) by the taste, of a viticulture, oenological defect of product | (6–2) |
| Positive intensity | Degree (magnitude) of full spectrum of qualitative odors perceived by taste. | (8–2) |
| Harmonious persistence | To measure the length of residual olfacto-gustatory sensation, corresponding to the sensation perceived when the product is in mouth and length of time is measured. | (8–4) |
| Quality | Degree (magnitude) of full spectrum of qualitative odors perceived by taste | (22–10) |
| Harmony—Overall judgement: Corresponds to overall appraisal of a product. | (11–7) | |
| Parameter | Jimbee Must | Okashi Must |
|---|---|---|
| TSS (°Brix) | 11.30 z ± 0.31 | 8.93 ± 0.07 |
| pH | 6.28 ± 0.02 | 6.05 ± 0.00 |
| TA (g CE/L) | 1.43 ± 0.01 | 1.96 ± 0.03 |
| L* | 34.73 ± 0.18 | 39.23 ± 0.25 |
| °Hue | 65.46 ± 0.44 | 109.00 ± 0.48 |
| Chroma | 15.82 ± 0.17 | 20.65 ± 0.61 |
| TPC | 305.58 ± 11.38 | 216.68 ± 5.36 |
| FRAP | 2.59 ± 0.06 | 1.60 ± 0.03 |
| ABTS | 2.44 ± 0.15 | 1.87 ± 0.26 |
| Parameter | Jimbee Wine | Okashi Wine |
|---|---|---|
| Alcohol (%; v/v) | 9.8 z ± 0.0 | 9.9 ± 0.0 |
| TSS (°Brix) | 11.30 ± 0.31 | 8.93 ± 0.07 |
| Residual sugar (g/L) | 1.82 ± 0.34 | 1.67 ± 0.22 |
| Sugar-free extract (g/L) | 53.38 ± 2.26 | 25.33 ± 0.28 |
| pH | 4.09 ± 0.02 | 4.01 ± 0.03 |
| TA (g TE/L) | 8.86 ± 0.31 | 6.88 ± 0.19 |
| Volatile acidity (g/L) | 0.38± 0.09 | 0.21 ± 0.52 |
| SO2 free (mg/L) | 131.84 ± 2.56 | 63.15 ± 0.74 |
| SO2 total (mg/L) | 142.40 ± 1.85 | 97.60 ± 1.07 |
| L* | 50.17 ± 0.21 | 61.32 ± 0.03 |
| °Hue | 95.67 ± 0.37 | 107.54 ± 0.07 |
| Chroma | 13.68 ± 0.15 | 10.06 ± 0.28 |
| TPC | 406.71 ± 3.26 | 242.17 ± 2.40 |
| FRAP | 5.33 ± 0.15 | 3.07 ± 0.02 |
| ABTS | 1.94 ± 0.01 | 1.04 ± 0.03 |
| Soluble Sugar | Jimbee Wine | Okashi Wine |
|---|---|---|
| Glucose | 0.04 z ± 0.00 | 0.04 ± 0.00 |
| Fructose | 1.02 ± 0.20 | 1.44 ± 0.21 |
| Saccharose | 0.63 ± 0.11 | LD < 0.01 |
| Maltose | 0.13 ± 0.04 | 0.18 ± 0.01 |
| Total | 1.82 ± 0.34 | 1.66 ± 0.22 |
| R.T. | Tentative ID | KI | Jimbee Must | Jimbee Wine | Okashi Must | Okashi Wine |
|---|---|---|---|---|---|---|
| Esters | ||||||
| 2.171 | Methyl acetate | <1100 | 117.08 z ± 1.57 | n.d. | n.d. | n.d. |
| 2.477 | Ethyl Acetate | <1100 | 219.19 ± 2.51 | 672.16 ± 117.71 | 151.56 ± 2.34 | 456.61 ± 59.24 |
| 3.079 | Ethyl propionate | <1100 | 15.24 ± 0.08 | n.d. | 19.25 ± 0.32 | n.d. |
| 3.276 | n-Propyl acetate | <1100 | 36.87 ± 0.53 | n.d. | 19.76 ± 0.34 | n.d. |
| 3.793 | Isobutyl acetate | <1100 | 132.65 ± 2.13 | n.d. | 113.95 ± 2.77 | n.d. |
| 4.187 | Ethyl butanoate | <1100 | 63.18 ± 0.69 | 36.10 ± 6.40 | 41.46 ± 0.77 | n.d. |
| 4.471 | Ethyl 2-methyl-butanoate | <1100 | 22.03 ± 0.60 | n.d. | 48.16 ± 0.80 | n.d. |
| 4.876 | Butyl acetate | <1100 | 257.51 ± 5.22 | 22.66 ± 7.37 | 87.90 ± 2.06 | n.d. |
| 6.047 | 2-Methyl-1-butyl acetate | <1100 | 40.69 ± 3.64 | 194.39 ± 45.14 | 64.60 ± 3.79 | 310.69 ± 91.07 |
| 6.582 | Propyl 2-methyl-butanoate | <1100 | n.d. | n.d. | 6.08 ± 0.41 | n.d. |
| 7.623 | Pentyl acetate | 1110 | n.d. | n.d. | 7.42 ± 0.92 | n.d. |
| 10.047 | Ethyl hexanoate | 1194 | 6.31 ± 0.23 | 156.95 ± 40.09 | 7.28 ± 0.11 | 405.28 ± 68.15 |
| 11.861 | Hexyl acetate | 1245 | 62.41 ± 1.08 | n.d. | 63.35 ± 3.68 | n.d. |
| 13.790 | 4-Hexen-1-ol acetate- | 1290 | n.d. | n.d. | 37.76 ± 1.17 | n.d. |
| 20.526 | Ethyl octanoate | 1423 | n.d. | 1271.05 ± 232.08 | n.d. | 3876.75 ± 276.55 |
| 21.683 | Heptyl formate | 1445 | 7.50 ± 0.55 | n.d. | n.d. | n.d. |
| 22.693 | Octyl acetate | 1463 | 17.02 ± 3.86 | n.d. | n.d. | n.d. |
| 23.312 | Butane-2,3-diyl diacetate | 1474 | 18.90 ± 0.29 | n.d. | n.d. | n.d. |
| 26.111 | Ethyl nonanoate | 1519 | n.d. | 27.32 ± 0.87 | n.d. | 25.20 ± 0.83 |
| 28.830 | Ethyl 3-Nonenoate | 1556 | n.d. | n.d. | n.d. | 29.31 ± 1.74 |
| 29.328 | Methyl decanoate | 1562 | n.d. | n.d. | n.d. | 49.65 ± 1.94 |
| 31.552 | Ethyl decanoate | 1590 | n.d. | 600.12 ± 79.84 | n.d. | 1197.58 ± 58.97 |
| 32.185 | 3-Methylbutyl octanoate | 1597 | n.d. | n.d. | n.d. | 63.40 ± 5.21 |
| 33.515 | Phenylmethyl acetate | 1695 | 4.82 ± 2.41 | n.d. | 6.08 ± 0.06 | n.d. |
| 33.005 | Ethyl 9-decenoate | 1651 | n.d. | n.d. | n.d. | 696.19 ± 72.37 |
| 33.881 | Ethyl methoxyacetate | 1711 | n.d. | 49.24 ± 14.90 | n.d. | 32.89 ± 0.81 |
| 35.245 | Phenethyl acetate | 1761 | n.d. | 74.92 ± 0.73 | n.d. | 258.95 ± 3.74 |
| 35.354 | Methyl laurate | 1765 | n.d. | 31.82 ± 4.70 | n.d. | n.d. |
| 36.056 | Ethyl laurate | 1790 | n.d. | 318.35 ± 41.66 | n.d. | n.d. |
| 36.361 | 3-Methylbutyl decanoate | 1802 | n.d. | n.d. | n.d. | 41.58 ± 1.58 |
| 38.724 | Methyl 2,4,6-trimethylnonanoate | 2005 | n.d. | 101.73 ± 2.15 | n.d. | n.d. |
| 39.783 | Ethyl nonanoate | 2102 | n.d. | n.d. | n.d. | 10.37 ± 1.07 |
| 40.420 | Methyl palmitate | 2159 | n.d. | n.d. | n.d. | 44.54 ± 0.81 |
| 40.827 | Ethyl palmitate | 2195 | n.d. | 67.14 ± 12.19 | n.d. | 377.98 ± 10.05 |
| Alcohols | ||||||
| 3.009 | Ethanol | <1100 | n.d. | 4407.75 ± 133.36 | n.d. | 6472.94 ± 94.29 |
| 4.308 | 1-Propanol | <1100 | n.d. | n.d. | n.d. | 19.06 ± 5.84 |
| 5.639 | Isobutanol | <1100 | n.d. | 107.13 ± 9.25 | n.d. | 144.24 ± 4.95 |
| 9.057 | 2-Methyl-1-butanol | 1163 | 38.64 ± 1.14 | n.d. | 15.40 ± 0.73 | n.d. |
| 9.237 | 3-Methyl-1-butanol | 1169 | n.d. | 1425.50 ± 69.14 | n.d. | 2800.05 ± 92.86 |
| 16.037 | 1-Hexanol | 1339 | 93.73 ± 2.23 | 67.06 ± 1.42 | 7.43 ± 0.73 | n.d. |
| 21.383 | 1-Nonen-3-ol | 1439 | 13.74 ± 1.42 | n.d. | n.d. | n.d. |
| 27.558 | 1-Octanol | 1539 | 96.66 ± 1.03 | 51.96 ± 1.53 | n.d. | n.d. |
| 31.010 | Nonen-1-ol isomer | 1583 | 8.73 ± 1.16 | n.d. | 5.31 ± 1.53 | n.d. |
| 32.325 | 1-Nonanol | 1599 | 14.48 ± 1.07 | n.d. | 11.45 ± 0.31 | n.d. |
| 32.828 | Nonen-1-ol isomer | 1636 | 13.71 ± 0.35 | n.d. | 18.27 ± 1.48 | n.d. |
| 33.697 | Nonen-1-ol isomer | 1705 | 48.79 ± 4.14 | n.d. | 45.82 ± 2.67 | n.d. |
| 34.295 | 3,6-Nonadien-1-ol | 1727 | 40.72 ± 0.09 | n.d. | n.d. | n.d. |
| 34.702 | Citronellol | 1742 | n.d. | 38.99 ± 1.58 | n.d. | n.d. |
| 36.210 | Benzyl alcohol | 1796 | 29.19 ± 0.67 | n.d. | 12.38 ± 0.28 | 344.52 ± 27.24 |
| 36.772 | Phenylethyl alcohol | 1835 | 6.34 ± 0.28 | 330.77 ± 15.06 | 2.04 ± 0.10 | 648.11 ± 7.16 |
| 41.083 | 2,4-Bis(1,1-dimethylethyl)-phenol | 2218 | n.d. | 356.03 ± 7.23 | n.d. | 512.36 ± 16.06 |
| Unsaturated aliphatic | ||||||
| 9.240 | 1-Heptene | 1169 | n.d. | n.d. | 15.94 ± 1.47 | n.d. |
| 32.208 | 6-Methyl-1-Octene | 1598 | n.d. | 35.18 ± 3.19 | n.d. | n.d. |
| 39.595 | 2-Methoxy-2-methylbut-3-ene | 2085 | n.d. | 19.62 ± 5.80 | n.d. | n.d. |
| Aldehyde | ||||||
| 12.465 | Octanal | 1260 | n.d. | n.d. | 35.84 ± 0.34 | n.d. |
| 18.052 | Nonanal | 1377 | n.d. | n.d. | 17.02 ± 1.57 | n.d. |
| 21.090 | 6-Nonenal | 1434 | n.d. | n.d. | 236.10 ± 6.84 | n.d. |
| 24.126 | Benzaldehyde | 1488 | n.d. | n.d. | 5.51 ± 0.37 | n.d. |
| 28.256 | 2,6-Nonadienal, isomer | 1548 | 16.90 ± 1.81 | n.d. | 97.13 ± 4.07 | n.d. |
| Fatty acids | ||||||
| 20.714 | Acetic acid | 1427 | n.d. | 701.42 ± 15.40 | n.d. | 314.59 ± 9.67 |
| 35.914 | Hexanoic acid | 1785 | n.d. | n.d. | n.d. | 78.13 ± 2.74 |
| 38.621 | Octanoic acid | 1995 | n.d. | 118.19 ± 14.62 | n.d. | 509.21 ± 18.20 |
| Miscellaneous | ||||||
| 10.934 | 3-Octanone | 1220 | 7.83 ± 1.07 | n.d. | 5.99 ± 0.20 | n.d. |
| 19.754 | Bis(1,1-dimethylethyl)-benzene isomer | 1408 | 36.68 ± 2.54 | 152.42 ± 8.11 | n.d. | 193.27 ± 9.13 |
| 22.620 | S-(3-Hydroxypropyl) thioacetate | 1462 | n.d. | n.d. | 13.94 ± 2.81 | n.d. |
| 30.469 | 3-(Methylthio)propyl acetate | 1577 | 35.18 ± 1.15 | n.d. | 14.88 ± 0.66 | n.d. |
| 37.142 | 1-Ethenyl-2-methylbenzene | 1863 | n.d. | n.d. | n.d. | 48.13 ± 2.02 |
| TOTAL | 2145.56 ± 56.55 | 12,788.44 ± 1.228.65 | 1769.79 ± 26.15 | 20,504.38 ± 1.477.05 |
| Tentative ID | Threshold (mg/L) | Odor Description | OAV | ROC | ||
|---|---|---|---|---|---|---|
| Jimbee | Okashi | Jimbee | Okashi | |||
| Ethyl butanoate a | 20 | Pineapple, apple | 1.81 z | <1 | 0.06 | <0.01 |
| Ethyl hexanoate b | 8 | Fruity, green, wine-like | 19.62 | 50.66 | 0.68 | 0.44 |
| Ethyl octanoate a | 580 | Sweet, flora, fruity, pear | 2.19 | 6.68 | 0.08 | 0.06 |
| Ethyl decanoate a | 200 | Floral, fruity, | 3.00 | 5.99 | 0.10 | 0.05 |
| Ethyl 9-decenoate b | 100 | Rose | <1 | 6.96 | <0.01 | 0.06 |
| Phenethyl acetate c | 250 | Floral, honey, rose | <1 | 1.04 | 0.01 | 0.01 |
| Organoleptical Characteristics | Jimbee Wine | Okashi Wine | Range (Excellent to Inadequate) |
|---|---|---|---|
| Visual | 7.33 z ± 1.82 | 13.67 ± 0.61 | (15–3) |
| Limpidity | 2.00 ± 0.68 | 4.67 ± 0.21 | (5–1) |
| Aspect other than limpidity | 5.33 ± 1.23 | 9.00 ± 0.45 | (10–2) |
| Nose | 22.5 ± 1.61 | 23.83 ± 2.12 | (30–12) |
| Genuineness | 3.83 ± 0.31 | 5.33 ± 0.21 | (6–2) |
| Positive intensity | 6.33 ± 0.56 | 6.50 ± 0.56 | (8–2) |
| Quality | 12.33 ± 0.95 | 12.00 ± 1.71 | (16–8) |
| Taste | 29.83 ± 2.55 | 35.5 ± 0.96 | (44–18) |
| Genuineness | 3.67 ± 0.56 | 4.17 ± 0.48 | (6–2) |
| Positive intensity | 4.83 ± 0.75 | 6.83 ± 0.17 | (8–2) |
| Harmonious persistence | 5.83 ± 0.48 | 6.50 ± 0.34 | (8–4) |
| Quality | 15.50 ± 1.20 | 18.00 ± 0.63 | (22–10) |
| Harmony—Overall judgement | 8.50 ± 0.50 | 9.83 ± 0.17 | (11–7) |
| TOTAL | 68.17 ± 3.22 | 82.83 ± 1.96 | (100–40) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salas-Millán, J.Á.; Aznar, A.; Conesa, E.; Conesa-Bueno, A.; Aguayo, E. Fruit Wine Obtained from Melon by-Products: Physico-Chemical and Sensory Analysis, and Characterization of Key Aromas by GC-MS. Foods 2022, 11, 3619. https://doi.org/10.3390/foods11223619
Salas-Millán JÁ, Aznar A, Conesa E, Conesa-Bueno A, Aguayo E. Fruit Wine Obtained from Melon by-Products: Physico-Chemical and Sensory Analysis, and Characterization of Key Aromas by GC-MS. Foods. 2022; 11(22):3619. https://doi.org/10.3390/foods11223619
Chicago/Turabian StyleSalas-Millán, José Ángel, Arantxa Aznar, Encarnación Conesa, Andrés Conesa-Bueno, and Encarna Aguayo. 2022. "Fruit Wine Obtained from Melon by-Products: Physico-Chemical and Sensory Analysis, and Characterization of Key Aromas by GC-MS" Foods 11, no. 22: 3619. https://doi.org/10.3390/foods11223619