Current Advancements in Pectin: Extraction, Properties and Multifunctional Applications
Abstract
:1. Introduction
2. Basic Structure of Pectin
3. Chains of Pectin
4. Types of Pectin
5. Sources of Pectin
6. Extraction of Pectin
Challenges in Pectin Extraction at a Commercial Scale
7. Interactions of Pectin
7.1. Solubility and Dispersibility
7.2. Gelation
7.3. Breakdown of Pectin
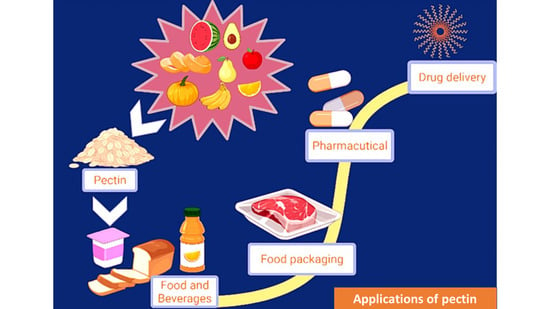
8. Potential Applications of Pectin
8.1. Food Industry
8.1.1. Jams, Jellies and Emulsifying Agent
8.1.2. Bakery Products
8.1.3. Prebiotic Properties
8.1.4. Stabilizing Acidified Milk Products
8.2. Health and Pharmaceuticals
8.2.1. Reduction in LDL Plasma Concentrations
8.2.2. Antioxidant Activity
8.2.3. Therapeutic and Pharmaceutical Uses
8.2.4. Glycemic Control
8.2.5. Pectin as an Encapsulating Agent
8.2.6. Metal Binding Properties
8.3. Food Packaging
8.3.1. Packaging Film
8.3.2. Coating Material
9. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Freitas, C.M.P.; Coimbra, J.S.R.; Souza, V.G.L.; Sousa, R.C.S. Structure and Applications of Pectin in Food, Biomedical, and Pharmaceutical Industry: A Review. Coatings 2021, 11, 922. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, P.; Zhang, H. Pectin in Cancer Therapy: A Review. Trends Food Sci. Technol. 2015, 44, 258–271. [Google Scholar] [CrossRef]
- Ngouémazong, E.D.; Christiaens, S.; Shpigelman, A.; Van Loey, A.; Hendrickx, M. The Emulsifying and Emulsion-Stabilizing Properties of Pectin: A Review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 705–718. [Google Scholar] [CrossRef]
- Martau, G.A.; Mihai, M.; Vodnar, D.C. The Use of Chitosan, Alginate, and Pectin in the Biomedical and Food Sector—Biocompatibility, Bioadhesiveness, and Biodegradability. Polymers 2019, 11, 1837. [Google Scholar] [CrossRef]
- Torres-León, C.; Ramírez-Guzman, N.; Londoño-Hernandez, L.; Martinez-Medina, G.A.; Díaz-Herrera, R.; Navarro-Macias, V.; Alvarez-Pérez, O.B.; Picazo, B.; Villarreal-Vázquez, M.; Ascacio-Valdes, J.; et al. Food Waste and Byproducts: An Opportunity to Minimize Malnutrition and Hunger in Developing Countries. Front. Sustain. Food Syst. 2018, 2, 52. [Google Scholar] [CrossRef]
- Dominiak, M.; Søndergaard, K.M.; Wichmann, J.; Vidal-Melgosa, S.; Willats, W.G.T.; Meyer, A.S.; Mikkelsen, J.D. Application of Enzymes for Efficient Extraction, Modification, and Development of Functional Properties of Lime Pectin. Food Hydrocoll. 2014, 40, 273–282. [Google Scholar] [CrossRef]
- Tanhatan Naseri, A.; Thibault, J.-F.; Ralet-Renard, M.-C.; Tanhatan Nasseri, A.; Ralet, M.-C. Citrus Pectin: Structure and Application in Acid Dairy Drinks. In Tree and Forestry Science and Biotechnology; Global Science Books: Carrollton, GA, USA, 2008. [Google Scholar]
- Voragen, A.G.J.; Coenen, G.J.; Verhoef, R.P.; Schols, H.A. Pectin, a Versatile Polysaccharide Present in Plant Cell Walls. Struct. Chem. 2009, 20, 263. [Google Scholar] [CrossRef]
- Sakai, T.; Sakamoto, T.; Hallaert, J.; Vandamme, E.J. ⌈Pectin, Pectinase, and Protopectinase: Production,⌈ Properties, and Applications. Adv. Appl. Microbiol. 1993, 39, 213–294. [Google Scholar] [CrossRef]
- Xiao, C.; Anderson, C.T. Roles of Pectin in Biomass Yield and Processing for Biofuels. Front. Plant Sci. 2013, 4, 67. [Google Scholar] [CrossRef]
- Goldberg, R.; Morvan, C.; Jauneau, A.; Jarvis, M.C. Methyl-Esterification, de-Esterification and Gelation of Pectins in the Primary Cell Wall. Prog. Biotechnol. 1996, 14, 151–172. [Google Scholar] [CrossRef]
- Wang, W.; Chen, W.; Zou, M.; Lv, R.; Wang, D.; Hou, F.; Feng, H.; Ma, X.; Zhong, J.; Ding, T.; et al. Applications of Power Ultrasound in Oriented Modification and Degradation of Pectin: A Review. J. Food Eng. 2018, 234, 98–107. [Google Scholar] [CrossRef]
- Kameshwar, A.K.S.; Qin, W. Structural and Functional Properties of Pectin and Lignin–Carbohydrate Complexes de-Esterases: A Review. Bioresour. Bioprocess. 2018, 5, 43. [Google Scholar] [CrossRef]
- Sakamoto, T.; Yoshinaga, J.; Shogaki, T.; Sakai, T. Studies on Protopectinase-C Mode of Action: Analysis of the Chemical Structure of the Specific Substrate in Sugar Beet Protopectin and Characterization of the Enzyme Activity. Biosci. Biotechnol. Biochem. 1993, 57, 1832–1837. [Google Scholar] [CrossRef]
- Babbar, N.; Dejonghe, W.; Gatti, M.; Sforza, S.; Elst, K. Pectic Oligosaccharides from Agricultural By-Products: Production, Characterization and Health Benefits. Crit. Rev. Biotechnol. 2015, 36, 594–606. [Google Scholar] [CrossRef]
- Fracasso, A.F.; Perussello, C.A.; Carpiné, D.; Petkowicz, C.L.d.O.; Haminiuk, C.W.I. Chemical Modification of Citrus Pectin: Structural, Physical and Rheologial Implications. Int. J. Biol. Macromol. 2018, 109, 784–792. [Google Scholar] [CrossRef]
- Koriem, K.M.; Arbid, M.; Emam, K.R. Therapeutic effect of pectin on octylphenol induced kidney dysfunction, oxidative stress and apoptosis in rats. Environ. Toxicol. Pharmacol. 2014, 38, 14–23. [Google Scholar] [CrossRef]
- Zhu, R.; Wang, C.; Zhang, L.; Wang, Y.; Chen, G.; Fan, J.; Jia, Y.; Yan, F.; Ning, C. Pectin oligosaccharides from fruit of Actinidia arguta: Structure-activity relationship of prebiotic and antiglycation potentials. Carbohydr. Polym. 2019, 217, 90–97. [Google Scholar] [CrossRef]
- De Moura, F.A.; Macagnan, F.T.; Petkowicz, C.L.D.O.; da Silva, L.P. Partially hydrolyzed pectin extracted from passion fruit peel: Molar mass and physicochemical properties. Bioact. Carbohydr. Diet. Fibre 2020, 21, 100206. [Google Scholar] [CrossRef]
- Bartolazzi, A. Galectins in Cancer and Translational Medicine: From Bench to Bedside. Int. J. Mol. Sci. 2018, 19, 2934. [Google Scholar] [CrossRef]
- Giusto, G.; Vercelli, C.; Comino, F.; Caramello, V.; Tursi, M.; Gandini, M. A new, easy-to-make pectin-honey hydrogel enhances wound healing in rats. BMC Complement. Altern. Med. 2017, 17, 266. [Google Scholar] [CrossRef] [Green Version]
- Christiaens, S.; Van Buggenhout, S.; Houben, K.; Jamsazzadeh Kermani, Z.; Moelants, K.R.N.; Ngouémazong, E.D.; Van Loey, A.; Hendrickx, M.E.G. Process–Structure–Function Relations of Pectin in Food. Crit. Rev. Food Sci. Nutr. 2016, 56, 1021–1042. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Hu, Z.; Hu, L.; Li, G.; Yao, Q.; Hu, Y. Pectin-based active packaging: A critical review on preparation, physical properties and novel application in food preservation. Trends Food Sci. Technol. 2021, 118, 167–178. [Google Scholar] [CrossRef]
- Li, D.-Q.; Li, J.; Dong, H.-L.; Li, X.; Zhang, J.-Q.; Ramaswamy, S.; Xu, F. Pectin in biomedical and drug delivery applications: A review. Int. J. Biol. Macromol. 2021, 185, 49–65. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, M.S.; Deshmukh, R.; Choudhari, P.; Bhatia, N.M. Chemical Modification of Pectins, Characterization and Evaluation for Drug Delivery. Sci. Pharm. 2008, 76, 775–784. [Google Scholar] [CrossRef]
- Willats, W.G.; Knox, J.P.; Mikkelsen, J.D. Pectin: New insights into an old polymer are starting to gel. Trends Food Sci. Technol. 2006, 17, 97–104. [Google Scholar] [CrossRef]
- Novosel’Skaya, I.L.; Voropaeva, N.L.; Semenova, L.N.; Rashidova, S.S. Trends in the science and applications of pectins. Chem. Nat. Compd. 2000, 36, 1–10. [Google Scholar] [CrossRef]
- Mohnen, D. Pectin structure and biosynthesis. Curr. Opin. Plant Biol. 2008, 11, 266–277. [Google Scholar] [CrossRef]
- Christiaens, S.; Uwibambe, D.; Uyttebroek, M.; Van Droogenbroeck, B.; Van Loey, A.M.; Hendrickx, M.E. Pectin characterisation in vegetable waste streams: A starting point for waste valorisation in the food industry. LWT Food Sci. Technol. 2015, 61, 275–282. [Google Scholar] [CrossRef]
- Sila, D.; Van Buggenhout, S.; Duvetter, T.; Fraeye, I.; De Roeck, A.; Van Loey, A.; Hendrickx, M. Pectins in Processed Fruits and Vegetables: Part II-Structure-Function Relationships. Compr. Rev. Food Sci. Food Saf. 2009, 8, 86–104. [Google Scholar] [CrossRef]
- Leijdekkers, A.; Bink, J.; Geutjes, S.; Schols, H.; Gruppen, H. Enzymatic saccharification of sugar beet pulp for the production of galacturonic acid and arabinose; a study on the impact of the formation of recalcitrant oligosaccharides. Bioresour. Technol. 2013, 128, 518–525. [Google Scholar] [CrossRef]
- Zhang, S.; Hu, H.; Wang, L.; Liu, F.; Pan, S. Preparation and prebiotic potential of pectin oligosaccharides obtained from citrus peel pectin. Food Chem. 2018, 244, 232–237. [Google Scholar] [CrossRef]
- Karaki, N.; Aljawish, A.; Humeau, C.; Muniglia, L.; Jasniewski, J. Enzymatic modification of polysaccharides: Mechanisms, properties, and potential applications: A review. Enzym. Microb. Technol. 2016, 90, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Eliaz, I.; Raz, A. Pleiotropic Effects of Modified Citrus Pectin. Nutrients 2019, 11, 2619. [Google Scholar] [CrossRef] [PubMed]
- Yapo, B.M.; Koffi, K.L. Extraction and Characterization of Highly Gelling Low Methoxy Pectin from Cashew Apple Pomace. Foods 2014, 3, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kurita, O.; Fujiwara, T.; Yamazaki, E. Characterization of the pectin extracted from citrus peel in the presence of citric acid. Carbohydr. Polym. 2008, 74, 725–730. [Google Scholar] [CrossRef]
- Belkheiri, A.; Forouhar, A.; Ursu, A.; Dubessay, P.; Pierre, G.; Delattre, C.; Djelveh, G.; Abdelkafi, S.; Hamdami, N.; Michaud, P. Extraction, Characterization, and Applications of Pectins from Plant By-Products. Appl. Sci. 2021, 11, 6596. [Google Scholar] [CrossRef]
- Capel, F.; Nicolai, T.; Durand, D.; Boulenguer, P.; Langendorff, V. Calcium and acid induced gelation of (amidated) low methoxyl pectin. Food Hydrocoll. 2006, 20, 901–907. [Google Scholar] [CrossRef]
- Lootens, D.; Capel, F.; Durand, D.; Nicolai, T.; Boulenguer, P.; Langendorff, V. Influence of pH, Ca concentration, temperature and amidation on the gelation of low methoxyl pectin. Food Hydrocoll. 2003, 17, 237–244. [Google Scholar] [CrossRef]
- Marenda, F.R.B.; Mattioda, F.; Demiate, I.M.; de Francisco, A.; Petkowicz, C.L.D.O.; Canteri, M.H.G.; Amboni, R.D.D.M.C. Advances in Studies Using Vegetable Wastes to Obtain Pectic Substances: A Review. J. Polym. Environ. 2019, 27, 549–560. [Google Scholar] [CrossRef]
- Cho, E.-H.; Jung, H.-T.; Lee, B.-H.; Kim, H.-S.; Rhee, J.-K.; Yoo, S.-H. Green process development for apple-peel pectin production by organic acid extraction. Carbohydr. Polym. 2019, 204, 97–103. [Google Scholar] [CrossRef]
- Sousa, A.G.; Ahl, L.I.; Pedersen, H.L.; Fangel, J.U.; Sørensen, S.O.; Willats, W.G. A multivariate approach for high throughput pectin profiling by combining glycan microarrays with monoclonal antibodies. Carbohydr. Res. 2015, 409, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Imaizumi, T.; Szymańska-Chargot, M.; Pieczywek, P.M.; Chylińska, M.; Kozioł, A.; Ganczarenko, D.; Tanaka, F.; Uchino, T.; Zdunek, A. Evaluation of pectin nanostructure by atomic force microscopy in blanched carrot. LWT 2017, 84, 658–667. [Google Scholar] [CrossRef]
- Round, A.N.; Rigby, N.M.; MacDougall, A.J.; Morris, V.J. A new view of pectin structure revealed by acid hydrolysis and atomic force microscopy. Carbohydr. Res. 2010, 345, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Kirby, A.; MacDougall, A.; Morris, V. Atomic force microscopy of tomato and sugar beet pectin molecules. Carbohydr. Polym. 2008, 71, 640–647. [Google Scholar] [CrossRef]
- Peaucelle, A.; Braybrook, S.A.; Le Guillou, L.; Bron, E.; Kuhlemeier, C.; Höfte, H. Pectin-Induced Changes in Cell Wall Mechanics Underlie Organ Initiation in Arabidopsis. Curr. Biol. 2011, 21, 1720–1726. [Google Scholar] [CrossRef]
- Ramli, N. Effect of ammonium oxalate and acetic acid at several extraction time and pH on some physicochemical properties of pectin from cocoa husks (Theobroma cacao). Afr. J. Food Sci. 2011, 5, 790–798. [Google Scholar] [CrossRef]
- Thakur, B.R.; Singh, R.K.; Handa, A.K.; Rao, M.A. Chemistry and uses of pectin—A review. Crit. Rev. Food Sci. Nutr. 1997, 37, 47–73. [Google Scholar] [CrossRef]
- Sandarani, M. A Review: Different Extraction Techniques of Pectin. J. Pharmacogn. Nat. Prod. 2017, 3, 143. [Google Scholar] [CrossRef]
- Mollea, C.; Chiampo, F.; Conti, R. Extraction and characterization of pectins from cocoa husks: A preliminary study. Food Chem. 2008, 107, 1353–1356. [Google Scholar] [CrossRef]
- Shi, X.; Chang, K.; Schwarz, J.; Wiesenborn, D.; Shih, M. Optimizing pectin extraction from sunflower heads by alkaline washing. Bioresour. Technol. 1996, 58, 291–297. [Google Scholar] [CrossRef]
- Funami, T.; Nakauma, M.; Ishihara, S.; Tanaka, R.; Inoue, T.; Phillips, G.O. Structural modifications of sugar beet pectin and the relationship of structure to functionality. Food Hydrocoll. 2011, 25, 221–229. [Google Scholar] [CrossRef]
- Cui, S.W.; Chang, Y.H. Emulsifying and structural properties of pectin enzymatically extracted from pumpkin. LWT—Food Sci. Technol. 2014, 58, 396–403. [Google Scholar] [CrossRef]
- Petkowicz, C.; Vriesmann, L.; Williams, P. Pectins from food waste: Extraction, characterization and properties of watermelon rind pectin. Food Hydrocoll. 2017, 65, 57–67. [Google Scholar] [CrossRef]
- Franchi, M.L.; Marzialetti, M.B.; Pose, G.N.; Cavalitto, S.F. Evaluation of Enzymatic Pectin Extraction by a Recombinant Polygalacturonase (PGI) From Apples and Pears Pomace of Argentinean Production and Characterization of the Extracted Pectin. J. Food Process. Technol. 2014, 5, 352. [Google Scholar] [CrossRef]
- Yang, J.-S.; Mu, T.-H.; Ma, M.-M. Extraction, structure, and emulsifying properties of pectin from potato pulp. Food Chem. 2018, 244, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Gawkowska, D.; Cybulska, J.; Zdunek, A. Structure-Related Gelling of Pectins and Linking with Other Natural Compounds: A Review. Polymers 2018, 10, 762. [Google Scholar] [CrossRef]
- Lara-Espinoza, C.; Carvajal-Millán, E.; Balandrán-Quintana, R.; López-Franco, Y.; Rascón-Chu, A. Pectin and Pectin-Based Composite Materials: Beyond Food Texture. Molecules 2018, 23, 942. [Google Scholar] [CrossRef] [PubMed]
- Ishii, T. Feruloyl Oligosaccharides from Cell Walls of Suspension-Cultured Spinach Cells and Sugar Beet Pulp. Plant Cell Physiol. 1994, 35, 701–704. [Google Scholar] [CrossRef] [PubMed]
- Bunzel, M.; Ralph, J.; Lu, F.; Hatfield, A.R.D.; Steinhart, H. Lignins and Ferulate−Coniferyl Alcohol Cross-Coupling Products in Cereal Grains. J. Agric. Food Chem. 2004, 52, 6496–6502. [Google Scholar] [CrossRef]
- Yoo, S.H.; Lee, B.H.; Lee, H.; Lee, S.; Bae, I.Y.; Lee, H.G.; Fishman, M.L.; Chau, H.K.; Savary, B.J.; Hotchkiss, A.T. Structural Characteristics of Pumpkin Pectin Extracted by Microwave Heating. J. Food Sci. 2012, 77. [Google Scholar] [CrossRef]
- Li, D.-Q.; Du, G.-M.; Jing, W.-W.; Li, J.-F.; Yan, J.-Y.; Liu, Z.-Y. Combined effects of independent variables on yield and protein content of pectin extracted from sugar beet pulp by citric acid. Carbohydr. Polym. 2015, 129, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Funami, T.; Zhang, G.; Hiroe, M.; Noda, S.; Nakauma, M.; Asai, I.; Cowman, M.K.; Al-Assaf, S.; Phillips, G.O. Effects of the proteinaceous moiety on the emulsifying properties of sugar beet pectin. Food Hydrocoll. 2007, 21, 1319–1329. [Google Scholar] [CrossRef]
- Drusch, S. Sugar beet pectin: A novel emulsifying wall component for microencapsulation of lipophilic food ingredients by spray-drying. Food Hydrocoll. 2007, 21, 1223–1228. [Google Scholar] [CrossRef]
- Iglesias, M.; Lozano, J. Extraction and characterization of sunflower pectin. J. Food Eng. 2004, 62, 215–223. [Google Scholar] [CrossRef]
- Lin, M.J.Y.; Humbert, E.S.; Sosulski, F.W.; Downey, R.K. Distribution and composition of pectins in sunflower plants. Can. J. Plant Sci. 1975, 55, 507–513. [Google Scholar] [CrossRef]
- Turquois, T.; Rinaudo, M.; Taravel, F.; Heyraud, A. Extraction of highly gelling pectic substances from sugar beet pulp and potato pulp: Influence of extrinsic parameters on their gelling properties. Food Hydrocoll. 1999, 13, 255–262. [Google Scholar] [CrossRef]
- Ptichkina, N.; Markina, O.; Rumyantseva, G. Pectin extraction from pumpkin with the aid of microbial enzymes. Food Hydrocoll. 2008, 22, 192–195. [Google Scholar] [CrossRef]
- Pagán, J.; Ibarz, A.; Llorca, M.; Pagán, A.; Barbosa-Cánovas, G. Extraction and characterization of pectin from stored peach pomace. Food Res. Int. 2001, 34, 605–612. [Google Scholar] [CrossRef]
- Díaz-Rojas, E.; Pacheco-Aguilar, R.; Lizardi, J.; Argüelles-Monal, W.; Valdez, M.; Rinaudo, M.; Goycoolea, F. Linseed pectin: Gelling properties and performance as an encapsulation matrix for shark liver oil. Food Hydrocoll. 2004, 18, 293–304. [Google Scholar] [CrossRef]
- Zouambia, Y.; Ettoumi, K.Y.; Krea, M.; Moulai-Mostefa, N. A new approach for pectin extraction: Electromagnetic induction heating. Arab. J. Chem. 2017, 10, 480–487. [Google Scholar] [CrossRef] [Green Version]
- Rodsamran, P.; Sothornvit, R. Microwave heating extraction of pectin from lime peel: Characterization and properties compared with the conventional heating method. Food Chem. 2019, 278, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, L.; Bailina, Y.; Ge, Z.; Ding, T.; Ye, X.; Liu, D. Effects of ultrasound and/or heating on the extraction of pectin from grapefruit peel. J. Food Eng. 2014, 126, 72–81. [Google Scholar] [CrossRef]
- Marcon, M.V.; Vriesmann, L.C.; Wosiacki, G.; Beleski-Carneiro, E.; Petkowicz, C.L.O. Pectins from apple pomace. Polímeros 2005, 15, 127–129. [Google Scholar] [CrossRef]
- Canteri-Schemin, M.H.; Cristina, H.; Fertonani, R.; Waszczynskyj, N.; Wosiacki, G. BRAZILIAN ARCHIVES OF BIOLOGY AND TECHNOLOGY Extraction of Pectin From Apple Pomace. Braz. Arch. Biol. Technol. 2005, 48, 259–266. [Google Scholar] [CrossRef]
- Chan, S.-Y.; Choo, W.-S. Effect of extraction conditions on the yield and chemical properties of pectin from cocoa husks. Food Chem. 2013, 141, 3752–3758. [Google Scholar] [CrossRef]
- Abou-Elseoud, W.S.; Hassan, E.A.; Hassan, M.L. Extraction of pectin from sugar beet pulp by enzymatic and ultrasound-assisted treatments. Carbohydr. Polym. Technol. Appl. 2021, 2, 100042. [Google Scholar] [CrossRef]
- Pacheco, M.T.; Villamiel, M.; Moreno, R.; Moreno, F.J. Structural and Rheological Properties of Pectins Extracted from Industrial Sugar Beet By-Products. Molecules 2019, 24, 392. [Google Scholar] [CrossRef]
- Méndez, D.; Fabra, M.; Gómez-Mascaraque, L.; López-Rubio, A.; Martinez-Abad, A. Modelling the Extraction of Pectin towards the Valorisation of Watermelon Rind Waste. Foods 2021, 10, 738. [Google Scholar] [CrossRef]
- Hassan, M.L.; Berglund, L.; Abou Elseoud, W.S.; Hassan, E.A.; Oksman, K. Effect of Pectin Extraction Method on Properties of Cellulose Nanofibers Isolated from Sugar Beet Pulp. Cellulose 2021, 28, 10905–10920. [Google Scholar] [CrossRef]
- Khamsucharit, P.; Laohaphatanalert, K.; Gavinlertvatana, P.; Sriroth, K.; Sangseethong, K. Characterization of pectin extracted from banana peels of different varieties. Food Sci. Biotechnol. 2018, 27, 623–629. [Google Scholar] [CrossRef]
- Wongkaew, M.; Sommano, S.R.; Tangpao, T.; Rachtanapun, P.; Jantanasakulwong, K. Mango Peel Pectin by Microwave-Assisted Extraction and Its Use as Fat Replacement in Dried Chinese Sausage. Foods 2020, 9, 450. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Nisar, T.; Hou, Y.; Gou, X.; Sun, L.; Guo, Y. Pomegranate peel pectin can be used as an effective emulsifier. Food Hydrocoll. 2018, 85, 30–38. [Google Scholar] [CrossRef]
- Sylvie, A.; Louise, W. Quality assessment of Borassus aethiopum Mart fruit pulp pectin precipitated with various solvents. Afr. J. Food Sci. 2020, 14, 222–232. [Google Scholar] [CrossRef]
- Yuliarti, O.; Matia-Merino, L.; Goh, K.T.; Mawson, J.; Brennan, C. Characterisation of gold kiwifruit pectin isolated by enzymatic treatment. Int. J. Food Sci. Technol. 2012, 47, 633–639. [Google Scholar] [CrossRef]
- Kazemi, M.; Khodaiyan, F.; Labbafi, M.; Hosseini, S.S. Ultrasonic and heating extraction of pistachio by-product pectin: Physicochemical, structural characterization and functional measurement. J. Food Meas. Charact. 2019, 14, 679–693. [Google Scholar] [CrossRef]
- Wathoni, N.; Shan, C.Y.; Shan, W.Y.; Rostinawati, T.; Indradi, R.B.; Pratiwi, R.; Muchtaridi, M. Characterization and antioxidant activity of pectin from Indonesian mangosteen (Garcinia mangostana L.) rind. Heliyon 2019, 5, e02299. [Google Scholar] [CrossRef]
- Sabater, C.; Blanco-Doval, A.; Montilla, A.; Corzo, N. Optimisation of an enzymatic method to obtain modified artichoke pectin and pectic oligosaccharides using artificial neural network tools. In silico and in vitro assessment of the antioxidant activity. Food Hydrocoll. 2021, 110, 106161. [Google Scholar] [CrossRef]
- Thibault, J.-F.; Renard, C.M.; Axelos, M.A.; Roger, P.; Crépeau, M.-J. Studies of the length of homogalacturonic regions in pectins by acid hydrolysis. Carbohydr. Res. 1993, 238, 271–286. [Google Scholar] [CrossRef]
- Yeoh, S.; Zhang, S.; Shi, J.; Langrish, T.A.G. A COMPARISON OF DIFFERENT TECHNIQUES FOR WATER-BASED EXTRACTION OF PECTIN FROM ORANGE PEELS. Chem. Eng. Commun. 2008, 195, 511–520. [Google Scholar] [CrossRef]
- Monsoor, M.A. Effect of drying methods on the functional properties of soy hull pectin. Carbohydr. Polym. 2005, 61, 362–367. [Google Scholar] [CrossRef]
- Ma, Y.; Luo, J.; Xu, Y. Co-Preparation of Pectin and Cellulose from Apple Pomace by a Sequential Process. J. Food Sci. Technol. 2019, 56, 4091. [Google Scholar] [CrossRef] [PubMed]
- Oosterveld, A.; Beldman, G.; Voragen, A.G. Oxidative cross-linking of pectic polysaccharides from sugar beet pulp. Carbohydr. Res. 2000, 328, 199–207. [Google Scholar] [CrossRef]
- Munarin, F.; Tanzi, M.C.; Petrini, P. Advances in biomedical applications of pectin gels. Int. J. Biol. Macromol. 2012, 51, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Raji, Z.; Khodaiyan, F.; Rezaei, K.; Kiani, H.; Hosseini, S.S. Extraction optimization and physicochemical properties of pectin from melon peel. Int. J. Biol. Macromol. 2017, 98, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Garna, H.; Mabon, N.; Robert, C.; Cornet, C.; Nott, K.; Legros, H.; Wathelet, B.; Paquot, M. Effect of Extraction Conditions on the Yield and Purity of Apple Pomace Pectin Precipitated but Not Washed by Alcohol. J. Food Sci. 2007, 72, C001–C009. [Google Scholar] [CrossRef]
- Karbuz, P.; Tugrul, N. Microwave and ultrasound assisted extraction of pectin from various fruits peel. J. Food Sci. Technol. 2021, 58, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Panouillé, M.; Thibault, J.-F.; Bonnin, E. Cellulase and Protease Preparations Can Extract Pectins from Various Plant Byproducts. J. Agric. Food Chem. 2006, 54, 8926–8935. [Google Scholar] [CrossRef] [PubMed]
- Yuliarti, O.; Matia-Merino, L.; Goh, K.K.; Mawson, J.; Williams, M.A.; Brennan, C. Characterization of gold kiwifruit pectin from fruit of different maturities and extraction methods. Food Chem. 2015, 166, 479–485. [Google Scholar] [CrossRef]
- Humerez-Flores, J.N.; Kyomugasho, C.; Gutiérrez-Ortiz, A.A.; De Bie, M.; Panozzo, A.; Van Loey, A.M.; Moldenaers, P.; Hendrickx, M.E. Production and molecular characterization of tailored citrus pectin-derived compounds. Food Chem. 2021, 367, 130635. [Google Scholar] [CrossRef]
- Picot-Allain, M.C.N.; Ramasawmy, B.; Emmambux, M.N. Extraction, Characterisation, and Application of Pectin from Tropical and Sub-Tropical Fruits: A Review. Food Rev. Int. 2020, 38, 282–312. [Google Scholar] [CrossRef]
- Kirtchev, N.; Panchev, I.; Kratchanov, C. Pectin extraction in the presence of alcohols. Carbohydr. Polym. 1989, 11, 257–263. [Google Scholar] [CrossRef]
- Attri, B.; Maini, S. Pectin from galgal (Citrus pseudolimon Tan.) peel. Bioresour. Technol. 1996, 55, 89–91. [Google Scholar] [CrossRef]
- Benassi, L.; Alessandri, I.; Vassalini, I. Assessing Green Methods for Pectin Extraction from Waste Orange Peels. Molecules 2021, 26, 1766. [Google Scholar] [CrossRef] [PubMed]
- Mierczyńska, J.; Cybulska, J.; Pieczywek, P.; Zdunek, A. Effect of Storage on Rheology of Water-Soluble, Chelate-Soluble and Diluted Alkali-Soluble Pectin in Carrot Cell Walls. Food Bioprocess Technol. 2015, 8, 171–180. [Google Scholar] [CrossRef]
- Fischer, M.; Amadò, R. Changes in the pectic substances of apples during development and postharvest ripening. Part 1: Analysis of the alcohol-insoluble residue. Carbohydr. Polym. 1994, 25, 161–166. [Google Scholar] [CrossRef]
- Diaz, J.V.; Anthon, G.E.; Barrett, D.M. Nonenzymatic Degradation of Citrus Pectin and Pectate during Prolonged Heating: Effects of pH, Temperature, and Degree of Methyl Esterification. J. Agric. Food Chem. 2007, 55, 5131–5136. [Google Scholar] [CrossRef]
- May, C.D. Industrial pectins: Sources, production and applications. Carbohydr. Polym. 1990, 12, 79–99. [Google Scholar] [CrossRef]
- Kastner, H.; Einhorn-Stoll, U.; Senge, B. Structure formation in sugar containing pectin gels—Influence of Ca2+ on the gelation of low-methoxylated pectin at acidic pH. Food Hydrocoll. 2012, 27, 42–49. [Google Scholar] [CrossRef]
- Alba, K.; Kasapis, S.; Kontogiorgos, V. Influence of pH on mechanical relaxations in high solids LM-pectin preparations. Carbohydr. Polym. 2015, 127, 182–188. [Google Scholar] [CrossRef]
- Wei, J.; Ma, F.; Shi, S.; Qi, X.; Zhu, X.; Yuan, J. Changes and postharvest regulation of activity and gene expression of enzymes related to cell wall degradation in ripening apple fruit. Postharvest Biol. Technol. 2010, 56, 147–154. [Google Scholar] [CrossRef]
- Jayani, R.S.; Saxena, S.; Gupta, R. Microbial pectinolytic enzymes: A review. Process Biochem. 2005, 40, 2931–2944. [Google Scholar] [CrossRef]
- Akusu, O.M.; Chibor, B.S.; Mastanjevic, K. Pectin Strength of Common Varieties of Plantain Peels Used in the Production of Jam/Marmalade. Asian Food Sci. J. 2020, 19, 1–9. [Google Scholar] [CrossRef]
- Zhilinskaya, N.V.; Sarkisyan, V.A.; Vorobieva, V.M.; Vorobieva, I.S.; Kochetkova, A.A.; Smirnova, E.A.; Glazkova, I.V. Development of a marmalade for patients with type 2 diabetes: Sensory characteristics and acceptability. Food Sci. Technol. Int. 2018, 24, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Mendez, D.; Fabra, M.; Martínez-Abad, A.; Μartínez-Sanz; Gorria, M.; López-Rubio, A. Understanding the different emulsification mechanisms of pectin: Comparison between watermelon rind and two commercial pectin sources. Food Hydrocoll. 2021, 120, 106957. [Google Scholar] [CrossRef]
- Isusi, G.I.S.; Weilandt, M.; Majollari, I.; Karbstein, H.P.; van der Schaaf, U.S. Emulsions stabilised with pectin-based microgels: Investigations into the effect of pH and ionic strength on emulsion stability. Food Funct. 2021, 12, 7227–7238. [Google Scholar] [CrossRef]
- Donchenko, L.V.; Sokol, N.V.; Sanzharovskaya, N.S.; Khrapko, O.P.; Mikhaylova, T.A. Functional role of pectin in the bakery technology. IOP Conf. Ser. Earth Environ. Sci. 2020, 488, 012010. [Google Scholar] [CrossRef]
- Ajibade, B.O.; Ijabadeniyi, O.A. Effects of pectin and emulsifiers on the physical and nutritional qualities and consumer acceptability of wheat composite dough and bread. J. Food Sci. Technol. 2019, 56, 83–92. [Google Scholar] [CrossRef]
- Khubber, S.; Chaturvedi, K.; Thakur, N.; Sharma, N.; Yadav, S.K. Low-methoxyl pectin stabilizes low-fat set yoghurt and improves their physicochemical properties, rheology, microstructure and sensory liking. Food Hydrocoll. 2021, 111, 106240. [Google Scholar] [CrossRef]
- Yuliarti, O.; Mei, K.H.; Ting, Z.K.X.; Yi, K.Y. Influence of combination carboxymethylcellulose and pectin on the stability of acidified milk drinks. Food Hydrocoll. 2019, 89, 216–223. [Google Scholar] [CrossRef]
- Gómez, B.; Gullón, B.; Yáñez, R.; Schols, H.; Alonso, J.L. Prebiotic potential of pectins and pectic oligosaccharides derived from lemon peel wastes and sugar beet pulp: A comparative evaluation. J. Funct. Foods 2016, 20, 108–121. [Google Scholar] [CrossRef]
- Rodsamran, P.; Sothornvit, R. Lime peel pectin integrated with coconut water and lime peel extract as a new bioactive film sachet to retard soybean oil oxidation. Food Hydrocoll. 2019, 97, 105173. [Google Scholar] [CrossRef]
- Liu, H.; Li, J.; Zhu, D.; Wang, Y.; Zhao, Y.; Li, J. Preparation of Soy Protein Isolate (SPI)-Pectin Complex Film Containing Cinnamon Oil and Its Effects on Microbial Growth of Dehydrated Soybean Curd (Dry Tofu). J. Food Process. Preserv. 2014, 38, 1371–1376. [Google Scholar] [CrossRef]
- Ravishankar, S.; Jaroni, D.; Zhu, L.; Olsen, C.; McHugh, T.; Friedman, M. Inactivation of Listeria monocytogenes on Ham and Bologna Using Pectin-Based Apple, Carrot, and Hibiscus Edible Films Containing Carvacrol and Cinnamaldehyde. J. Food Sci. 2012, 77, M377–M382. [Google Scholar] [CrossRef]
- Xiong, Y.; Li, S.; Warner, R.D.; Fang, Z. Effect of oregano essential oil and resveratrol nanoemulsion loaded pectin edible coating on the preservation of pork loin in modified atmosphere packaging. Food Control 2020, 114, 107226. [Google Scholar] [CrossRef]
- Moradi, L.T.; Sharifan, A.; Larijani, K. The Effect of Multilayered Chitosan–Pectin–Mentha Piperita and Lemon Essential Oil on Oxidation Effects and Quality of Rainbow Trout Fillet (Oncorhynchus Mykiss) during Refrigeration at 4±1 °C Storage. Iran. J. Fish. Sci. 2020, 19, 2544–2559. [Google Scholar] [CrossRef]
- Çavdaroğlu, E.; Farris, S.; Yemenicioğlu, A. Development of pectin–eugenol emulsion coatings for inhibition of Listeria on webbed-rind melons: A comparative study with fig and citrus pectins. Int. J. Food Sci. Technol. 2020, 55, 1448–1457. [Google Scholar] [CrossRef]
- Sumonsiri, N.; Danpongprasert, W.; Thaidech, K. Comparison of Sweet Orange (Citrus sinensis) and Lemon (Citrus limonum) Essential Oils on Qualities of Fresh-Cut Apples during Storage. Chem. Chem. Eng. Biotechnol. Food Ind. 2020, 21, 47–57. [Google Scholar]
- Brouns, F.; Theuwissen, E.; Adam, A.; Bell, M.; Berger, A.; Mensink, R.P. Cholesterol-lowering properties of different pectin types in mildly hyper-cholesterolemic men and women. Eur. J. Clin. Nutr. 2012, 66, 591–599. [Google Scholar] [CrossRef]
- Celus, M.; Salvia-Trujillo, L.; Kyomugasho, C.; Maes, I.; Van Loey, A.M.; Grauwet, T.; Hendrickx, M.E. Structurally modified pectin for targeted lipid antioxidant capacity in linseed/sunflower oil-in-water emulsions. Food Chem. 2018, 241, 86–96. [Google Scholar] [CrossRef]
- Sun, D.; Chen, X.; Zhu, C. Physicochemical properties and antioxidant activity of pectin from hawthorn wine pomace: A comparison of different extraction methods. Int. J. Biol. Macromol. 2020, 158, 1239–1247. [Google Scholar] [CrossRef]
- Wang, R.; Li, Y.; Shuai, X.; Chen, J.; Liang, R.; Liu, C. Development of Pectin-Based Aerogels with Several Excellent Properties for the Adsorption of Pb2+. Foods 2021, 10, 3127. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, M.E.; Mohamed, A.K. Novel derived pectin hydrogel from mandarin peel based metal-organic frameworks composite for enhanced Cr(VI) and Pb(II) ions removal. Int. J. Biol. Macromol. 2020, 164, 920–931. [Google Scholar] [CrossRef]
- Sibiya, N.; Ngubane, P.; Mabandla, M. Cardioprotective effects of pectin-insulin patch in streptozotocin-induced diabetic rats. J. Diabetes 2017, 9, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Sanaka, M.; Yamamoto, T.; Anjiki, H.; Nagasawa, K.; Kuyama, Y. Effects of Agar and Pectin on Gastric Emptying and Post-Prandial Glycaemic Profiles in Healthy Human Volunteers. Clin. Exp. Pharmacol. Physiol. 2007, 34, 1151–1155. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Kaur, K.; Kumar, P. Optimizing microencapsulation of α-tocopherol with pectin and sodium alginate. J. Food Sci. Technol. 2018, 55, 3625–3631. [Google Scholar] [CrossRef]
- Hartini, N.; Ponrasu, T.; Wu, J.-J.; Sriariyanun, M.; Cheng, Y.-S. Microencapsulation of Curcumin in Crosslinked Jelly Fig Pectin Using Vacuum Spray Drying Technique for Effective Drug Delivery. Polymers 2021, 13, 2583. [Google Scholar] [CrossRef]
- Li, K.; Zhu, L.; Li, H.; Zhu, Y.; Pan, C.; Gao, X.; Liu, W. Structural characterization and rheological properties of a pectin with anti-constipation activity from the roots of Arctium lappa L. Carbohydr. Polym. 2019, 215, 119–129. [Google Scholar] [CrossRef]
- Valle, K.Z.M.; Acuña, R.A.S.; Arana, J.V.R.; Lobo, N.; Rodriguez, C.; Cuevas-Gonzalez, J.C.; Tovar-Carrillo, K.L. Natural Film Based on Pectin and Allantoin for Wound Healing: Obtaining, Characterization, and Rat Model. BioMed Res. Int. 2020, 2020, 6897497. [Google Scholar] [CrossRef]
- Hossein, G.; Keshavarz, M.; Ahmadi, S.; Naderi, N. Synergistic Effects of PectaSol-C Modified Citrus Pectin an Inhibitor of Galectin-3 and Paclitaxel on Apoptosis of Human SKOV-3 Ovarian Cancer Cells. Asian Pac. J. Cancer Prev. 2013, 14, 7561–7568. [Google Scholar] [CrossRef]
- Palko-Łabuz, A.; Maksymowicz, J.; Sobieszczańska, B.; Wikiera, A.; Skonieczna, M.; Wesołowska, O.; Środa-Pomianek, K. Newly Obtained Apple Pectin as an Adjunct to Irinotecan Therapy of Colorectal Cancer Reducing E. coli Adherence and β-Glucuronidase Activity. Cancers 2021, 13, 2952. [Google Scholar] [CrossRef]
- Nishikawa, H.; Liu, L.; Nakano, F.; Kawakita, F.; Kanamaru, H.; Nakatsuka, Y.; Okada, T.; Suzuki, H. Modified Citrus Pectin Prevents Blood-Brain Barrier Disruption in Mouse Subarachnoid Hemorrhage by Inhibiting Galectin-3. Stroke 2018, 49, 2743–2751. [Google Scholar] [CrossRef] [PubMed]
- Wicker, L.; Kim, Y.; Kim, M.-J.; Thirkield, B.; Lin, Z.; Jung, J. Pectin as a bioactive polysaccharide—Extracting tailored function from less. Food Hydrocoll. 2014, 42, 251–259. [Google Scholar] [CrossRef]
- Nakauma, M.; Funami, T.; Noda, S.; Ishihara, S.; Al-Assaf, S.; Nishinari, K.; Phillips, G.O. Comparison of sugar beet pectin, soybean soluble polysaccharide, and gum arabic as food emulsifiers. 1. Effect of concentration, pH, and salts on the emulsifying properties. Food Hydrocoll. 2008, 22, 1254–1267. [Google Scholar] [CrossRef]
- Correa, M.J.; Pérez, G.T.; Ferrero, C. Pectins as Breadmaking Additives: Effect on Dough Rheology and Bread Quality. Food Bioprocess Technol. 2011, 5, 2889–2898. [Google Scholar] [CrossRef]
- Bush, P.L. Pectin: Chemical Properties, Uses and Health Benefits; Nova Science Publishers: Hauppauge, NY, USA, 2014. [Google Scholar]
- Flander, L.; Salmenkallio-Marttila, M.; Suortti, T.; Autio, K. Optimization of ingredients and baking process for improved wholemeal oat bread quality. LWT-Food Sci. Technol. 2007, 40, 860–870. [Google Scholar] [CrossRef]
- Pectic Substances and Their Functional Role in Bread-Making from Frozen Semi-Finished Products|Kenijz|European Online Journal of Natural and Social Sciences. Available online: https://european-science.com/eojnss/article/view/101 (accessed on 12 July 2022).
- Eduardo, M.; Svanberg, U.; Oliveira, J.; Ahrné, L. Effect of Cassava Flour Characteristics on Properties of Cassava-Wheat-Maize Composite Bread Types. Int. J. Food Sci. 2013, 2013, 305407. [Google Scholar] [CrossRef] [PubMed]
- Rosell, C.M.; Santos, E. Impact of fibers on physical characteristics of fresh and staled bake off bread. J. Food Eng. 2010, 98, 273–281. [Google Scholar] [CrossRef]
- Ferreira-Lazarte, A.; Moreno, F.J.; Cueva, C.; Gil-Sánchez, I.; Villamiel, M. Behaviour of citrus pectin during its gastrointestinal digestion and fermentation in a dynamic simulator (simgi®). Carbohydr. Polym. 2019, 207, 382–390. [Google Scholar] [CrossRef]
- Gullón, B.; Gómez, B.; Martínez-Sabajanes, M.; Yáñez, R.; Parajó, J.; Alonso, J. Pectic oligosaccharides: Manufacture and functional properties. Trends Food Sci. Technol. 2013, 30, 153–161. [Google Scholar] [CrossRef]
- Moon, J.S.; Shin, S.Y.; Choi, H.S.; Joo, W.; Cho, S.K.; Li, L.; Kang, J.-H.; Kim, T.-J.; Han, N.S. In vitro digestion and fermentation properties of linear sugar-beet arabinan and its oligosaccharides. Carbohydr. Polym. 2015, 131, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Islamova, Z.I.; Ogai, D.K.; Abramenko, O.I.; Lim, A.L.; Abduazimov, B.B.; Malikova, M.K.; Rakhmanberdyeva, R.K.; Khushbaktova, Z.A.; Syrov, V.N. Comparative Assessment of the Prebiotic Activity of Some Pectin Polysaccharides. Pharm. Chem. J. 2017, 51, 288–291. [Google Scholar] [CrossRef]
- Shtriker, M.G.; Hahn, M.; Taieb, E.; Nyska, A.; Moallem, U.; Tirosh, O.; Madar, Z. Fenugreek galactomannan and citrus pectin improve several parameters associated with glucose metabolism and modulate gut microbiota in mice. Nutrition 2018, 46, 134–142.e3. [Google Scholar] [CrossRef]
- Chung, W.S.F.; Meijerink, M.; Zeuner, B.; Holck, J.; Louis, P.; Meyer, A.S.; Wells, J.M.; Flint, H.J.; Duncan, S.H. Prebiotic potential of pectin and pectic oligosaccharides to promote anti-inflammatory commensal bacteria in the human colon. FEMS Microbiol. Ecol. 2017, 93, fix127. [Google Scholar] [CrossRef] [PubMed]
- Sabater, C.; Abad-García, C.; Delgado-Fernández, P.; Corzo, N.; Montilla, A. Carbohydrate fraction characterisation of functional yogurts containing pectin and pectic oligosaccharides through convolutional networks. J. Food Compos. Anal. 2020, 90, 103484. [Google Scholar] [CrossRef]
- Wusigale; Liang, L.; Luo, Y. Casein and pectin: Structures, interactions, and applications. Trends Food Sci. Technol. 2020, 97, 391–403. [Google Scholar] [CrossRef]
- Arioui, F.; Saada, D.A.; Cheriguene, A. Physicochemical and sensory quality of yogurt incorporated with pectin from peel of Citrus sinensis. Food Sci. Nutr. 2017, 5, 358–364. [Google Scholar] [CrossRef]
- Moslemi, M.; Fard, R.M.N.; Hosseini, S.M.; Rad, A.H.; Mortazavian, A.M. Incorporation of Propionibacteria in Fermented Milks as a Probiotic. Crit. Rev. Food Sci. Nutr. 2013, 56, 1290–1312. [Google Scholar] [CrossRef]
- Rtibi, K.; Selmi, S.; Grami, D.; Sebai, H.; Marzouki, L. Laxative and anti-purgative bioactive compounds in prevention and treatment of functional gastrointestinal disorders, constipation and diarrhea. J. Nutr. Health Food Eng. 2018, 8, 1. [Google Scholar] [CrossRef]
- Vowden, K.; Vowden, P. Wound dressings: Principles and practice. Surgery 2017, 35, 489–494. [Google Scholar] [CrossRef]
- Günter, E.A.; Popeyko, O.V.; Istomina, E.I. Encapsulated drug system based on the gels obtained from callus cultures modified pectins. J. Biotechnol. 2019, 289, 7–14. [Google Scholar] [CrossRef]
- Leclere, L.; Van Cutsem, P.; Michiels, C. Anti-Cancer Activities of PH- or Heat-Modified Pectin. Front. Pharmacol. 2013, 4, 128. [Google Scholar] [CrossRef] [Green Version]
- Dong, R.; Zhang, M.; Hu, Q.; Zheng, S.; Soh, A.; Zheng, Y.; Yuan, H. Galectin-3 as a novel biomarker for disease diagnosis and a target for therapy (Review). Int. J. Mol. Med. 2018, 41, 599–614. [Google Scholar] [CrossRef]
- Farhad, M.; Rolig, A.S.; Redmond, W.L. The role of Galectin-3 in modulating tumor growth and immunosuppression within the tumor microenvironment. OncoImmunology 2018, 7, e1434467. [Google Scholar] [CrossRef]
- Wdowiak, K.; Francuz, T.; Gallego-Colon, E.; Ruiz-Agamez, N.; Kubeczko, M.; Grochoła, I.; Wojnar, J. Galectin Targeted Therapy in Oncology: Current Knowledge and Perspectives. Int. J. Mol. Sci. 2018, 19, 210. [Google Scholar] [CrossRef]
- Jones, M.; Gu, X.; Stebbins, N.; Crandall, P.; Ricke, S.; Lee, S. Effects of soybean pectin on blood glucose and insulin responses in healthy men. FASEB J. 2015, 29, 596.16. [Google Scholar] [CrossRef]
- Makarova, E.; Górnaś, P.; Konrade, I.; Tirzite, D.; Cirule, H.; Gulbe, A.; Pugajeva, I.; Seglina, D.; Dambrova, M. Acute anti-hyperglycaemic effects of an unripe apple preparation containing phlorizin in healthy volunteers: A preliminary study. J. Sci. Food Agric. 2015, 95, 560–568. [Google Scholar] [CrossRef]
- Javan, A.J.; Moslemi, M.; Salimirad, S.; Soleymanpour, S. Effect of chitosan and Trachyspermum ammi essential oil on microbial growth, proteolytic spoilage, lipid oxidation and sensory attributes of chicken fillet during refrigerated storage. Iran. J. Vet. Med. 2018, 12, 69–78. [Google Scholar] [CrossRef]
- Encina, C.; Vergara, C.; Giménez, B.; Oyarzún-Ampuero, F.; Robert, P. Conventional spray-drying and future trends for the microencapsulation of fish oil. Trends Food Sci. Technol. 2016, 56, 46–60. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Mirmoghtadaie, L.; Cheraghali, F.; Shojaee-Aliabadi, S.; Mortazavian, A.M.; Ghanati, K.; Abedi, A.-S.; Moslemi, M. Characterization of microcapsule containing walnut (Juglans regia L.) green husk extract as preventive antioxidant and antimicrobial agent. Int. J. Prev. Med. 2018, 9, 101. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Pires, J.R.A.; Rodrigues, C.; Coelhoso, I.M.; Fernando, A.L. Chitosan Composites in Packaging Industry—Current Trends and Future Challenges. Polymers 2020, 12, 417. [Google Scholar] [CrossRef]
- Rivera-Hernández, L.; Chavarría-Hernández, N.; Cuellar, M.D.R.L.; Martínez-Juárez, V.M.; Rodríguez-Hernández, A.-I. Pectin-gellan films intended for active food packaging: Release kinetics of nisin and physico-mechanical characterization. J. Food Sci. Technol. 2020, 58, 2973–2981. [Google Scholar] [CrossRef] [PubMed]
- Norcino, L.; Mendes, J.; Natarelli, C.; Manrich, A.; Oliveira, J.; Mattoso, L. Pectin films loaded with copaiba oil nanoemulsions for potential use as bio-based active packaging. Food Hydrocoll. 2020, 106, 105862. [Google Scholar] [CrossRef]
- Zaitseva, O.; Khudyakov, A.; Sergushkina, M.; Solomina, O.; Polezhaeva, T. Pectins as a universal medicine. Fitoterapia 2020, 146, 104676. [Google Scholar] [CrossRef] [PubMed]
- Khotimchenko, Y.; Khozhaenko, E.; Kovalev, V.; Khotimchenko, M. Cerium Binding Activity of Pectins Isolated from the Seagrasses Zostera marina and Phyllospadix iwatensis. Mar. Drugs 2012, 10, 834–848. [Google Scholar] [CrossRef] [PubMed]
- Espitia, P.J.P.; Du, W.-X.; Avena-Bustillos, R.D.J.; Soares, N.D.F.F.; McHugh, T.H. Edible films from pectin: Physical-mechanical and antimicrobial properties—A review. Food Hydrocoll. 2014, 35, 287–296. [Google Scholar] [CrossRef]
- Sakooei-Vayghan, R.; Peighambardoust, S.H.; Hesari, J.; Peressini, D. Effects of osmotic dehydration (with and without sonication) and pectin-based coating pretreatments on functional properties and color of hot-air dried apricot cubes. Food Chem. 2020, 311, 125978. [Google Scholar] [CrossRef]
- Gaona-Sánchez, V.A.; Calderón-Domínguez, G.; Morales-Sánchez, E.; Moreno-Ruiz, L.A.; Terrés-Rojas, E.; Salgado-Cruz, M.D.L.P.; Escamilla-García, M.; Barrios-Francisco, R. Physicochemical and superficial characterization of a bilayer film of zein and pectin obtained by electrospraying. J. Appl. Polym. Sci. 2021, 138, 50045. [Google Scholar] [CrossRef]
- Sucheta; Chaturvedi, K.; Sharma, N.; Yadav, S.K. Composite edible coatings from commercial pectin, corn flour and beetroot powder minimize post-harvest decay, reduces ripening and improves sensory liking of tomatoes. Int. J. Biol. Macromol. 2019, 133, 284–293. [Google Scholar] [CrossRef]
- Anastas, P.; Eghbali, N. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef]
- Roy, S.; Priyadarshi, R.; Rhim, J.-W. Development of Multifunctional Pullulan/Chitosan-Based Composite Films Reinforced with ZnO Nanoparticles and Propolis for Meat Packaging Applications. Foods 2021, 10, 2789. [Google Scholar] [CrossRef]
- Roy, S.; Priyadarshi, R.; Rhim, J.-W. Gelatin/agar-based multifunctional film integrated with copper-doped zinc oxide nanoparticles and clove essential oil Pickering emulsion for enhancing the shelf life of pork meat. Food Res. Int. 2022, 160, 111690. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Preparation of pectin/agar-based functional films integrated with zinc sulfide nano petals for active packaging applications. Colloids Surf. B Biointerfaces 2021, 207, 111999. [Google Scholar] [CrossRef] [PubMed]
- Lazaridou, A.; Biliaderis, C.G. Edible Films and Coatings with Pectin. In Pectin: Technological and Physiological Properties; Springer: Cham, Switzerland, 2020; pp. 99–123. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Fabrication of pectin/agar blended functional film: Effect of reinforcement of melanin nanoparticles and grapefruit seed extract. Food Hydrocoll. 2021, 118, 106823. [Google Scholar] [CrossRef]
- Ezati, P.; Roy, S.; Rhim, J.-W. Pectin/gelatin-based bioactive composite films reinforced with sulfur functionalized carbon dots. Colloids Surf. A Physicochem. Eng. Asp. 2021, 636, 128123. [Google Scholar] [CrossRef]
- Nastasi, J.R.; Kontogiorgos, V.; Daygon, V.D.; Fitzgerald, M.A. Pectin-based films and coatings with plant extracts as natural preservatives: A systematic review. Trends Food Sci. Technol. 2022, 120, 193–211. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Genipin-Crosslinked Gelatin/Chitosan-Based Functional Films Incorporated with Rosemary Essential Oil and Quercetin. Materials 2022, 15, 3769. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Starch/agar-based functional films integrated with enoki mushroom-mediated silver nanoparticles for active packaging applications. Food Biosci. 2022, 49, 101867. [Google Scholar] [CrossRef]
- Ezati, P.; Roy, S.; Rhim, J.-W. Effect of Saffron on the Functional Property of Edible Films for Active Packaging Applications. ACS Food Sci. Technol. 2022, 2, 1318–1325. [Google Scholar] [CrossRef]
- Kim, H.-J.; Roy, S.; Rhim, J.-W. Gelatin/agar-based color-indicator film integrated with Clitoria ternatea flower anthocyanin and zinc oxide nanoparticles for monitoring freshness of shrimp. Food Hydrocoll. 2022, 124, 107294. [Google Scholar] [CrossRef]
- Roy, S.; Priyadarshi, R.; Ezati, P.; Rhim, J.-W. Curcumin and its uses in active and smart food packaging applications—A comprehensive review. Food Chem. 2022, 375, 131885. [Google Scholar] [CrossRef]
- Roy, S.; Van Hai, L.; Kim, H.C.; Zhai, L.; Kim, J. Preparation and characterization of synthetic melanin-like nanoparticles reinforced chitosan nanocomposite films. Carbohydr. Polym. 2020, 231, 115729. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Roy, S.; Yoon, K.S.; Rhim, J. Preparation of low-density polyethylene- and poly (lactide)/poly (butylene adipate-co-terephthalate)-based antibacterial films integrated with elemental sulfur and sulfur nanoparticles. Packag. Technol. Sci. 2021, 34, 505–516. [Google Scholar] [CrossRef]
- Chalapud, M.C.; Baümler, E.; Carelli, A.A. Edible films based on aqueous emulsions of low-methoxyl pectin with recovered and purified sunflower waxes. J. Sci. Food Agric. 2020, 100, 2675–2687. [Google Scholar] [CrossRef] [PubMed]
- Suhag, R.; Kumar, N.; Petkoska, A.T.; Upadhyay, A. Film formation and deposition methods of edible coating on food products: A review. Food Res. Int. 2020, 136, 109582. [Google Scholar] [CrossRef]
- Ishwarya, P.; Sandhya, R.; Nisha, P. Advances and Prospects in the Food Applications of Pectin Hydrogels. Crit. Rev. Food Sci. Nutr. 2021, 62, 4393–4417. [Google Scholar] [CrossRef]
- Melgarejo-Flores, B.; Ortega-Ramírez, L.; Silva-Espinoza, B.; González-Aguilar, G.; Miranda, M.; Ayala-Zavala, J. Antifungal protection and antioxidant enhancement of table grapes treated with emulsions, vapors, and coatings of cinnamon leaf oil. Postharvest Biol. Technol. 2013, 86, 321–328. [Google Scholar] [CrossRef]
- Da Silva, I.S.V.; Prado, N.S.; de Melo, P.G.; Arantes, D.C.; Andrade, M.Z.; Otaguro, H.; Pasquini, D. Edible Coatings Based on Apple Pectin, Cellulose Nanocrystals, and Essential Oil of Lemongrass: Improving the Quality and Shelf Life of Strawberries (Fragaria Ananassa). J. Renew. Mater. 2019, 7, 73–87. [Google Scholar] [CrossRef] [Green Version]


| Source | Pectin Yield | Extraction Method | References |
|---|---|---|---|
| Citrange | 29% (w/w) of dried albedos | Acidic extraction with a solution of 1 M H2SO4 and electromagnetic induction heating | [71] |
| Lime peel | 5.20 to 23.59% | Acidic extraction with hydrochloric or citric acid and microwave and conventional heating methods | [72] |
| Grapefruit peel | 23.44–26.74% | Acidic extraction with 0.5 M HCl using ultrasound-assisted heating extraction | [73] |
| Apple pomace | 5.7–16.8% | Acidic extraction with 5% (w/v) citric acid | [74] |
| Apple pomace | 13.75–17.82 g % of pectin on a dry basis | Acidic extraction with citric or nitric acids | [75] |
| Cocoa husk | 2.0–9.0% | Acidic extraction with HCl using microwave heating | [50] |
| Cocoa husks | 3.38–7.62% | Acidic extraction with citric acid or hydrochloric acid at pH 2.5 or 4.0 | [76] |
| Sunflower heads | 15–25% | Alkaline washing, 16 °C for 25 min at pH 5–7, 28:1 solvent:solid ratio | [51] |
| Sugar beet | 4.37–28.84% | Enzymatic extraction with xylanase, cellulase and their mixtures (1–4 h), and ultrasound-assisted treatments | [77] |
| Sugar beet (pressed, ensiled and dried pulp) | 13–19% | Acid method or a commercial cellulase | [78] |
| Pumpkin | 10.03 and 8.08 g/100 g | Enzymatic extraction by cellulase and α-amylase | [53] |
| Watermelon | WRP yield (13.4%), | Acidic extraction by 1 M HCl solution | [79] |
| Watermelon | 14.2–19.35% | Acidic extraction with 0.1 M nitric acid for 1 h | [54] |
| Pears | 68.40–42.00 (g ethanol insoluble material/100 g material) dry basis of material processed; 6.84–4.20 (g ethanol insoluble material/100 g material) dry basis of material processed | Enzymatic extraction by a recombinant polygalacturonase | [55] |
| Potato pulp | 14.34–4.08% | Acidic extraction with HCl, H2SO4, HNO3, citric acid and acetic acid | [56] |
| Sugar beet pulp | 6.3% to 23.0%, | Acidic extraction by citric acid | [62,80] |
| Banana peels | 15.89–24.08% | Acidic extraction by citric acid | [81] |
| Mango peel pectin | 13.85% | Acidic extraction at pH 1.5 by 2 M HCl using the microwave-assisted technique | [82] |
| Linseed seed | 0.35–0.65% | Alkaline extraction procedure, 0.1 M HCl at pH 2 | [70] |
| Pomegranate peel pectin | 8.5%, | Acidic extraction using 1 M nitric acid. | [83] |
| Palmyra palm | 102.3–105.8 (g kg−1) | Acidic extraction using 0.1 mol/L HNO3 | [84] |
| Cashew apple pomace | 10.7% to 25.3% dried raw material | Acid extraction conditions with 1 N HNO3 | [35] |
| Gold kiwifruit pectin | 4.00 to 5.40% (w/w) on dry matter basis | Enzymatic extraction (Celluclast 1.5 L, Cytolase CL, Cellulyve TR 400 and NS33048) | [85] |
| Pistachio | 10.3–12.0% | Acidic extraction using citric acid, hydrochloric acid and sulfuric acid with ultrasound-assisted extraction | [86] |
| Mangosteen rind pectin | 1.16 ± 0.17% | Acidic extraction using H2SO4 at pH 2 | [87] |
| Artichoke | 65.9 ± 2.1 mg/100 mg | Enzyme extraction using Viscozyme® L Novozymes | [88] |
| Application Type | Pectin Utility | Key Finding | References |
|---|---|---|---|
| Food Industry | |||
| Jams and Jellies | |||
| Jams and marmalade from French Plantain peel | French Plantain peel was successfully utilized for jam and marmalade preparation with nice spreadability and overall acceptability. | [113] | |
| Marmalade for patients with type 2 diabetes | The marmalade was prepared with agar-gelatin, and pectin-based marmalades with maltitol, dried fruits and berries for glycemic control. The marmalade was successfully developed with textural parameters such as ‘‘gumminess,’’ ‘‘springiness,’’ and ‘‘homogeneity”, and organoleptic properties with comparable overall consumer acceptance for both healthy people and people suffering with type 2 diabetes. | [114] | |
| Emulsifying agent | |||
| Watermelon rind pectin as emulsifying agent | The watermelon rind pectin displayed exceptional emulsification capacity, incorporating up to 60% (v/v) oil in the emulsions. The emulsions were stable for longer periods because of its protein content acting as surface active materials and the steric repulsions between droplets caused by long chain branches of RG-1 enriched pectin. | [115] | |
| Pectin-based microgels as emulsions | The pectin-based microgel sensitivity varied with changes in pH and ionic strength and influenced the stability of emulsions. After emulsification, the pH of the emulsions was adjusted from pH 4.2 to 4, 3 or 2, and they remained stable for at least three weeks. | [116] | |
| Bakery products | |||
| Wheat flour bread | Apple pectin was successfully used for the preparation of wheat flour bread quality with improvement in the activation of fermentation and acid accumulation processes. The bread crust had a thin-walled crumb, with high porosity and sorption capacity. | [117] | |
| Wheat composite dough and bread | Composite flour of wheat, pearl millet, and Bambara groundnut were used for bread production, along with apple pectin. The pectin exhibited up to 1.5% improved dough stability, whereas the highest overall acceptability for composite bread was observed at 2% pectin addition. | [118] | |
| Prebiotic properties and stabilizing acidified milk products | |||
| Low fat yoghurt | The low-fat set yoghurt, with enhanced bacterial counts, was prepared with the addition of low methoxyl pectin contributing towards metabolite production, thus accountable for the higher acidity and antioxidant potential. This resulted in enhanced physico-chemical quality, rheology (elastic, viscous modulus or complex viscosity) and sensory liking. | [119] | |
| Carboxymethylcellulose and pectin effect on the stability of acidified milk drinks | The acidified skim milk drinks were not stabilised after a period of time, but the whole milk drinks exhibited a noticeably reduced formation of serum phase after the addition of the combination of high methoxyl pectin (HMP) and carboxymethylcellulose (CMC) polysaccharides. The drink stability was enhanced when the amount of HMP increased in the polysaccharide ratio. | [120] | |
| Sugar beet pulp pectin and lemon peel waste prebiotic potential | Sugar beet pulp and lemon peel waste pectic oligosaccharides have prebiotic properties; joint populations of bifidobacteria and lactobacilli increased from 19% to 29%, 34% and 32% in cultures, respectively. | [121] | |
| Packaging industry | |||
| Food packaging film | |||
| Pectin/lime peel extract/coconut water-based film | Pectin-based functionalized film was useful in the retardation of vegetable oil during storage. | [122] | |
| Pectin/cinnamon oil film | The film, when used for tofu storage, showed enhancement in shelf-life by reducing the growth of unwanted microbes. | [123] | |
| Pectin/Carvacrol/Cinnamaldehyde film | The pectin-based film reduced the growth Listeria in the ham and bologna, and it shows a better response towards ham. | [124] | |
| Pectin/carbon quantum dot film | The film showed excellent antioxidant activity and good antimicrobial potential, which could be useful for food packaging applications. | ||
| Food coating | |||
| Pectin/oregano oil/ resveratrol | The pectin formulation-coated pork loin showed less lipid oxidation and low microbial growth compared to uncoated counterparts. | [125] | |
| Pectin/ lemon EO/mint EO | The rainbow trout coated with pectin-based solution preserved the texture and color, as well as delayed the oxidation. | [126] | |
| Pectin/eugenol | The melon coated with functionalized pectin solution reduced the growth of Listeria while in storage. | [127] | |
| Pectin/lemon EO/orange EO | The formulation-coated apple slice showed lowered microbial count and less weight loss compared to the untreated sample. | [128] | |
| Health and Pharmaceutical industry | |||
| Reduction in LDL plasma concentrations | |||
| Cholesterol-lowering properties of different pectin types | The trials revealed that a high degree of esterification and high molecular weight pectin were important for cholesterol lowering in mildly hyper-cholesterolemic persons. In a successive 3-week trial with 6 g/day pectin, citrus DE-70 and high MW pectin DE-70 reduced low-density lipoprotein by 6–7% as compared to the control (without changes in total cholesterol). | [129] | |
| Antioxidant activity | |||
| Mangosteen pectin antioxidant activity | The mangosteen pectin showed antioxidant activity with an IC50 of about 161.94 ± 31.57 ppm. | [87] | |
| Structurally modified pectin for lipid antioxidant capacity in linseed/sunflower oil-in-water emulsions | The citrus pectin 5% (w/v) was added in linseed/sunflower oil emulsions stabilized with 0.5% (w/v) Tween 80, and examined during two weeks of storage at 35 °C. The higher antioxidant capacity was observed in low demethylesterified pectin (≤33%) than high demethylesterified pectin (≥58%), probably due to its higher chelating capacity of pro-oxidative metal ions (Fe2+), whereas the arrangement of methylesters along the pectin chain slightly affected the antioxidant capacity. | [130] | |
| Antioxidant activity of pectin from hawthorn wine pomace | The antioxidant activity was evaluated for hawthorn wine pomace pectin extracted by different methods by using the concentration (IC50) index, DPPH scavenging ability, and the IC50 values were 0.01 (VC, ascorbic acid), 2.63 (hydrochloric acid method), 2.10 (citric acid method), 2.24 (cellulase method) and 3.11 (microwave-assisted chelating agent method) mg/mL. | [131] | |
| Metal binding properties | |||
| Pectin-based aerogels properties for adsorption of Pb2+ | Novel porous pectin-based aerogels (PPEAs), prepared by incorporating polyethylenimine (PEI) using ethylene glycol diglycidyl ether (EGDE) as a cross-linker, have several desirable features, such as a maximum Pb2+ adsorption capacity (373.7 mg/g, tested at pH 5.0), are ultralight (as low as 63.4 mg/cm3), with high mechanical strength (stress above 0.24 MPa at 50% strain), and easy recyclability. | [132] | |
| Pectin hydrogel from mandarin peel-based metalorganic frameworks composite | The pectin hydrogel from mandarin peel-based metalorganic frameworks composite was successfully examined for adsorptive removal of both Cr(VI)/Pb(II) ions from aqueous samples at pH 5.0 and 1.0, respectively, and proved to be useful as an adsorbent for toxic heavy metal elimination from wastewater. | [133] | |
| Glycemic control | |||
| Cardio-protective effects of pectin-insulin patch in streptozotocin-induced diabetic rats | The use of the pectin-insulin matrix patches (82.9 μg/kg) resulted in decreased blood glucose concentration and diabetes-induced disturbances in the lipid profile. Diabetes evoked an increase in MAP, which was attenuated in patch (82.9 μg/kg)-treated animals and decreased heart-to-body weight ratio, as well as cardiotropin-1, TNFα and hisCRP concentration. | [134] | |
| Agar and pectin on gastric emptying and post-prandial glycemic profiles | The gastric emptying and post-prandial glycemic profiles were examined for ten healthy male volunteers with three different test meals (450 kcal/500 mL): (i) a fiber-free meal; (ii) a meal with 2.0 g agar; (iii) a meal with 5.2 g pectin. The participants went through a [13C]-acetate breath test, along with serial blood sampling each time, and it was observed that agar and pectin delayed gastric emptying but have no impact on the post-prandial glucose response. | [135] | |
| Encapsulating agent | |||
| Microencapsulation of a-tocopherol with pectin and sodium alginate | The encapsulation efficiency of α-tocopherol in microencapsules formed using sodium alginate 1.5% w/v was 52.91% and pectin 2.0% w/v. α-Tocopherol microencapsules gave an encapsulation efficiency of 55.97% with the encapsulator, and 52.11% with the syringe method. | [136] | |
| Microencapsulation of curcumin in crosslinked jelly using fig pectin | Microencapsulation of curcumin in 0.75 w/w% jelly fig pectin was carried out by the vacuum spray drying (VSD) technique with 80–90 °C inlet temperature and 0.01 mPa pressure. The best encapsulation efficiency was observed with the VSD technique, and yield and loading efficiency was up to 91.56 ± 0.80%, 70.02 ± 1.96% and 5.45 ± 0.14%, respectively. | [137] | |
| Therapeutic and pharmaceutical uses | |||
| Pectin with anti-constipation activity | The pectin extracted from the roots of Arctium lappa L. with dosages of 200 mg/kg and 400 mg/kg exhibited strong anti-constipation activity in vivo. The Arctium lappa L. pectin-treated groups perhaps had improved small intestinal movement rate, and had significantly increased weight of feces in constipated mice. | [138] | |
| Pectin–honey hydrogel enhances wound healing | The wound area reduction rate was faster in rats treated with the pectin–honey hydrogel, liquid Manuka honey and pectin hydrogel compared to the control group, was significantly faster in the pectin–honey hydrogel group; unexpectedly, the pectin hydrogel displayed more rapid wound healing than the liquid Manuka honey. | [21] | |
| Natural film based on pectin and allantoin for wound healing | The pectin–allantoin films comprise two well-differentiated layers, one-layer rich in allantoin (regenerative layer), and one rich in pectin as an antimicrobial and protective layer to the wound. An in vivo assay illustrated a notable decrease of time period in the wound healing process by approximately 25%. | [139] | |
| PectaSol-C-modified citrus pectin, an inhibitor of galectin-3 | Approximately 41% increased cell proliferation, 36% decreased caspase-3 activity and 33.6% increased substrate-dependent adhesion was observed in the presence of rhGal-3 compared to the control case (p < 0.001). Treatment of cells with a non-effective dose of PTX (100 nM) and 0.1% PectaSol-C-modified citrus pectin in combination revealed synergistic cytotoxic effects, with 75% reduced cell viability and a subsequent 3.9-fold increase in caspase-3 activity. | [140] | |
| Apple pectin as an adjunct to irinotecan therapy of colorectal cancer | The novel enzymatically extracted apple pectin reduced the viability of HCT 116 and Caco-2 colorectal cancer cells, induced apoptosis and increased intracellular reactive oxygen species production. Furthermore, enzymatically extracted apple pectin enhanced the cytotoxic and proapoptotic effect of irinotecan (at concentrations below its IC50), and exhibited potent anti-inflammatory properties. | [141] | |
| Modified citrus pectin prevents blood–brain barrier disruption in mouse subarachnoid haemorrhage by inhibiting galectin-3 | Four micrograms of MCP attenuated post-SAH blood–brain barrier disruption and galectin-3 upregulation in brain capillary endothelial cells. Coimmunoprecipitation assay confirmed physical interactions between galectin-3 and TLR (toll-like receptor) 4. R-galectin-3 blocked the neuroprotective effects of MCP. | [142] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chandel, V.; Biswas, D.; Roy, S.; Vaidya, D.; Verma, A.; Gupta, A. Current Advancements in Pectin: Extraction, Properties and Multifunctional Applications. Foods 2022, 11, 2683. https://doi.org/10.3390/foods11172683
Chandel V, Biswas D, Roy S, Vaidya D, Verma A, Gupta A. Current Advancements in Pectin: Extraction, Properties and Multifunctional Applications. Foods. 2022; 11(17):2683. https://doi.org/10.3390/foods11172683
Chicago/Turabian StyleChandel, Vinay, Deblina Biswas, Swarup Roy, Devina Vaidya, Anil Verma, and Anil Gupta. 2022. "Current Advancements in Pectin: Extraction, Properties and Multifunctional Applications" Foods 11, no. 17: 2683. https://doi.org/10.3390/foods11172683