Improvement of the Ultrasound-Assisted Extraction of Polyphenols from Welsh Onion (Allium fistulosum) Leaves Using Response Surface Methodology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
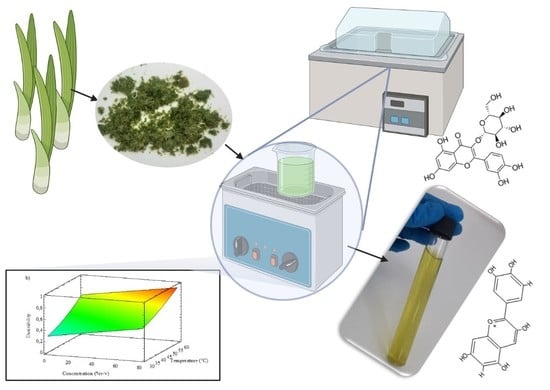
2.2. Sample Preparation
2.3. Ultrasound-Assisted Extraction
2.4. Total Polyphenols Content
2.5. Antioxidant Activity
2.5.1. DPPH Assay
2.5.2. ABTS Assay
2.5.3. FRAP Assay
2.6. UHPLC-ESI+-Orbitrap-MS Analysis
2.7. Experimental Design
2.8. Scanning Electron Microscopy (SEM)
2.9. Fourier Transform Infrared Spectroscopy (FTIR)
2.10. Statistical Analysis
3. Results and Discussion
3.1. Effect of the UAE Conditions on Total Polyphenols Content
3.2. Effect of the UAE Conditions on the DPPH Radical Scavenging Activity
3.3. Simultaneous Multi-Response Optimization
3.4. Antioxidant Activity by ABTS and FRAP
3.5. Phenolic Composition for the Extracts at the Optimal UAE Conditions Obtained by UHPLC-ESI+-Orbitrap-MS Analysis
3.6. Chemical Conformation of the Extract Obtained at the Optimal UAE Conditions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. Food and Agriculture Data. Available online: http://www.fao.org/faostat/en/?#home (accessed on 7 March 2022).
- Poojary, M.M.; Putnik, P.; Kovačević, D.B.; Barba, F.J.; Lorenzo, J.M.; Dias, D.A.; Shpigelman, A. Stability and Extraction of Bioactive Sulfur Compounds from Allium Genus Processed by Traditional and Innovative Technologies. J. Food Compos. Anal. 2017, 61, 28–39. [Google Scholar] [CrossRef]
- Kurnia, D.; Ajiati, D.; Heliawati, L.; Sumiarsa, D. Antioxidant Properties and Structure-Antioxidant Activity Relationship of Allium Species Leaves. Molecules 2021, 26, 7175. [Google Scholar] [CrossRef] [PubMed]
- Vlase, L.; Parvu, M.; Parvu, E.A.; Toiu, A. Phytochemical Analysis of Allium Fistulosum L. and A. Ursinum L. Dig. J. Nanomater. Biostructures 2013, 8, 457–467. [Google Scholar]
- Singh, B.K.; Ramakrishna, Y. Welsh Onion (Allium Fistulosum L.): A Promising Spicing-Culinary Herb of Mizoram. Indian J. Hill Farming 2017, 30, 201–208. [Google Scholar]
- Rubatzky, V.E.; Yamaguchi, M. World Vegetables Principles, Production, and Nutritive Values. Fruits 1997, 5, 381. [Google Scholar]
- Medina-Jaramillo, C.; Usgame-Fagua, K.; Franco-González, N.; López-Córdoba, A. Single and Combined Effect of Mild-Heat Treatment and Alginate Coatings on Quality Preservation of Minimally Processed Bunching Green Onions. Foods 2022, 11, 641. [Google Scholar] [CrossRef]
- El-Hadidy, E.M.; Mossa, M.E.A.; Habashy, H.N. Effect of Freezing on the Pungency and Antioxidants Activity in Leaves and Bulbs of Green Onion in Giza 6 and Photon Varieties. Ann. Agric. Sci. 2014, 59, 33–39. [Google Scholar] [CrossRef]
- Ajayi, G.O.; Akinsanya, M.A.; Agbabiaka, A.T.; Oyebanjo, K.S.; Hungbo, T.D.; Olagunju, J.A. D-Limonene: A Major Bioactive Constituent in Allium Fistulosum Identified by GC-MS Analysis. J. Phytopharm. 2019, 8, 257–259. [Google Scholar] [CrossRef]
- De Whalley, C.V.; Rankin, S.M.; Hoult, J.R.S.; Jessup, W.; Leake, D.S. Flavonoids Inhibit the Oxidative Modification of Low Density Lipoproteins by Macrophages. Biochem. Pharmacol. 1990, 39, 1743–1750. [Google Scholar] [CrossRef]
- Piechowiak, T.; Grzelak-Błaszczyk, K.; Bonikowski, R.; Balawejder, M. Optimization of Extraction Process of Antioxidant Compounds from Yellow Onion Skin and Their Use in Functional Bread Production. LWT 2020, 117, 108614. [Google Scholar] [CrossRef]
- Zolfaghari, B.; Yazdiniapour, Z.; Sadeghi, M.; Akbari, M.; Troiano, R.; Lanzotti, V. Cinnamic Acid Derivatives from Welsh Onion (Allium Fistulosum) and Their Antibacterial and Cytotoxic Activities. Phytochem. Anal. 2021, 32, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Lanzotti, V.; Scala, F.; Bonanomi, G. Compounds from Allium Species with Cytotoxic and Antimicrobial Activity. Phytochem. Rev. 2014, 13, 769–791. [Google Scholar] [CrossRef]
- Țigu, A.B.; Moldovan, C.S.; Toma, V.-A.; Farcaș, A.D.; Moț, A.C.; Jurj, A.; Fischer-Fodor, E.; Mircea, C.; Pârvu, M. Phytochemical Analysis and in Vitro Effects of Allium Fistulosum L. And Allium Sativum L. Extracts on Human Normal and Tumor Cell Lines: A Comparative Study. Molecules 2021, 26, 574. [Google Scholar] [CrossRef]
- Chang, T.-C.; Jang, H.-D.; Lin, W.-D.; Duan, P.-F. Antifungal Activities of Commercial Rice Wine Extracts of Taiwanese Allium Fistulosum. Adv. Microbiol. 2016, 6, 471. [Google Scholar] [CrossRef]
- Karabegović, I.; Nikolova, M.; Veličković, D.; Stojičević, S.; Veljković, V.; Lazić, M. Comparison of Antioxidant and Antimicrobial Activities of Methanolic Extracts of the Artemisia Sp. Recovered by Different Extraction Techniques. Chin. J. Chem. Eng. 2011, 19, 504–511. [Google Scholar] [CrossRef]
- Štajner, D.; Milić, N.; Čanadanović-Brunet, J.; Kapor, A.; Štajner, M.; Popović, B.M. Exploring Allium Species as a Source of Potential Medicinal Agents. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2006, 20, 581–584. [Google Scholar]
- Yusoff, I.M.; Mat Taher, Z.; Rahmat, Z.; Chua, L.S. A Review of Ultrasound-Assisted Extraction for Plant Bioactive Compounds: Phenolics, Flavonoids, Thymols, Saponins and Proteins. Food Res. Int. 2022, 157, 111268. [Google Scholar] [CrossRef]
- Carrera, C.; Ruiz-Rodríguez, A.; Palma, M.; Barroso, C.G. Ultrasound Assisted Extraction of Phenolic Compounds from Grapes. Anal. Chim. Acta 2012, 732, 100–104. [Google Scholar] [CrossRef]
- Barbosa-Cánovas, G.V.; Donsì, F.; Yildiz, S.; Candoğan, K.; Pokhrel, P.R.; Guadarrama-Lezama, A.Y. Nonthermal Processing Technologies for Stabilization and Enhancement of Bioactive Compounds in Foods. Food Eng. Rev. 2021, 14, 63–99. [Google Scholar] [CrossRef]
- Chemat, F.; Khan, M.K. Applications of Ultrasound in Food Technology: Processing, Preservation and Extraction. Ultrason. Sonochem. 2011, 18, 813–835. [Google Scholar] [CrossRef]
- Chen, F.; Sun, Y.; Zhao, G.; Liao, X.; Hu, X.; Wu, J.; Wang, Z. Optimization of Ultrasound-Assisted Extraction of Anthocyanins in Red Raspberries and Identification of Anthocyanins in Extract Using High-Performance Liquid Chromatography—Mass Spectrometry. Ultrason. Sonochem. 2007, 14, 767–778. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Baik, O.D. Application of Ultrasound as Pretreatment for Extraction of Podophyllotoxin from Rhizomes of Podophyllum Peltatum. Ultrason. Sonochem. 2012, 19, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Cirak, C.; Radusiene, J.; Raudone, L.; Vilkickyte, G.; Seyis, F.; Marksa, M.; Ivanauskas, L.; Yayla, F. Phenolic Compounds and Antioxidant Activity of Achillea Arabica Populations. S. Afr. J. Bot. 2022, 147, 425–433. [Google Scholar] [CrossRef]
- Ghitescu, R.E.; Volf, I.; Carausu, C.; Bühlmann, A.M.; Gilca, I.A.; Popa, V.I. Optimization of Ultrasound-Assisted Extraction of Polyphenols from Spruce Wood Bark. Ultrason. Sonochem. 2015, 22, 535–541. [Google Scholar] [CrossRef]
- Paz, J.E.W.; Márquez, D.B.M.; Ávila, G.C.G.M.; Cerda, R.E.B.; Aguilar, C.N. Ultrasound-Assisted Extraction of Polyphenols from Native Plants in the Mexican Desert. Ultrason. Sonochem. 2015, 22, 474–481. [Google Scholar]
- D’Alessandro, L.G.; Kriaa, K.; Nikov, I.; Dimitrov, K. Ultrasound Assisted Extraction of Polyphenols from Black Chokeberry. Sep. Purif Technol. 2012, 93, 42–47. [Google Scholar] [CrossRef]
- Chien, W.-J.; Saputri, D.S.; Lin, H.-Y. Valorization of Taiwan’s Citrus Depressa Hayata Peels as a Source of Nobiletin and Tangeretin Using Simple Ultrasonic-Assisted Extraction. Curr. Res. Food Sci. 2022, 5, 278–287. [Google Scholar] [CrossRef]
- Hemwimol, S.; Pavasant, P.; Shotipruk, A. Ultrasound-Assisted Extraction of Anthraquinones from Roots of Morinda Citrifolia. Ultrason. Sonochem. 2006, 13, 543–548. [Google Scholar] [CrossRef]
- Mrkonjić, Ž.; Rakić, D.; Olgun, E.O.; Canli, O.; Kaplan, M.; Teslić, N.; Zeković, Z.; Pavlić, B. Optimization of Antioxidants Recovery from Wild Thyme (Thymus Serpyllum L.) by Ultrasound-Assisted Extraction: Multi-Response Approach. J. Appl. Res. Med. Aromat. Plants 2021, 24, 100333. [Google Scholar] [CrossRef]
- Altemimi, A.; Watson, D.G.; Choudhary, R.; Dasari, M.R.; Lightfoot, D.A. Ultrasound Assisted Extraction of Phenolic Compounds from Peaches and Pumpkins. PLoS ONE 2016, 11, e0148758. [Google Scholar] [CrossRef]
- Shreelakshmi, S.V.; Nazareth, M.S.; Matam, P.; Dorairaj, D.; Shetty, N.P. Chemometric Evaluation of Functional Components and Anti-quorum Sensing Activity of Mulberry Leaves from Indian Cultivars: A Potential Contribution to the Food Industry. J. Sci. Food Agric. 2022, 102, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- ICH. Guideline Q2(R2) on Validation of Analytical Procedures. ICH Qual. Guidel. 2017, 5, 127–166. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.-E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Sierra, L.J.; Córdoba, Y.; Mejía, J.J.; Rueda, E.Q.; Ocazionez, R.E.; Avila-Acevedo, J.G.; García-Bores, A.M.; Espinosa-González, A.M.; del Carmen Benítez-Flores, J.; del Rosario González-Valle, M.; et al. Photoprotective Activity of Ipomoea Horsfalliae Flower Extract. Rev. Bras. Farmacogn. 2020, 30, 69–79. [Google Scholar] [CrossRef]
- Martínez-Patiño, J.C.; Gullón, B.; Romero, I.; Ruiz, E.; Brnčić, M.; Žlabur, J.Š.; Castro, E. Optimization of Ultrasound-Assisted Extraction of Biomass from Olive Trees Using Response Surface Methodology. Ultrason. Sonochem. 2019, 51, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound Assisted Extraction (UAE) of Bioactive Compounds from Fruit and Vegetable Processing by-Products: A Review. Ultrason. Sonochem. 2021, 70, 105325. [Google Scholar] [CrossRef]
- Hanen, N.; Fattouch, S.; Ammar, E.; Neffati, M. Allium Species, Ancient Health Food for the Future? In Scientific, Health and Social Aspects of the Food Industry; Valdez, B., Ed.; Intech: London, UK, 2012; pp. 343–354. [Google Scholar]
- Gallo, M.; Formato, A.; Giacco, R.; Riccardi, G.; Luongo, D.; Formato, G.; Amoresano, A.; Naviglio, D. Mathematical Optimization of the Green Extraction of Polyphenols from Grape Peels through a Cyclic Pressurization Process. Heliyon 2019, 5, e01526. [Google Scholar] [CrossRef]
- Tomšik, A.; Pavlić, B.; Vladić, J.; Ramić, M.; Brindza, J.; Vidović, S. Optimization of Ultrasound-Assisted Extraction of Bioactive Compounds from Wild Garlic (Allium Ursinum L.). Ultrason. Sonochem. 2016, 29, 502–511. [Google Scholar] [CrossRef]
- González-De-peredo, A.V.; Vázquez-Espinosa, M.; Espada-Bellido, E.; Ferreiro-González, M.; Carrera, C.; Barbero, G.F.; Palma, M. Development of Optimized Ultrasound-Assisted Extraction Methods for the Recovery of Total Phenolic Compounds and Anthocyanins from Onion Bulbs. Antioxidants 2021, 10, 1755. [Google Scholar] [CrossRef] [PubMed]
- Bordin Viera, V.; Piovesan, N.; Mello, R.D.O.; Barin, J.S.; Fogaça, A.D.O.; Bizzi, C.A.; de Moraes Flores, É.M.; dos Santos Costa, A.C.; Pereira, D.E.; Soares, J.K.B.; et al. Ultrasonic _assisted Extraction of Phenolic Compounds with Evaluation of Red Onion Skin (Allium Cepa L.) Antioxidant Capacity. J. Culin. Sci. Technol. 2021, 1–17. [Google Scholar] [CrossRef]
- Siddeeg, A.; AlKehayez, N.M.; Abu-Hiamed, H.A.; Al-Sanea, E.A.; AL-Farga, A.M. Mode of Action and Determination of Antioxidant Activity in the Dietary Sources: An Overview. Saudi J. Biol. Sci. 2021, 28, 1633–1644. [Google Scholar] [CrossRef] [PubMed]
- Sadeer, N.B.; Montesano, D.; Albrizio, S.; Zengin, G.; Mahomoodally, M.F. The Versatility of Antioxidant Assays in Food Science and Safety—Chemistry, Applications, Strengths, and Limitations. Antioxidants 2020, 9, 709. [Google Scholar] [CrossRef]
- Siddiq, M.; Roidoung, S.; Sogi, D.S.; Dolan, K.D. Total Phenolics, Antioxidant Properties and Quality of Fresh-Cut Onions (Allium Cepa L.) Treated with Mild-Heat. Food Chem. 2013, 136, 803–806. [Google Scholar] [CrossRef] [PubMed]
- Santas, J.; Carbó, R.; Gordon, M.H.; Almajano, M.P. Comparison of the Antioxidant Activity of Two Spanish Onion Varieties. Food Chem. 2008, 107, 1210–1216. [Google Scholar] [CrossRef]
- Rodríguez Galdón, B.; Rodríguez Rodríguez, E.M.; Díaz Romero, C. Flavonoids in Onion Cultivars (Allium Cepa L.). J. Food Sci. 2008, 73, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Slimestad, R.; Fossen, T.; Vågen, I.M. Onions: A Source of Unique Dietary Flavonoids. J. Agric. Food Chem. 2007, 55, 10067–10080. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.-S.; Towantakavanit, K.; Kowalska, T.; Jung, S.-T.; Ham, K.-S.; Heo, B.-G.; Cho, J.-Y.; Yun, J.-G.; Kim, H.-J.; Gorinstein, S. Bioactive Compounds and Antioxidant and Antiproliferative Activities of Korean White Lotus Cultivars. J. Med. Food 2009, 12, 1057–1064. [Google Scholar] [CrossRef] [PubMed]
- Kuntzler, S.G.; Costa, J.A.V.; de Morais, M.G. Development of Electrospun Nanofibers Containing Chitosan/PEO Blend and Phenolic Compounds with Antibacterial Activity. Int. J. Biol. Macromol. 2018, 117, 800–806. [Google Scholar] [CrossRef] [PubMed]
- Baltacıoğlu, H.; Baltacıoğlu, C.; Okur, I.; Tanrıvermiş, A.; Yalıç, M. Optimization of Microwave-Assisted Extraction of Phenolic Compounds from Tomato: Characterization by FTIR and HPLC and Comparison with Conventional Solvent Extraction. Vib. Spectrosc. 2021, 113, 103204. [Google Scholar] [CrossRef]







| n | Ethanol Concentration (% v/v) | Extraction Time (min) | Temperature (°C) | TPC a,* (mg GAE/100 g) | DPPH b,* (mg Trolox/100 g) |
|---|---|---|---|---|---|
| 1 | 35.0 | 10.0 | 30.0 | 16.87 (±1.66) | 13.09 (±1.38) |
| 2 | 0.0 | 10.0 | 30.0 | 25.32 (±1.56) | 16.69 (±0.69) |
| 3 | 70.0 | 30.0 | 30.0 | 40.35 (±1.97) | 20.54 (±0.46) |
| 4 | 0.0 | 30.0 | 30.0 | 41.14 (±0.43) | 18.00 (±0.32) |
| 5 | 0.0 | 20.0 | 30.0 | 38.95 (±0.58) | 19.09 (±1.65) |
| 6 | 35.0 | 20.0 | 60.0 | 45.05 (±2.68) | 20.56 (±3.21) |
| 7 | 0.0 | 10.0 | 45.0 | 41.03 (±0.47) | 26.49 (±3.60) |
| 8 | 35.0 | 30.0 | 60.0 | 42.32 (±1.29) | 28.61 (±1.13) |
| 9 | 0.0 | 30.0 | 60.0 | 40.43 (±2.07) | 29.90 (±0.81) |
| 10 | 70.0 | 20.0 | 45.0 | 20.56 (±4.12) | 16.13 (±1.99) |
| 11 | 70.0 | 30.0 | 45.0 | 27.85 (±1.75) | 22.34 (±0.82) |
| 12 | 70.0 | 30.0 | 60.0 | 42.88 (±4.28) | 22.21 (±2.77) |
| 13 | 35.0 | 20.0 | 45.0 | 47.47 (±4.17) | 25.42 (±1.71) |
| 14 | 35.0 | 10.0 | 60.0 | 44.06 (±2.79) | 24.19 (±1.62) |
| 15 | 0.0 | 20.0 | 45.0 | 46.29 (±1.71) | 25.61 (±1.62) |
| 16 | 70.0 | 20.0 | 60.0 | 46.52 (±4.21) | 32.59 (±0.94) |
| 17 | 0.0 | 10.0 | 60.0 | 44.89 (±1.15) | 30.81 (±2.50) |
| 18 | 70.0 | 20.0 | 30.0 | 45.76 (±0.54) | 30.93 (±0.55) |
| 19 | 70.0 | 10.0 | 30.0 | 36.30 (±1.10) | 22.26 (±1.25) |
| 20 | 35.0 | 20.0 | 30.0 | 40.05 (±0.59) | 24.63 (±2.77) |
| 21 | 70.0 | 10.0 | 45.0 | 46.33 (±1.46) | 25.93 (±0.74) |
| 22 | 0.0 | 20.0 | 60.0 | 39.93 (±2.44) | 23.52 (±4.10) |
| 23 | 35.0 | 30.0 | 45.0 | 43.53 (±0.95) | 25.33 (±0.44) |
| 24 | 35.0 | 10.0 | 45.0 | 43.15 (±2.40) | 22.57 (±2.26) |
| 25 | 0.0 | 30.0 | 45.0 | 50.99 (±1.59) | 33.78 (±1.80) |
| 26 | 35.0 | 30.0 | 30.0 | 53.83 (±2.36) | 33.00 (±0.60) |
| 27 | 70.0 | 10.0 | 60.0 | 50.08 (±2.11) | 32.09 (±2.73) |
| Source | Sum of Squares | Degrees Freedom | Mean Square | Error | F-Value | p-Value |
|---|---|---|---|---|---|---|
| x1: Concentration | 882.013 | 1 | 882.013 | 0.92113 | 77.00 | 0.0000 |
| x2: time | 590.346 | 1 | 590.346 | 0.92113 | 51.54 | 0.0000 |
| x3: temperature | 2374.35 | 1 | 2374.35 | 0.92113 | 207.28 | 0.0000 |
| x2x3 | 844.309 | 1 | 844.309 | 1.12815 | 73.71 | 0.0000 |
| x32 | 247.87 | 1 | 247.870 | 1.59545 | 21.64 | 0.0014 |
| Lack of fit | 1070.07 | 21 | 50.956 | 4.45 | 0.0000 | |
| Pure error | 618.548 | 54 | 11.455 | |||
| Total | 6627.51 | 80 |
| Source | Sum of Squares | Degrees Freedom | Mean Square | Error | F-Value | p-Value |
|---|---|---|---|---|---|---|
| x1: Concentration | 418.964 | 1 | 418.964 | 0.52780 | 111.40 | 0.0000 |
| x2: time | 60.3885 | 1 | 60.3885 | 0.52780 | 16.06 | 0.0002 |
| x3: temperature | 1484.14 | 1 | 1484.14 | 0.52780 | 394.64 | 0.0000 |
| x2x3 | 73.4735 | 1 | 73.4735 | 0.64642 | 19.54 | 0.0000 |
| x32 | 158.717 | 1 | 158.717 | 0.91418 | 42.20 | 0.0026 |
| Lack of fit | 204.384 | 21 | 9.73256 | 2.59 | ||
| Pure error | 203.081 | 54 | 3.76077 | |||
| Total | 2603.14 | 80 |
| Optimal Conditions | Results | |||
|---|---|---|---|---|
| a [Et] (% v/v) | b time (min) | c T (°C) | d TPC (mg GAE/100 g) | e DPPH (mg Trolox/100 g) |
| Experimental values | ||||
| 70 | 10 | 60 | 51.33 (±2.54) * | 32.35 (±1.47) * |
| Predicted values | ||||
| 70 | 10 | 60 | 51.78 | 34.07 |
| Phenolic Compounds | tR (min) | Concentration (mg kg−1) |
|---|---|---|
| p-Coumaric acid | 3.6 | 0.70 (±0.01) |
| Ferulic acid | 3.7 | 1.85 (±0.21) |
| Quercetin | 4.5 | 0.55 (±0.21) |
| Cyanidin | 3.8 | 6.00 (±0.56) |
| Kaempferol | 4.9 | 0.30 (±0.14) |
| Quercetin-3-glucoside | 3.6 | 4.40 (±0.28) |
| Kaempferol-3-glucoside | 3.5 | 1.20 (±0.14) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medina-Jaramillo, C.; Gomez-Delgado, E.; López-Córdoba, A. Improvement of the Ultrasound-Assisted Extraction of Polyphenols from Welsh Onion (Allium fistulosum) Leaves Using Response Surface Methodology. Foods 2022, 11, 2425. https://doi.org/10.3390/foods11162425
Medina-Jaramillo C, Gomez-Delgado E, López-Córdoba A. Improvement of the Ultrasound-Assisted Extraction of Polyphenols from Welsh Onion (Allium fistulosum) Leaves Using Response Surface Methodology. Foods. 2022; 11(16):2425. https://doi.org/10.3390/foods11162425
Chicago/Turabian StyleMedina-Jaramillo, Carolina, Edward Gomez-Delgado, and Alex López-Córdoba. 2022. "Improvement of the Ultrasound-Assisted Extraction of Polyphenols from Welsh Onion (Allium fistulosum) Leaves Using Response Surface Methodology" Foods 11, no. 16: 2425. https://doi.org/10.3390/foods11162425