Algae as Food in Europe: An Overview of Species Diversity and Their Application †
Abstract
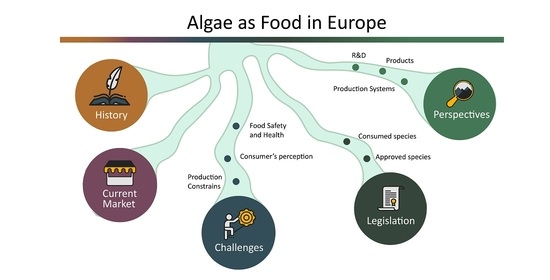
:1. Introduction
2. Algae as Food, Not as a Novelty
2.1. Historical Perspective
2.2. Current EU Market Data
2.2.1. Microalgae
2.2.2. Seaweed
3. Challenges of Algae as Food
3.1. Production Constraints
3.2. Food Safety and Health
3.2.1. Toxicity and Allergens
3.2.2. Mineral Contaminants
3.2.3. Microbiological Contaminants
3.3. Consumer’s Perception
4. Legislation on Edible Algae Species
4.1. Legislation for Algae Consumption in Some Algae-Consuming Countries
4.2. EU Legislation
4.3. Edible Algae Species in Europe
5. Perspectives of the Consumption of New Species
5.1. Research and Development
- i.
- To increase the risk-benefit seaweed analyses, with added knowledge on the speciation of iodine/chemical form, and bioavailability;
- ii.
- To standardize and define the chemical compound classes, activities, traceability, methods, and species identification;
- iii.
- To further investigate the domestication of new species, the effects of preservation methods and treatments on biomass, and to define the best storage procedures and best practices for the evaluation of product shelf-life; and
- iv.
- To implement sensory evaluation panels [65].
5.2. Advances in Technology Production
5.3. Algae Food Products
5.4. Future Perspectives
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Caporgno, M.P.; Mathys, A. Trends in Microalgae Incorporation Into Innovative Food Products With Potential Health Benefits. Front. Nutr. 2018, 5, 58. [Google Scholar] [CrossRef] [PubMed]
- Bleakley, S.; Hayes, M. Algal Proteins: Extraction, Application, and Challenges Concerning Production. Foods 2017, 6, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dillehay, T.D.; Ramírez, C.; Pino, M.; Collins, M.B.; Rossen, J.; Pino-Navarro, J.D. Monte Verde: Seaweed, Food, Medicine, and the Peopling of South America. Science 2008, 320, 784–786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalilieh, H.S.; Boulos, A. A glimpse on the uses of seaweeds in islamic science and daily life during the classical period. Arab. Sci. Philos. 2006, 16, 91–101. [Google Scholar] [CrossRef]
- Pereira, L. Edible Seaweeds of the World; CRC Press: Boca Raton, FL, USA, 2016; ISBN 9780429154041. [Google Scholar]
- Richmond, A.E.; Soeder, C.J. Microalgaculture. Crit. Rev. Biotechnol. 1986, 4, 369–438. [Google Scholar] [CrossRef]
- Boukid, F.; Castellari, M. Food and Beverages Containing Algae and Derived Ingredients Launched in the Market from 2015 to 2019: A Front-of-Pack Labeling Perspective with a Special Focus on Spain. Foods 2021, 10, 173. [Google Scholar] [CrossRef]
- Alexandratos, N.; Bruinsma, J. World Agriculture towards 2030/2050: The 2012 Revision; ESA Working Paper No. 12-03; FAO: Rome, Italy, 2012. [Google Scholar]
- Norsker, N.-H.; Barbosa, M.J.; Vermuë, M.H.; Wijffels, R.H. Microalgal production—A close look at the economics. Biotechnol. Adv. 2011, 29, 24–27. [Google Scholar] [CrossRef]
- Smith, A.G.; Tredici, M.R.; Boussiba, S.; Verdelho, V.; Cadoret, J.-P.; Davey, M.P.; Huete-Ortega, M.; Acien, F.G.; Schmid-Staiger, U.; Rodriguez, H.; et al. EABA—Position Paper—What Are Algae? EABA: Florence, Italy, 2020. [Google Scholar]
- Araújo, R.; Vázquez Calderón, F.; Sánchez López, J.; Azevedo, I.C.; Bruhn, A.; Fluch, S.; Garcia Tasende, M.; Ghaderiardakani, F.; Ilmjärv, T.; Laurans, M.; et al. Current Status of the Algae Production Industry in Europe: An Emerging Sector of the Blue Bioeconomy. Front. Mar. Sci. 2021, 7, 626389. [Google Scholar] [CrossRef]
- Fernández, F.G.A.; Reis, A.; Wijffels, R.H.; Barbosa, M.; Verdelho, V.; Llamas, B. The role of microalgae in the bioeconomy. New Biotechnol. 2021, 61, 99–107. [Google Scholar] [CrossRef]
- Fédération des Spiruliniers de France. Available online: https://www.spiruliniersdefrance.fr/ (accessed on 9 May 2022).
- Mouritsen, O.G.; Rhatigan, P.; Pérez-Lloréns, J.L. The rise of seaweed gastronomy: Phycogastronomy. Bot. Mar. 2019, 62, 195–209. [Google Scholar] [CrossRef]
- ALGAplus Products. Available online: https://www.algaplus.pt/produtos/ (accessed on 10 April 2021).
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef] [Green Version]
- Peñalver, R.; Lorenzo, J.M.; Ros, G.; Amarowicz, R.; Pateiro, M.; Nieto, G. Seaweeds as a functional ingredient for a healthy diet. Marine Drugs. Mar. Drugs 2020, 18, 301. [Google Scholar] [CrossRef]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Nova, P.; Martins, A.P.; Teixeira, C.; Abreu, H.; Silva, J.G.; Silva, A.M.; Freitas, A.C.; Gomes, A.M. Foods with microalgae and seaweeds fostering consumers health: A review on scientific and market innovations. J. Appl. Phycol. 2020, 32, 1789–1802. [Google Scholar] [CrossRef]
- Ścieszka, S.; Klewicka, E. Algae in food: A general review. Crit. Rev. Food Sci. Nutr. 2019, 59, 3538–3547. [Google Scholar] [CrossRef]
- Barbier, M.; Charrier, B.; Araujo, R.; Holdt, S.L.; Bertrand, J.; Céline, R. PEGASUS-PHYCOMORPH European Guidelines for a Sustainable Aquaculture of Seaweeds. COST Action FA1406; Barbier, M., Charrier, B., Eds.; HAL Open Science: Lyon, France, 2019. [Google Scholar]
- Mintel. Seaweed-flavoured food and drink launches increased by 147% in Europe between 2011 and 2015. Available online: http://www.mintel.com/press-centre/food-and-drink/seaweed-flavoured-food-and-drink-launches-increased-by-147-in-europe-between-2011-and-2015 (accessed on 9 March 2016).
- Kumari, P.; Kumar, M.; Reddy, C.R.K.; Jha, B. Algal lipids, fatty acids and sterols. In Functional Ingredients from Algae for Foods and Nutraceuticals; Elsevier: Amsterdam, The Netherlands, 2013; pp. 87–134. [Google Scholar]
- Wielgosz-Collin, G.; Kendel, M.; Couzinet-Mossion, A. Lipids, fatty acids, glycolipids, and phospholipids. In Seaweed in Health and Disease Prevention; Fleurence, J., Levine, I., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 185–221. [Google Scholar]
- Marik, P.E.; Varon, J. Omega-3 Dietary Supplements and the Risk of Cardiovascular Events: A Systematic Review. Clin. Cardiol. 2009, 32, 365–372. [Google Scholar] [CrossRef]
- Huang, H.-L.; Wang, B.-G. Antioxidant Capacity and Lipophilic Content of Seaweeds Collected from the Qingdao Coastline. J. Agric. Food Chem. 2004, 52, 4993–4997. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Ascherio, A.; Hu, F.B.; Stampfer, M.J.; Willett, W.C.; Siscovick, D.S.; Rimm, E.B. Interplay Between Different Polyunsaturated Fatty Acids and Risk of Coronary Heart Disease in Men. Circulation 2005, 111, 157–164. [Google Scholar] [CrossRef] [Green Version]
- Sakamoto, A.; Saotome, M.; Iguchi, K.; Maekawa, Y. Marine-derived omega-3 polyunsaturated fatty acids and heart failure: Current understanding for basic to clinical relevance. Int. J. Mol. Sci. 2019, 20, 4025. [Google Scholar] [CrossRef] [Green Version]
- Freitas, R.; Campos, M.M. Protective Effects of Omega-3 Fatty Acids in Cancer-Related Complications. Nutrients 2019, 11, 945. [Google Scholar] [CrossRef] [Green Version]
- Rennie, K.L.; Hughes, J.; Lang, R.; Jebb, S.A. Nutritional management of rheumatoid arthritis: A review of the evidence. J. Hum. Nutr. Diet. 2003, 16, 97–109. [Google Scholar] [CrossRef]
- SanGiovanni, J.P.; Parra-Cabrera, S.; Colditz, G.A.; Berkey, C.S.; Dwyer, J.T. Meta-analysis of Dietary Essential Fatty Acids and Long-Chain Polyunsaturated Fatty Acids as They Relate to Visual Resolution Acuity in Healthy Preterm Infants. Pediatrics 2000, 105, 1292–1298. [Google Scholar] [CrossRef]
- Barros, R.; Moreira, A.; Fonseca, J.; Delgado, L.; Graça Castel-Branco, M.; Haahtela, T.; Lopes, C.; Moreira, P. Dietary intake of α-linolenic acid and low ratio of n-6:n-3 PUFA are associated with decreased exhaled NO and improved asthma control. Br. J. Nutr. 2011, 106, 441–450. [Google Scholar] [CrossRef] [Green Version]
- Tsai, C.-J.; Sun Pan, B. Identification of Sulfoglycolipid Bioactivities and Characteristic Fatty Acids of Marine Macroalgae. J. Agric. Food Chem. 2012, 60, 8404–8410. [Google Scholar] [CrossRef]
- da Costa, E.; Melo, T.; Moreira, A.; Bernardo, C.; Helguero, L.; Ferreira, I.; Cruz, M.; Rego, A.; Domingues, P.; Calado, R.; et al. Valorization of Lipids from Gracilaria sp. through Lipidomics and Decoding of Antiproliferative and Anti-Inflammatory Activity. Mar. Drugs 2017, 15, 62. [Google Scholar] [CrossRef] [Green Version]
- Paiva, L.; Lima, E.; Patarra, R.F.; Neto, A.I.; Baptista, J. Edible Azorean macroalgae as source of rich nutrients with impact on human health. Food Chem. 2014, 164, 128–135. [Google Scholar] [CrossRef]
- Patarra, R.F.; Leite, J.; Pereira, R.; Baptista, J.; Neto, A.I. Fatty acid composition of selected macrophytes. Nat. Prod. Res. 2013, 27, 665–669. [Google Scholar] [CrossRef]
- Paiva, L.; Lima, E.; Neto, A.I.; Marcone, M.; Baptista, J. Health-promoting ingredients from four selected Azorean macroalgae. Food Res. Int. 2016, 89, 432–438. [Google Scholar] [CrossRef]
- Paiva, L.; Lima, E.; Neto, A.I.; Marcone, M.; Baptista, J. Nutritional and functional bioactivity value of selected Azorean macroalgae: Ulva compressa, Ulva rigida, Gelidium microdon, and Pterocladiella capillacea. J. Food Sci. 2017, 82, 1757–1764. [Google Scholar] [CrossRef]
- Torres-Tiji, Y.; Fields, F.J.; Mayfield, S.P. Microalgae as a future food source. Biotechnol. Adv. 2020, 41, 107536. [Google Scholar] [CrossRef]
- Becker, E.W. Micro-algae as a source of protein. Biotechnol. Adv. 2007, 25, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Koyande, A.K.; Chew, K.W.; Rambabu, K.; Tao, Y.; Chu, D.-T.; Show, P.-L. Microalgae: A potential alternative to health supplementation for humans. Food Sci. Hum. Wellness 2019, 8, 16–24. [Google Scholar] [CrossRef]
- Samarakoon, K.; Jeon, Y.-J. Bio-functionalities of proteins derived from marine algae—A review. Food Res. Int. 2012, 48, 948–960. [Google Scholar] [CrossRef]
- Admassu, H.; Gasmalla, M.A.A.; Yang, R.; Zhao, W. Bioactive Peptides Derived from Seaweed Protein and Their Health Benefits: Antihypertensive, Antioxidant, and Antidiabetic Properties. J. Food Sci. 2018, 83, 6–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ursu, A.-V.; Marcati, A.; Sayd, T.; Sante-Lhoutellier, V.; Djelveh, G.; Michaud, P. Extraction, fractionation and functional properties of proteins from the microalgae Chlorella vulgaris. Bioresour. Technol. 2014, 157, 134–139. [Google Scholar] [CrossRef]
- Ovando, C.A.; de Carvalho, J.C.; Vinícius de Melo Pereira, G.; Jacques, P.; Soccol, V.T.; Soccol, C.R. Functional properties and health benefits of bioactive peptides derived from Spirulina: A review. Int. Food Res. J. 2018, 34, 34–51. [Google Scholar] [CrossRef]
- Fleurence, J. Seaweed proteins. Trends Food Sci. Technol. 1999, 10, 25–28. [Google Scholar] [CrossRef]
- Canelli, G.; Tarnutzer, C.; Carpine, R.; Neutsch, L.; Bolten, C.J.; Dionisi, F.; Mathys, A. Biochemical and nutritional evaluation of Chlorella and Auxenochlorella biomasses relevant for food application. Front. Nutr. 2020, 7, 168. [Google Scholar] [CrossRef]
- Usman, A.; Khalid, S.; Usman, A.; Hussain, Z.; Wang, Y. Algal Polysaccharides, Novel Application, and Outlook. In Algae Based Polymers, Blends, and Composites: Chemistry, Biotechnology and Materials Science; Elsevier: Amsterdam, The Netherlands, 2017; pp. 115–153. ISBN 9780128123607. [Google Scholar]
- Xu, S.-Y.; Huang, X.; Cheong, K.-L. Recent Advances in Marine Algae Polysaccharides: Isolation, Structure, and Activities. Mar. Drugs 2017, 15, 388. [Google Scholar] [CrossRef] [Green Version]
- Hayes, M.; Tiwari, B. Bioactive Carbohydrates and Peptides in Foods: An Overview of Sources, Downstream Processing Steps and Associated Bioactivities. Int. J. Mol. Sci. 2015, 16, 22485–22508. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Vaquero, M.; Rajauria, G.; O’Doherty, J.V.; Sweeney, T. Polysaccharides from macroalgae: Recent advances, innovative technologies and challenges in extraction and purification. Int. Food Res. J. 2017, 99, 1011–1020. [Google Scholar] [CrossRef] [Green Version]
- MacArtain, P.; Gill, C.; Brooks, M.; Campbell, R.; Rowland, I.R. Nutritional Value of Edible Seaweeds. Nutr. Rev. 2007, 65, 535–543. [Google Scholar] [CrossRef]
- Devillé, C.; Gharbi, M.; Dandrifosse, G.; Peulen, O. Study on the effects of laminarin, a polysaccharide from seaweed, on gut characteristics. J. Sci. Food Agric. 2007, 87, 1717–1725. [Google Scholar] [CrossRef]
- Walsh, A.M.; Sweeney, T.; O’Shea, C.J.; Doyle, D.N.; O’Doherty, J.V. Effect of dietary laminarin and fucoidan on selected microbiota, intestinal morphology and immune status of the newly weaned pig. Br. J. Nutr. 2013, 110, 1630–1638. [Google Scholar] [CrossRef] [Green Version]
- Koyande, A.K.; Show, P.L.; Guo, R.; Tang, B.; Ogino, C.; Chang, J.S. Bio-processing of algal bio-refinery: A review on current advances and future perspectives. Bioengineered 2019, 10, 574–592. [Google Scholar] [CrossRef] [Green Version]
- Abe, J.-I.; Kotla, S.; Yu, J.; Baker, A.B.; Patil, N.P.; Le, V.; Sligar, A.D.; Mei, L.; Chavarria, D.; Yang, E.Y. Algal Polysaccharides as Therapeutic Agents for Atherosclerosis. Front. Cardiovasc. Med. 2018, 5, 153. [Google Scholar] [CrossRef] [Green Version]
- Shanura Fernando, I.P.; Kim, K.-N.; Kim, D.; Jeon, Y.-J. Algal polysaccharides: Potential bioactive substances for cosmeceutical applications. Crit. Rev. Biotechnol. 2019, 39, 99–113. [Google Scholar] [CrossRef]
- Kain (Jones), J.M.; Dawes, C.P. Useful European seaweeds: Past hopes and present cultivation. Hydrobiologia 1987, 151–152, 173–181. [Google Scholar] [CrossRef]
- Glicksman, M. Utilization of seaweed hydrocolloids in the food industry. In Twelfth International Seaweed Symposium; Ragan, M.A., Bird, C.J., Eds.; Springer: Dordrecht, The Netherlands, 1987; pp. 31–47. [Google Scholar]
- Melo, R.A. Exploração dos Recursos Algológicos em Portugal. In Fragmentos em Ecologia; Martins-Loução, A., Ed.; FCUL e Livraria Escolar Editora: Lisbon, Portugal, 2002; pp. 45–65. [Google Scholar]
- Bogacz-Radomska, L.; Harasym, J.; Piwowar, A. Commercialization aspects of carotenoids. In Carotenoids: Properties, Processing and Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 327–357. [Google Scholar]
- Cotas, J.; Leandro, A.; Pacheco, D.; Gonçalves, A.M.M.; Pereira, L. A Comprehensive Review of the Nutraceutical and Therapeutic Applications of Red Seaweeds (Rhodophyta). Life 2020, 10, 19. [Google Scholar] [CrossRef] [Green Version]
- Vílchez, C.; Forján, E.; Cuaresma, M.; Bédmar, F.; Garbayo, I.; Vega, J.M. Marine Carotenoids: Biological Functions and Commercial Applications. Mar. Drugs 2011, 9, 319–333. [Google Scholar] [CrossRef] [Green Version]
- Catarina Guedes, A.; Barbosa, C.R.; Amaro, H.M.; Pereira, C.I.; Xavier Malcata, F. Microalgal and cyanobacterial cell extracts for use as natural antibacterial additives against food pathogens. Int. J. Food Sci. Technol. 2011, 46, 862–870. [Google Scholar] [CrossRef]
- Pangestuti, R.; Kim, S.-K. Biological activities and health benefit effects of natural pigments derived from marine algae. J. Funct. Foods 2011, 3, 255–266. [Google Scholar] [CrossRef]
- European Comission EU Novel Food Catalogue. Available online: https://ec.europa.eu/food/safety/novel_food/catalogue/search/public/index.cfm (accessed on 1 March 2022).
- Mouritsen, O.G.; Dawczynski, C.; Duelund, L.; Jahreis, G.; Vetter, W.; Schröder, M. On the human consumption of the red seaweed dulse (Palmaria palmata (L.) Weber & Mohr). J. Appl. Phycol. 2013, 25, 1777–1791. [Google Scholar] [CrossRef]
- Hallsson, S. The uses of seaweeds in iceland. In Proceedings of the Fourth International Seaweed Symposium, Biarritz, France, 18–24 September 1961. [Google Scholar]
- Johnston, H. The Biological and Economic Importance of Algae, Part 2. Tuatara 1966, 14, 4–6. [Google Scholar]
- Klnc, B.; Cirik, S.; Turan, G.; Tekogul, H.; Koru, E. Seaweeds for Food and Industrial Applications. In Food Industry; InTech: London, UK, 2013; pp. 735–748. [Google Scholar]
- Haroun, R.; Gil-Rodríguez, M.C.; Neto, A.I.; Machín-Sánchez, M.; Viera-Rodríguez, M.A. A review of current uses and potential biotechnological applications of seaweeds from the Macaronesian region (Central-East Atlantic Ocean). J. Appl. Phycol. 2019, 31, 3777–3790. [Google Scholar] [CrossRef]
- Kraan, S.; Lyons, H.J.; Steele, S.; Guiry, M. How Seaweed Is Used in Ireland: The Seaweed of Ireland’s Coastline; An Chomhairle Oidhreachta The Heritage Council: Kilkenny, Ireland, 2010. [Google Scholar]
- Pereira, L. Seaweed Flora of the European North Atlantic and Mediterranean. In Hb25_Springer Handbook of Marine Biotechnology; Springer: Berlin/Heidelberg, Germany, 2015; pp. 65–178. [Google Scholar] [CrossRef]
- Teas, J.; Pino, S.; Critchley, A.; Braverman, L.E. Variability of iodine content in common commercially available edible seaweeds. Thyroid 2004, 14, 836–841. [Google Scholar] [CrossRef] [PubMed]
- McDowell, L.R. Iodine. In Minerals in Animal and Human Nutrition; McDowell, L.R., Ed.; Academic Press: San Diego, CA, USA, 1992; pp. 224–245. [Google Scholar]
- Pérez-Lloréns, J.L.; Mouritsen, O.G.; Rhatigan, P.; Cornish, M.L.; Critchley, A.T. Seaweeds in mythology, folklore, poetry, and life. J. Appl. Phycol. 2020, 32, 3157–3182. [Google Scholar] [CrossRef]
- (BioMara) The Importance of Seaweed across the Ages. Available online: http://www.biomara.org/understanding-seaweed/the-importance-of-seaweed-across-the-ages.html (accessed on 1 March 2021).
- Baldwin, J.R. Working with Seaweed in North-West Sutherland. In The Province of Strathnaver; Baldwin, J.R., Thomson, W.P.L., Eds.; Scottish Society for Northern Studies: Edinburgh, Scotland, 2000; pp. 116–141. [Google Scholar]
- (Nordic Seaweed Tang) Nordic Seaweed: Nutrition in Seaweed. Available online: http://nordicseaweed.com/engelsk/eng_nutrition.html (accessed on 5 April 2021).
- Lloréns, J.L.P.; Carrero, I.H.; Oñate, J.J.V.; Murillo, F.G.B.; González, Á.L. ¿Las Algas Se Comen? Un Periplo por La Biología, La Historia, Las Curiosidades y La Gastronomía (Ceimar), 1st ed.; Servicio de Publicaciones de la Universidad de Cádiz: Cádiz, Spain, 2016. [Google Scholar]
- Patarra, A.R.F. Culture Studies of Economically Important Seaweeds; University of Azores: Ponta Delgada, Portugal, 2018. [Google Scholar]
- (Netalgae) Overview of the Seaweed Industry by Country Ireland, France, Norway, Portugal, Spain, United Kingdom; Neatalgae Report; European Union, European Regional Development Fund (EDRF), Nordland County Council: Nordland, Norway, 2012.
- Patarra, R.F.; Paiva, L.; Neto, A.I.; Lima, E.; Baptista, J. Nutritional value of selected macroalgae. J. Appl. Phycol. 2011, 23, 205–208. [Google Scholar] [CrossRef] [Green Version]
- Neto, A.I.; Tittley, I.; Raposeiro, P.M. Flora Marinha do Litoral dos Açores = Rocky Shore Marine Flora of the Azores; Secretaria Regional do Ambiente e do Mar: Horta, Portugal, 2005. [Google Scholar]
- Sousa-Pinto, I. The Seaweed Resources of Portugal. In Seaweed Resources of the World; JICA: Tokyo, Japan, 1998; pp. 176–184. [Google Scholar]
- Michanek, G. Seaweed Resources of the Ocean, 138th–, 139th ed.; Food and Agriculture Organization of the United Nations: Rome, Italy, 1975; Volume 138. [Google Scholar]
- Seaver, K.A. The Last Vikings: The Epic Story of the Great Norse Voyages; I.B.Tauris & Co., Ltd.: London, UK, 2010. [Google Scholar]
- Mouritsen, O.G.; Rhatigan, P.; Cornish, M.L.; Critchley, A.T.; Pérez-Lloréns, J.L. Saved by seaweeds: Phyconomic contributions in times of crises. J. Appl. Phycol. 2021, 33, 443–458. [Google Scholar] [CrossRef] [PubMed]
- Dickens, C.; Robertson, J.; Dickens, C. The Purple Shore. Househ. Words 1856, XIV, 391–395. [Google Scholar]
- (Mara Seaweed) A Short History of Dulse Seaweed. Available online: https://maraseaweed.com/blogs/news/a-short-history-of-dulse-seaweed (accessed on 28 March 2021).
- Pereira, L. MACOI, Portuguese Seaweeds Website; World-wide Electronic Publication, MARE UC, Department of Life Sciences, University of Coimbra: Coimbra, Portugal, 2021; Available online: http://macoi.ci.uc.pt/spec_list_detail.php?spec_id=125 (accessed on 2 April 2021).
- McHugh, D.J. Seaweeds uses as Human Foods. In A Guide to the Seaweed Industry; FAO: Rome, Italy, 2003; p. 105. [Google Scholar]
- Walsh, M.; Watson, L. A Market Analysis towards the Further Development of Seaweed Aquaculture in Ireland; Irish Sea Fisheries Board: Dublin, Ireland, 2011; Volume 1. [Google Scholar]
- Gaspar, R.; Pereira, L.; Sousa-Pinto, I. The seaweed resources of Portugal. Bot. Mar. 2019, 62, 499–525. [Google Scholar] [CrossRef]
- Palmieri, N.; Forleo, M.B. The potential of edible seaweed within the western diet. A segmentation of Italian consumers. Int. J. Gastron. Food Sci. 2020, 20, 100202. [Google Scholar] [CrossRef]
- Lentini, F.; Venza, F. Wild food plants of popular use in Sicily. J. Ethnobiol. Ethnomed. 2007, 3, 15. [Google Scholar] [CrossRef] [Green Version]
- Cappellani, S. Il Mauru Ossia Alghe Rosse Commestibili Nella Sicilia Centro-Orientale; CITEM: Metro Manila, Philippines, 1960. [Google Scholar]
- Subba Rao, P.V.; Ganesan, K.; Suresh Kumar, K. Seaweeds as a Human Diet: An Emerging Trend in the New Millennium. Adv. Appl. Phycol. 2007, 1, 85–96. [Google Scholar] [CrossRef]
- Ferdouse, F.; Lovstad Holdt, S.; Smith, R.; Murúa, P.; Yang, Z.; (FAO). The global status of seaweed production, trade and utilization. Globefish Res. Programme 2018, 124, 120. [Google Scholar]
- Calado, R.M. Macrobiótica Como e Porquê; Grafitécnica: Lisbon, Portugal, 1976. [Google Scholar]
- Kushi, L.H.; Cunningham, J.E.; Hebert, J.R.; Lerman, R.H.; Bandera, E.V.; Teas, J. The Macrobiotic Diet in Cancer. J. Nutr. 2001, 131, 3056S–3064S. [Google Scholar] [CrossRef] [Green Version]
- Varatojo, F. As Algas-Os Legumes do Mar. Available online: https://www.institutomacrobiotico.com/pt-pt/imp/artigos/alimentacao/algas (accessed on 1 March 2021).
- Fralick, R.A.; Andrade, F. The growth, reproduction, harvesting and management of Pterocladia pinnata (Rhodophyceae) in the Azores, Portugal. In Proceedings of the 10th International Seaweed Symposium, Goteborg, Sweden, 11–15 August 1980; Levring, T., Ed.; Walter de Gruyter & Co.: Gothenburg, Sweden, 1981; pp. 289–295. [Google Scholar]
- Fralick, R.A. Algas agarófitas. In Exposição Oral Sobre Desenvolvimento das Algas nos Açores; Presidência do Governo, Departamento Regional de Estudos e Planeamento, Região Autónoma dos Açores: Horta, Portugal, 1980. [Google Scholar]
- Palminha, F. Exploração e utilização de Algas Marinhas na Plataforma Portuguesa e nas Ilhas do Arquipélago dos Açores. Junta Nac. Fom. Das Pescas 1971, 7, 25–36. [Google Scholar]
- Palminha, F.; Melo, R.; Santos, R. A existência de Gelidium sesquipedale (Clem.) Born et Thur. na costa sul do Algarve. I. Distribuição local. Bol. INIP. 1982, 8, 93–105. [Google Scholar]
- Palminha, F.; Melo, R.; Santos, R. A existência de Gelidium sesquipedale (Clem.) Born et Thur. na costa sul do Algarve. II. Biomassa total. Bol. INIP. 1985, 13, 77–91. [Google Scholar]
- Santos, R.; Duarte, P. Marine plant harvest in Portugal. J. Appl. Phycol. 1991, 3, 11–18. [Google Scholar] [CrossRef]
- Marshall, S.N.; Newton, L.; Orr, A.P. A Study of Certain British Seaweeds and Their Utilisation in the Preparation of Agar., 1st ed.; His Majesty’s Stationary Office: London, UK, 1949. [Google Scholar]
- Patarra, R.F.; Iha, C.; Pereira, L.; Neto, A.I. Concise review of the species Pterocladiella capillacea (S.G. Gmelin) Santelices & Hommersand. J. Appl. Phycol. 2020, 32, 787–808. [Google Scholar]
- Burlew, J.S. Algal Culture from Laboratory to Pilot Plant. AIBS Bull. 1953, 3, 11. [Google Scholar] [CrossRef]
- Powell, R.C.; Nevels, E.M.; McDowell, M.E. Algae Feeding in Humans. J. Nutr. 1961, 75, 7–12. [Google Scholar] [CrossRef] [Green Version]
- Melo, R.A. Gelidium commercial exploitation: Natural resources and cultivation. J. Appl. Phycol. 1998, 10, 303–314. [Google Scholar] [CrossRef]
- Gao, F.Z.; Ge, B.S.; Xiang, W.Z.; Qin, S. Development of microalgal industries in the past 60 years due to biotechnological research in China: A review. Sci. Sin. Vitae 2021, 51, 26–39. [Google Scholar] [CrossRef]
- Gantar, M.; Svirčev, Z. Microalgae and cyanobacteria: Food for thought. J. Phycol. 2008, 44, 260–268. [Google Scholar] [CrossRef]
- Takenaka, H.; Yamaguchi, Y.; Sakaki, S.; Watarai, K.; Tanaka, N.; Hori, M.; Seki, H.; Tsuchida, M.; Yamada, A.; Nishimori, T.; et al. Safety evaluation of Nostoc flagelliforme (nostocales, cyanophyceae) as a potential food. Food Chem. Toxicol. 1998, 36, 1073–1077. [Google Scholar] [CrossRef]
- Aaronson, S. A role for algae as human food in antiquity. Food Foodways 1986, 1, 311–315. [Google Scholar] [CrossRef]
- Farrar, W.V. Tecuitlatl; A Glimpse of Aztec Food Technology. Nature 1966, 211, 341–342. [Google Scholar] [CrossRef]
- Abdulqader, G.; Barsanti, L.; Tredici, M.R. Harvest of Arthrospira platensis from Lake Kossorom (Chad) and its household usage among the Kanembu. J. Appl. Phycol. 2000, 12, 493–498. [Google Scholar] [CrossRef]
- Ciferri, O. Spirulina, the edible microorganism. Microbiol. Rev. 1983, 47, 551–578. [Google Scholar] [CrossRef] [PubMed]
- Piccolo, A. Spirulina A Livehood and A Business Venture; FAO/IOC: Rome, Italy, 2012; Volume 45. [Google Scholar]
- Chacón-Lee, T.L.; González-Mariño, G.E. Microalgae for “Healthy” Foods-Possibilities and Challenges. Compr. Rev. Food Sci. Food Saf. 2010, 9, 655–675. [Google Scholar] [CrossRef] [PubMed]
- Sathasivam, R.; Radhakrishnan, R.; Hashem, A.; Abd_Allah, E.F. Microalgae metabolites: A rich source for food and medicine. Saudi J. Biol. Sci. 2019, 26, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.M.; Allsopp, P.J.; Magee, P.J.; Gill, C.I.; Nitecki, S.; Strain, C.R.; McSorley, E.M. Seaweed and human health. Nutr. Rev. 2014, 72, 205–216. [Google Scholar] [CrossRef]
- Enzing, C.; Ploeg, M.; Barbosa, M.; Sijtsma, L. Microalgae-Based Products for the Food and Feed Sector: An Outlook for Europe; Vigani, M., Parisi, C., Cerezo, E.R., Eds.; Publications Office of the European Union: Luxembourg, 2014. [Google Scholar]
- Santillan, C. Mass production of Spirulina. Experientia 1982, 38, 40–43. [Google Scholar] [CrossRef]
- Geoghegan, M.J. Unicellular Algae as a Source of Food. Nature 1951, 168, 426–427. [Google Scholar] [CrossRef]
- Algae as a Source of Human Food. Nutr. Rev. 1959, 17, 238–240. [CrossRef]
- Soeder, C.J. Zur Verwendung von Mikroalgen Für Ernährungszwecke. Naturwissenschaften 1976, 63, 131–138. [Google Scholar] [CrossRef]
- Krauss, R.W. Mass Culture of Algae for Food and Other Organic Compounds. Am. J. Bot. 1962, 49, 425. [Google Scholar] [CrossRef]
- Kihlberg, R. The Microbe as a Source of Food. Annu. Rev. Microbiol. 1972, 26, 427–466. [Google Scholar] [CrossRef]
- Soeder, C.J. Massive cultivation of microalgae: Results and prospects. Hydrobiologia 1980, 72, 197–209. [Google Scholar] [CrossRef]
- Tamiya, H. Growing Chlorella for Food and Feed. In Proceedings of the World Symposium on Applied Solar Energy, Phoenix, AZ, USA; 1955; pp. 231–241. [Google Scholar]
- Tamura, E.; Baba, H.; Tamura, A.; Kobatake, Y. Nutritional Studies on Chlorella. Jpn. J. Nutr. Diet. 1959, 17, 197–198. [Google Scholar] [CrossRef]
- Tamura, E.; Matsuno, N.; Morimoto, K. Nutritional Studies on Chlorella VII Absorption Experiment of Dried Chlorella on Human Subject. Jpn. J. Nutr. Diet 1959, 17, 91–93. [Google Scholar] [CrossRef] [Green Version]
- Tamura, E.; Morimoto, K.; Tsuchida, M. Nutritional Studies on Chlorella II. Practical Use of Chlorella on Cooking. Jpn. J. Nutr. Diet 1959, 17, 21–22. [Google Scholar] [CrossRef] [Green Version]
- Soeder, C.J.; Pabst, W. Gesichtspunkte Für Die Verwendung von Mikroalgen in Der Ernährung von Mensch Und Tier 1. Ber. Dtsch. Bot. Ges. 1970, 83, 607–625. [Google Scholar]
- Space Medicine. Scott. Med. J. 1959, 4, 462–465. [CrossRef]
- Helvey, T.C. Nutrition in Space and Related Waste Problems. In Proceedings of the Conference on Nutrition in Space and Related Waste problems, Universtiy of South Florida, Tampa, FL, USA, 27–30 April 1964. [Google Scholar]
- Kondrat’ev, I.I.; Bychkov, V.P.; Ushakov, A.S.; Boĭko, N.N.; Kliushkina, N.S.; Abaturova, E.A.; Terpilovskiĭ, A.M.; Korneeva, N.A.; Beliakova, M.I.; Kasatkina, A.G. The aus der Alge Dunaliella of dried biomass of unicellular algae in human food rations. Vopr. Pitan. 1966, 25, 9–14. [Google Scholar]
- Kondrat’ev, I.I.; Bychkov, V.P.; Ushakov, A.S.; Boĭko, N.N.; Kliushkina, N.S.; Abaturova, E.A.; Terpilovskiĭ, A.M.; Korneeva, N.W.; Beliakova, M.I.; Vorob’eva, E.S.; et al. The use of 150 g dry blomass of unicellular algae in human diet. Vopr. Pitan. 1966, 25, 14–19. [Google Scholar]
- Kofrányi, E.; Jekat, F. Zur Bestimmung der biologischen Wertigkeit von Nahrungsproteinen, XII. Die Mischung von Ei mit Reis, Mais, Soja, Algen. Hoppe-Seyler´S Z. Physiol. Chem. 1967, 348, 84–88. [Google Scholar] [CrossRef]
- Müller-Wecker, H.; Kofrányi, E. Zur Bestimmung der biologischen Wertigkeit von Nahrungsproteinen, XVIII. Einzeller als zusätzliche Nahrungsquelle. Hoppe-Seyler´S Z. Physiol. Chem. 1973, 354, 1034–1042. [Google Scholar] [CrossRef]
- Fink, H.; Herold, E. Über den Biologischen Wert der Einzelligen Grünalge Scenedesmus Obliquus—Frisch Und Verschieden Getrocknet—Und Ihre Diätetischen Und Therapeutischen Eigenschaften; Schlie, I., Ed.; VS Verlag für Sozialwissenschaften: Wiesbaden, Germany, 1963; ISBN 978-3-663-06425-1. [Google Scholar]
- Berkhoff, G.; Kramer, G. 156a. Untersuchungen über den Einfluß der Algennahrung auf den Eiweißhaushalt bei Intensivpflegefällen. Langenbecks Arch. Chir. 1971, 329, 523. [Google Scholar] [CrossRef] [Green Version]
- Shelef, G.; Moraine, R.; Oron, G.; Binsack, R.; Heussler, P.; Castillo, J.; Merino, F.; Becker, E.W.; Payer, H.J.; Pithakpol, B.; et al. Microalgae for food and feed. In Proceedings of the German-Israeli Workshop, Neuherberg, Germany, 17–18 October 1977; Soeder, C.J., Binsack, R., Eds.; FAO: Rome, Italy, 1979; p. 300, ISBN 978-3-510-47009-9. [Google Scholar]
- Ahsan, M.; Habib, B.; Parvin, M.; Huntington, T.C.; Hasan, M. A Review on Culture, Production and Use of Spirulina as Food for Humans and Feeds for Domestic Animals and Fish; FAO: Rome, Italy, 2008; Volume 1034, ISBN 9789251061060. [Google Scholar]
- Oliveira, J.C. Utilisation of Macro-Algae as Vegetables and Food Additives in Portugal; Instituto Nacional de Investigação das Pescas: Lisbon, Portugal, 1992; p. 7. [Google Scholar]
- AstaReal AB The World´s Leading Producer and Innovator of Natural Astaxanthin. Available online: http://www.astareal.se/about-us (accessed on 20 April 2021).
- Fuji Chemical Industries Co. Satisfying the Global Demand for Natural Astaxanthin. Available online: https://www.fujichemical.co.jp/english/whatsnew/archive/200609/whatsnew.html (accessed on 10 April 2021).
- Indergaard, M.; Minsaas, J. Animal and human nutrition. In Seaweed Resources in Europe: Uses and Potential; Guiry, M.D., Blunden, G., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 1991; pp. 21–64. ISBN 0 471 929 476. [Google Scholar]
- Blake, W. Jerusalem the Emanation of the Giant Albion; William Blake: London, UK, 1821. [Google Scholar]
- McIrvine, D. An Erne in Erin: William Blake Depicts a “Sea Eagle” and Dillisk from County Antrim’s ‘Giants Caus[e]way’ in Plate 78 of *Jerusalem*, and Japanese Women Abalone-Diving in Plate 89; 2019; Volume 1. Available online: https://www.academia.edu/41485060/An_Erne_in_Erin_William_Blake_Depicts_a_Sea_Eagle_and_Dillisk_from_County_Antrims_Giants_caus_e_way_in_Plate_78_of_Jerusalem_and_Japanese_Women_Abalone_Diving_in_Plate_89 (accessed on 10 April 2021).
- Kelly, F.C. Iodine in Medicine and Pharmacy Since its Discovery-1811–1961. Proc. R. Soc. Med. 1961, 54, 831–836. [Google Scholar] [CrossRef] [Green Version]
- Instituto Nacional de Estatística. Estatísticas da Pesca Continente e Ilhas Adjacentes. Junta Nac. Fom. Pescas 1973, 26–36. [Google Scholar]
- CEVA Edible Seaweed and Microalgae—Regulatory Status in France and Europe; 2019 Update; CEVA: Pleubian, France, 2019.
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as nutritional and functional food sources: Revisiting our understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef]
- Pérez-Lloréns, J.L. Microalgae: From staple foodstuff to avant-garde cuisine. Int. J. Gastron. Food Sci. 2020, 21, 100221. [Google Scholar] [CrossRef]
- Statista. Retail Sales Value of Fungi/Algae Meat Substitutes in Western Europe in 2015 and 2018, with a Forecast for 2019 and 2022. Available online: https://www.statista.com/statistics/1076190/retail-sales-value-of-fungi-algae-meat-substitutes-in-western-europe/ (accessed on 10 April 2021).
- Statista. Retail Sales Value of Fungi/Algae Meat Substitutes in Western Europe in 2018, by Category. Available online: https://www.statista.com/statistics/1076194/retail-sales-value-of-fungi-algae-meat-substitutes-in-western-europe/i-algae-meat-substitutes-in-western-europe/ (accessed on 10 April 2021).
- Lucas, S.; Gouin, S.; Lesueur, M. Seaweed Consumption and Label Preferences in France. Mar. Resour. Econ. 2019, 34, 143–162. [Google Scholar] [CrossRef]
- Mouritsen, O.G.; Williams, L.; Bjerregaard, R.; Duelund, L. Seaweeds for umami flavour in the New Nordic Cuisine. Flavour 2012, 1, 4. [Google Scholar] [CrossRef] [Green Version]
- Roleda, M.Y.; Marfaing, H.; Desnica, N.; Jónsdóttir, R.; Skjermo, J.; Rebours, C.; Nitschke, U. Variations in polyphenol and heavy metal contents of wild-harvested and cultivated seaweed bulk biomass: Health risk assessment and implication for food applications. Food Control 2019, 95, 121–134. [Google Scholar] [CrossRef]
- National Food Institute Denmark; Sá Monteiro, M.; Sloth, J.; Holdt, S.; Hansen, M. Analysis and Risk Assessment of Seaweed. EFSA J. 2019, 17, e170915. [Google Scholar] [CrossRef] [Green Version]
- (ALGAplus) Fava-Do-Mar Desidratada, 100g. Available online: https://algaplus.lojasonlinectt.pt/product/fava-do-mar-desidratada-100g (accessed on 10 May 2021).
- Li, X.; Li, J.; Wang, Y.; Fu, L.; Fu, Y.; Li, B.; Jiao, B. Aquaculture Industry in China: Current State, Challenges, and Outlook. Rev. Fish. Sci. 2011, 19, 187–200. [Google Scholar] [CrossRef]
- Statista. Estimated Value of Algae Market Worldwide from 2016 to 2023, by Region. Available online: https://www.statista.com/statistics/1032615/global-market-value-of-algae-by-region/ (accessed on 10 April 2021).
- Turan, G.; Cırık, S. Sea Vegetables. In Vegetables—Importance of Quality Vegetables to Human Health; InTech: London, UK, 2018. [Google Scholar]
- (MICHELIN Guide UK Editorial Team) Explore the World of the MICHELIN Guide. Available online: https://guide.michelin.com/en (accessed on 24 May 2021).
- Goulding, M.; Seeding the Ocean: Inside a Michelin-Starred Chef’s Revolutionary Quest to Harvest Rice From the Sea. Available online: https://time.com/5926780/chef-angel-leon-sea-rice/ (accessed on 10 April 2021).
- Rodrigues, J. Projecto Matéria. Available online: https://www.projectomateria.pt (accessed on 1 February 2022).
- Rumin, J.; Nicolau, E.; Gonçalves de Oliveira Junior, R.; Fuentes-Grünewald, C.; Picot, L. Analysis of Scientific Research Driving Microalgae Market Opportunities in Europe. Mar. Drugs 2020, 18, 264. [Google Scholar] [CrossRef] [PubMed]
- García, J.L.; de Vicente, M.; Galán, B. Microalgae, old sustainable food and fashion nutraceuticals. Microb. Biotechnol. 2017, 10, 1017–1024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- PwC. Vitamins & Dietary Supplements Market trends—Overview. Euromonitor International 2019, Press. Available online: https://www.pwc.com/it/it/publications/assets/docs/Vitamins-Dietary-Supplements-Market-Overview.pdf (accessed on 25 May 2022).
- Statista. Value of the Dietary Supplements Market in Europe in 2015 and 2020, by Region. Available online: https://www.statista.com/statistics/589374/dietary-supplements-market-value-europe/ (accessed on 10 April 2021).
- Statista. Retail Sales Forecast of Vitamin and Dietary Supplements in Europe from 2010 to 2020. Available online: https://www.statista.com/statistics/646795/retail-sales-forecast-of-supplements-european-union-eu/ (accessed on 10 April 2021).
- Schüler, L.; Greque de Morais, E.; Trovão, M.; Machado, A.; Carvalho, B.; Carneiro, M.; Maia, I.; Soares, M.; Duarte, P.; Barros, A.; et al. Isolation and Characterization of Novel Chlorella vulgaris Mutants With Low Chlorophyll and Improved Protein Contents for Food Applications. Front. Bioeng. Biotechnol. 2020, 8, 469. [Google Scholar] [CrossRef] [PubMed]
- Molino, A.; Iovine, A.; Casella, P.; Mehariya, S.; Chianese, S.; Cerbone, A.; Rimauro, J.; Musmarra, D. Microalgae Characterization for Consolidated and New Application in Human Food, Animal Feed and Nutraceuticals. Int. J. Environ. Res. Public Health 2018, 15, 2436. [Google Scholar] [CrossRef] [Green Version]
- Niccolai, A.; Chini Zittelli, G.; Rodolfi, L.; Biondi, N.; Tredici, M.R. Microalgae of interest as food source: Biochemical composition and digestibility. Algal Res. 2019, 42, 101617. [Google Scholar] [CrossRef]
- (Foodfrog) Astaxanthin Powder [Vacuumed] [1.2%] [5%] & [10%] Haematococcus pluvialis without Additives, Laboratory Tested. Available online: https://www.amazon.de/-/en/Astaxanthin-Haematococcus-laboratory-Resealable-03-01-2020/dp/B079G735CJ/ref=sr_1_6?dchild=1&keywords=Haematococcus&qid=1619540791&s=drugstore&sr=1-6 (accessed on 22 April 2021).
- (Vita World) Astaxantina 8mg 60 Cápsulas Vita World Producción en Farmacia en Alemania—Antioxidante—Haematococcus Pluvialis. Available online: https://www.amazon.es/Astaxantina-Cápsulas-Vita-World-Producción/dp/B0183YGDC8/ref=sr_1_1?__mk_es_ES=ÅMÅŽÕÑ&dchild=1&keywords=Haematococcus+pluvialis&qid=1619546645&sr=8-1 (accessed on 22 April 2021).
- (Igennus Healthcare) Nutrition Pure & Essential Astaxanthin Complex, 42 mg Astapure Providing 4 mg H. Pluvialis Astaxanthin, 90 Vegan Capsules. Available online: https://www.amazon.co.uk/Essential-Astaxanthin-Astapure-Delivering-Capsules/dp/B07BGHKG77/ref=sr_1_7?dchild=1&keywords=Haematococcus+pluvialis&qid=1619543962&s=drugstore&sr=1-7 (accessed on 22 April 2021).
- (Ekopura) Vegan Oméga 3—Huile d’Algues, 90 Capsules (250mg DHA/Capsule). Available online: https://www.amazon.fr/Vegan-Oméga-dAlgues-Capsules-Capsule/dp/B076PVW2LV/ref=sr_1_9?__mk_fr_FR=ÅMÅŽÕÑ&dchild=1&keywords=Haematococcus+pluvialis&qid=1619543956&sr=8-9 (accessed on 22 April 2021).
- NOVERG Antioxydant|Vitamines C & E, Zinc, CoEnzyme Q10, Sélénium, Pépins de Raisin OPC, Thé Vert, Dunaliella|Anti-Age & Belle Peau|Vegan|90 Gélules|Cure de 45 Jours|Fabriqué en France. Available online: https://www.amazon.fr/Antioxydant-Vitamines-CoEnzyme-Sélénium-Dunaliella/dp/B08RHY3J4X/ref=sr_1_10?__mk_fr_FR=ÅMÅŽÕÑ&dchild=1&keywords=Dunaliella&qid=1619543512&sr=8-10 (accessed on 22 April 2021).
- Anastore Vitamine A (Rétinol) * 16 mg/60 Kapseln * Trockenextrakt aus der Alge Dunaliella, zu 30 % aus β-Karotin titriert * Augen, Haut, Immun, Vegan. Available online: https://www.amazon.de/-/en/DN65/dp/B01IR7HDPM/ref=sr_1_3?dchild=1&keywords=Dunaliella&qid=1619540972&s=drugstore&sr=1-3 (accessed on 22 April 2021).
- Amazon Microalgae Powders. Available online: https://www.amazon.de/s?k=mikroalgaen+pulver&ref=nb_sb_noss (accessed on 22 April 2021).
- VeganBio Spirulina Products. Available online: https://www.veganobio.it/search-results/?action=search&productids=1559,4227,2355,2163,5669,463,2559,5578,1463,813,5824,2332,2555,2562,2558,5064,2575,5881,5828,4783,4775,5458 (accessed on 27 April 2021).
- Biosklep Mikroalgen Pulver Products. Available online: https://biosklep.com.pl/pl/searchquery/spirulina/1/phot/5?url=spirulina (accessed on 27 April 2021).
- Planeta Huerto Mikroalgen Pulver Products. Available online: https://www.planetahuerto.es/articulos-buscar?k=spirulina (accessed on 21 February 2021).
- Statista. Which of the Following Superfoods Do You Regularly Buy Because of Their Special Effect? Available online: https://www.statista.com/statistics/788851/superfoods-purchase-germany/ (accessed on 10 April 2021).
- Mattisson Chlorella sorokiniana Product of. Available online: https://www.mattisson.nl/product/absolute-chlorella-poeder-nederlands/ (accessed on 21 February 2021).
- MySuperfoods Marine Phytoplankton Powder (100 Grams), MySuperFoods, Purest Food on Earth, Cultivated from The Deep Sea, Rich in Micronutrients, Add to Juices, Smoothies, Shakes. Available online: https://www.amazon.nl/-/en/dp/B00M8F6QP8/ref=pd_sim_1?pd_rd_w=EerIM&pf_rd_p=14f79929-97cc-4510-b8a0-227891619eb7&pf_rd_r=9S9VQF5ZAYZ3WM8PEAMT&pd_rd_r=279be38e-7370-43d3-af05-3d08ddf36662&pd_rd_wg=ARZAK&pd_rd_i=B00M8F6QP8&psc=1 (accessed on 27 April 2021).
- Algova Algova Nannochloropsis Freeze Dried Phytoplankton—Food for Artemia, Daphnia, Whederanimals, Mussels, Shrimp Corals, Copepodes High Vitamin, Fatty Acid & Mineral Content. Available online: https://www.amazon.de/-/en/Nannochloropsis-Freeze-Dried-Phytoplankton-Artemia/dp/B00TVARE3G/ref=sr_1_3?dchild=1&keywords=nannochloropsis&qid=1619543022&sr=8-3 (accessed on 27 April 2021).
- Cai, J.; Lovatelli, A.; Gamarro, E.G.; Geehan, J.; Lucente, D.; Mair, G.; Miao, W.; Reantaso, M.; Roubach, R.; Yuan, X.; et al. Seaweeds and Microalgae: An Overview for Unlocking Their Potential in Global Aquaculture Development; FAO: Rome, Italy, 2021; ISBN 978-92-5-134710-2. [Google Scholar]
- Dos Santos Fernandes de Araujo, R. Brief on Algae Biomass Production; Lusser, M., Sanchez Lopez, J., Avraamides, M., Eds.; Publications Office of the European Union: Luxembourg, 2019; ISBN 978-92-76-12271-5. [Google Scholar]
- FAO. Fishery and Aquaculture Statistics 2019/FAO annuaire. Statistiques des pêches et de l’aquaculture 2019/FAO anuario. Estadísticas de pesca y acuicultura 2019; FAO: Rome, Italy, 2021. [Google Scholar] [CrossRef]
- FAO. Global Production Statistics Database. Available online: http://www.fao.org/fishery/topic/16140/en (accessed on 10 April 2021).
- Chopin, T.; Buschmann, A.H.; Halling, C.; Troell, M.; Kautsky, N.; Neori, A.; Kraemer, G.P.; Zertuche-González, J.A.; Yarish, C.; Neefus, C. Integrating Seaweeds into Marine Aquaculture Systems: A Key toward Sustainability. J. Phycol. 2001, 37, 975–986. [Google Scholar] [CrossRef]
- Bronswijk, L.; Vlottes, M.; Draisma, M.; Brouwers, E.; van Baelen, F. D4.1.1 Study on Existing Market for Algal Food Applications Part A: Seaweed. North Sea Farm Foundation: The Hague, Amsterdam, 2019. [Google Scholar]
- Organic Monitor. The European Market for Sea Vegetables; Report prepared for Bord Iascaigh Mhara (BIM). 2014. Available online: https://bim.ie/wp-content/uploads/2021/02/The,European,Market,for,Sea,Vegetables,-,2015.pdf (accessed on 10 April 2021).
- Vincent, A.; Stanley, A.; Ring, J. Hidden Champion of the Ocean: Seaweed as a Growth Engine for a Sustainable European Future: Seaweed for Europe. 2020. Available online: https://www.seaweedeurope.com/wp-content/uploads/2020/10/Seaweed_for_Europe-Hidden_Champion_of_the_ocean-Report.pdf (accessed on 10 April 2021).
- Paladin Maionese Do Mar. Available online: https://www.paladin.pt/pt-pt/produto/maionese-mar-225ml (accessed on 11 February 2022).
- Le Bras, Q.; Ritter, L.; Fasquel, D.; Lesueur, M.; Lucas, S. Etude de la consommation des algues alimentaires en France; Programme IDEALG Phase 1. Etude nationale. [Rapport Technique]; Agrocampus Ouest: Rennes, France, 2014. [Google Scholar]
- Simoes, J.G.; Hidalgo, C.A. Seaweeds and Other Algae: Not Fit for Human Consumption, Fresh, Chilled, Frozen or Dried, Whether or Not Ground. The Economic Complexity Observatory. Datawheel. Available online: https://oec.world/en/profile/hs/seaweeds-and-other-algae-not-fit-for-human-consumption-fresh-chilled-frozen-or-dried-whether-or-not-ground?countryComparisonMeasureSelector=Trade%20Value&countryComparisonGeoSelector=eu (accessed on 10 April 2021).
- Notpla Notpla Limited—We Make Packaging Disappear. Available online: https://www.notpla.com (accessed on 25 April 2021).
- Shen, Y.; Yuan, W.; Pei, Z.J.; Wu, Q.; Mao, E. Microalgae Mass Production Methods. Trans. ASABE 2009, 52, 1275–1287. [Google Scholar] [CrossRef]
- Larsson, C.; Axelsson, L. Bicarbonate uptake and utilization in marine macroalgae. Eur. J. Phycol. 1999, 34, 79–86. [Google Scholar] [CrossRef]
- Lüning, K.; Pang, S. Mass cultivation of seaweeds: Current aspects and approaches. J. Appl. Phycol. 2003, 15, 115–119. [Google Scholar] [CrossRef]
- Hurd, C.L.; Harrison, P.J.; Bischof, K.; Lobban, C.S. Seaweed Ecology and Physiology; Cambridge University Press: Cambridge, UK, 2014; ISBN 1139952013. [Google Scholar]
- Kim, J.K.; Yarish, C.; Hwang, E.K.; Park, M.; Kim, Y. Seaweed aquaculture: Cultivation technologies, challenges and its ecosystem services. ALGAE 2017, 32, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Grobbelaar, J.U. Microalgae mass culture: The constraints of scaling-up. J. Appl. Phycol. 2012, 24, 315–318. [Google Scholar] [CrossRef]
- Borowitzka, M.A. Microalgae for Aquaculture: Opportunities and Constraints. J. Appl. Phycol. 1997, 9, 393–401. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, W.; Chen, L.; Wang, J.; Liu, T. The contamination and control of biological pollutants in mass cultivation of microalgae. Bioresour. Technol. 2013, 128, 745–750. [Google Scholar] [CrossRef]
- Day, J.G.; Gong, Y.; Hu, Q. Microzooplanktonic grazers—A potentially devastating threat to the commercial success of microalgal mass culture. Algal Res. 2017, 27, 356–365. [Google Scholar] [CrossRef]
- Masó, M.; Garcés, E. Harmful microalgae blooms (HAB); problematic and conditions that induce them. Mar. Pollut. Bull. 2006, 53, 620–630. [Google Scholar] [CrossRef]
- Buschmann, A.H.; Retamales, C.A.; Figueroa, C. Ceramialean epiphytism in an intertidal Gracilaria chilensis (Rhodophyta) bed in southern Chile. J. Appl. Phycol. 1997, 9, 129–135. [Google Scholar] [CrossRef]
- Redmond, S.; Green, L.; Yarish, C.; Kim, J.; Neefus, C. New England Seaweed Culture Handbook. Seaweed Cultiv. 2014, 1–92. Available online: https://opencommons.uconn.edu/seagrant_weedcult/1 (accessed on 10 April 2021).
- Patarra, R.F.; Carreiro, A.S.; Lloveras, A.A.; Abreu, M.H.; Buschmann, A.H.; Neto, A.I. Effects of light, temperature and stocking density on Halopteris scoparia growth. J. Appl. Phycol. 2017, 29, 405–411. [Google Scholar] [CrossRef]
- Liu, X.; Bogaert, K.; Engelen, A.H.; Leliaert, F.; Roleda, M.Y.; De Clerck, O. Seaweed reproductive biology: Environmental and genetic controls. Bot. Mar. 2017, 60, 89–108. [Google Scholar] [CrossRef]
- Wang, H.; Lin, A.; Gu, W.; Huan, L.; Gao, S.; Wang, G. The sporulation of the green alga Ulva prolifera is controlled by changes in photosynthetic electron transport chain. Sci. Rep. 2016, 6, 24923. [Google Scholar] [CrossRef] [Green Version]
- Cherry, P.; O’Hara, C.; Magee, P.J.; McSorley, E.M.; Allsopp, P.J. Risks and benefits of consuming edible seaweeds. Nutr. Rev. 2019, 77, 307–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Costa, E.; Silva, J.; Mendonça, S.; Abreu, M.; Domingues, M. Lipidomic Approaches towards Deciphering Glycolipids from Microalgae as a Reservoir of Bioactive Lipids. Mar. Drugs 2016, 14, 101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sautier, C.; Trémolières, J.; Billion, J.; Flament, C.; Poivre, R. Valeur alimentaire des algues spirulines chez l’homme. Ann. Nutr. Aliment. 1975, 29, 517–534. [Google Scholar] [PubMed]
- Pokrovskaia, Y.E.I.; Tereshchenko, A.P.; Volynets, V.M. Effect of a Vegetable Diet Including a Biomass of Unicellular Algae on the Excretion and Balance of Mineral Elements (Vegetable Diet, Including 210 g of Dry Chlorella Biomass, Decreases Effect on Calcium and Magnesium Assimilation to Produce Insignificant. Kosm. Biol. I Meditsina 1968, 2, 78–81. [Google Scholar]
- Pereira, H.; Silva, J.; Santos, T.; Gangadhar, K.N.; Raposo, A.; Nunes, C.; Coimbra, M.A.; Gouveia, L.; Barreira, L.; Varela, J. Nutritional potential and toxicological evaluation of Tetraselmis sp. CtP4 microalgal biomass produced in industrial photobioreactors. Molecules 2019, 24, 3192. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Cyanobacterial Toxins: Anatoxin-a Background Document for Development of WHO Guidelines for Drinking-Water Quality and Guidelines for Safe Recreational Water Environments; WHO: Geneva, Switzerland, 2019; Available online: https://apps.who.int/iris/handle/10665/338060. (accessed on 11 April 2021).
- Sánchez-Parra, E.; Boutarfa, S.; Aboal, M. Are Cyanotoxins the Only Toxic Compound Potentially Present in Microalgae Supplements? Results from a Study of Ecological and Non-Ecological Products. Toxins 2020, 12, 552. [Google Scholar] [CrossRef]
- Zastepa, A.; Chemali, C. Bloom announcement: Late season cyanobacterial blooms co-dominated by Microcystis flos-aquae, Lyngbya birgei, and Aphanizomenon flos-aquae complex in Hamilton Harbour (Lake Ontario), an area of concern impacted by industrial effluent and residential waste. Data Br. 2021, 35, 106800. [Google Scholar] [CrossRef]
- (Quackwatch) Cell Tech Sued for Wrongful Death. Available online: https://quackwatch.org/cases/civil/celltech/complaint/ (accessed on 10 April 2021).
- Pelletreau, K.; Muller-Parker, G. Sulfuric acid in the phaeophyte alga Desmarestia munda deters feeding by the sea urchin Strongylocentrotus droebachiensis. Mar. Biol. 2002, 141, 1–9. [Google Scholar] [CrossRef]
- Rhodes, L.L.; Thomas, A.E. Coolia monotis (Dinophyceae): A toxic epiphytic microalgal species found in New Zealand (Note). N. Z. J. Mar. Freshw. Res. 1997, 31, 139–141. [Google Scholar] [CrossRef]
- AlgaeBase Coolia monotis Meunier 1919. Available online: https://www.algaebase.org/search/species/detail/?species_id=52206 (accessed on 10 May 2021).
- Thomas, I.; Siew, L.Q.C.; Watts, T.J.; Haque, R. Seaweed allergy. J. Allergy Clin. Immunol. Pract. 2019, 7, 714–715. [Google Scholar] [CrossRef]
- Fleurence, J.; Levine, I.A. Antiallergic and Allergic Properties. In Microalgae in Health and Disease Prevention; Levine, I.A., Fleurence, J., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 307–315. ISBN 978-0-12-811405-6. [Google Scholar]
- Motoyama, K.; Hamada, Y.; Nagashima, Y.; Shiomi, K. Allergenicity and allergens of amphipods found in nori (dried laver). Food Addit. Contam. 2007, 24, 917–922. [Google Scholar] [CrossRef]
- Hashimoto, H.; Hongo, T.; Hayashi, C.; Nakamura, K.; Nakanishi, K.; Ikeda, M.; Adachi, R.; Akiyama, H.; Teshima, R.; Yano, T. A method for the detection of shrimp/prawn and crab DNAs to identify allergens in dried seaweed products. Jpn. J. Food Chem. Saf. 2015, 22, 1–10. [Google Scholar] [CrossRef]
- Arulkumar, A.; Nigariga, P.; Paramasivam, S.; Rajaram, R. Metals accumulation in edible marine algae collected from Thondi coast of Palk Bay, Southeastern India. Chemosphere 2019, 221, 856–862. [Google Scholar] [CrossRef]
- Lin, Z.; Li, J.; Luan, Y.; Dai, W. Application of algae for heavy metal adsorption: A 20-year meta-analysis. Ecotoxicol. Environ. Saf. 2020, 190, 110089. [Google Scholar] [CrossRef]
- Packer, M.A.; Harris, G.C.; Adams, S.L. Food and Feed Applications of Algae. In Algae Biotechnology; Springer International Publishing: Cham, Switzerland, 2016; pp. 217–247. [Google Scholar]
- Arunakumara, K.K.I.U.; Zhang, X. Heavy Metal Bioaccumulation and Toxicity with Special Reference to Microalgae. J. Ocean Univ. China 2008, 7, 60–64. [Google Scholar] [CrossRef]
- Desideri, D.; Cantaluppi, C.; Ceccotto, F.; Meli, M.A.; Roselli, C.; Feduzi, L. Essential and toxic elements in seaweeds for human consumption. J. Toxicol. Environ. Health Part A 2016, 79, 112–122. [Google Scholar] [CrossRef]
- Rose, M.; Lewis, J.; Langford, N.; Baxter, M.; Origgi, S.; Barber, M.; MacBain, H.; Thomas, K. Arsenic in seaweed—Forms, concentration and dietary exposure. Food Chem. Toxicol. 2007, 45, 1263–1267. [Google Scholar] [CrossRef]
- Fu, Y.; Yin, N.; Cai, X.; Du, H.; Wang, P.; Sultana, M.S.; Sun, G.; Cui, Y. Arsenic speciation and bioaccessibility in raw and cooked seafood: Influence of seafood species and gut microbiota. Environ. Pollut. 2021, 280, 116958. [Google Scholar] [CrossRef]
- Vellinga, R.E.; Sam, M.; Verhagen, H.; Jakobsen, L.S.; Ravn-Haren, G.; Sugimoto, M.; Torres, D.; Katagiri, R.; Thu, B.J.; Granby, K.; et al. Increasing Seaweed Consumption in the Netherlands and Portugal and the Consequences for the Intake of Iodine, Sodium, and Exposure to Chemical Contaminants: A Risk-Benefit Study. Front. Nutr. 2022, 8, 792923. [Google Scholar] [CrossRef]
- Lähteenmäki-Uutela, A.; Rahikainen, M.; Camarena-Gómez, M.T.; Piiparinen, J.; Spilling, K.; Yang, B. European Union legislation on macroalgae products. Aquac. Int. 2021, 29, 487–509. [Google Scholar] [CrossRef]
- Banach, J.L.; Hoek-van den Hil, E.F.; Fels-Klerx, H.J. Food safety hazards in the European seaweed chain. Compr. Rev. Food Sci. Food Saf. 2020, 19, 332–364. [Google Scholar] [CrossRef]
- González, A.; Paz, S.; Rubio, C.; Gutiérrez, Á.J.; Hardisson, A. Human Exposure to Iodine from the Consumption of Edible Seaweeds. Biol. Trace Elem. Res. 2020, 197, 361–366. [Google Scholar] [CrossRef]
- Phaneuf, D.; Côté, I.; Dumas, P.; Ferron, L.A.; LeBlanc, A. Evaluation of the Contamination of Marine Algae (Seaweed) from the St. Lawrence River and Likely to Be Consumed by Humans. Environ. Res. 1999, 80, S175–S182. [Google Scholar] [CrossRef]
- Malea, P.; Chatziapostolou, A.; Kevrekidis, T. Trace element seasonality in marine macroalgae of different functional-form groups. Mar. Environ. Res. 2015, 103, 18–26. [Google Scholar] [CrossRef]
- Han, P.; Lu, Q.; Fan, L.; Zhou, W. A Review on the Use of Microalgae for Sustainable Aquaculture. Appl. Sci. 2019, 9, 2377. [Google Scholar] [CrossRef] [Green Version]
- Fabricant, D. Arsenic in Herbal Kelp Supplements: Concentration, Regulations, and Labeling. Environ. Health Perspect. 2007, 115, A574. [Google Scholar] [CrossRef]
- Mirzayeva, A.; Castro, R.; Barroso, C.G.; Durán-Guerrero, E. Characterization and differentiation of seaweeds on the basis of their volatile composition. Food Chem. 2021, 336, 127725. [Google Scholar] [CrossRef]
- Hou, X.; Chai, C.; Qian, Q.; Yan, X.; Fan, X. Determination of chemical species of iodine in some seaweeds (I). Sci. Total Environ. 1997, 204, 215–221. [Google Scholar] [CrossRef]
- Zava, T.T.; Zava, D.T. Assessment of Japanese iodine intake based on seaweed consumption in Japan: A literature-based analysis. Thyroid Res. 2011, 4, 14. [Google Scholar] [CrossRef] [Green Version]
- Badar, M.; Batool, F.; Idrees, M.; Iqbal, H.R.; Zia, M.A. Effect of Boiling on Removing of Botulinum Toxins from Drinking Water Samples. Int. J. Sci. Res. 2016, 5, 1969–1973. [Google Scholar] [CrossRef]
- Abraham, A.; Flewelling, L.J.; El Said, K.R.; Odom, W.; Geiger, S.P.; Granholm, A.A.; Jackson, J.T.; Bodager, D. An occurrence of neurotoxic shellfish poisoning by consumption of gastropods contaminated with brevetoxins. Toxicon 2021, 191, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Wu, S.; Yin, R.; Bai, X.; Bhunia, A.K.; Liu, C.; Zheng, Y.; Wang, F.; Blatchley, E.R. Effects of fulvic acid size on microcystin-LR photodegradation and detoxification in the chlorine/UV process. Water Res. 2021, 193, 116893. [Google Scholar] [CrossRef] [PubMed]
- Al-Thawadi, S. Public perception of algal consumption as an alternative food in the Kingdom of Bahrain. Arab J. Basic Appl. Sci. 2018, 25, 1–12. [Google Scholar] [CrossRef]
- Lee, J.; Chambers IV, E.; Chambers, D.H.; Chun, S.S.; Oupadissakoon, C.; Johnson, D.E. Consumer Acceptance for Green Tea by Consumers in the United States, Korea and Thailand. J. Sens. Stud. 2010, 25, 109–132. [Google Scholar] [CrossRef]
- Chapman, A.S.; Stévant, P.; Larssen, W.E. Food or fad? Challenges and opportunities for including seaweeds in a Nordic diet. Bot. Mar. 2015, 58, 423–433. [Google Scholar] [CrossRef]
- (Bord à Bord) La Cuisine Aux Algues. Available online: https://www.bord-a-bord.fr/-La-cuisine-aux-algues-.html?lang=fr (accessed on 10 April 2021).
- Milne, X. The Seaweed Cookbook; Joseph, M., Ed.; Penguin UK: London, UK, 2016; ISBN 978-0718183660. [Google Scholar]
- Morel, H. Vive Les Algues! Saveurs Iodées Pour Recettes Gourmandes, 1st ed.; Trop Mad: Lorient, France, 2019; ISBN 9782918068136. [Google Scholar]
- van den Burg, S.W.K.; Dagevos, H.; Helmes, R.J.K. Towards sustainable European seaweed value chains: A triple P perspective. ICES J. Mar. Sci. 2021, 78, 443–450. [Google Scholar] [CrossRef] [Green Version]
- Kulsdom, M.; Hanekroot, D.; van Langelaar, K.; Kreischer, L. The Dutch Weed Burger. Available online: https://dutchweedburger.com/en/ (accessed on 19 April 2021).
- Miyaki, T.; Retiveau-Krogmann, A.; Byrnes, E.; Takehana, S. Umami Increases Consumer Acceptability, and Perception of Sensory and Emotional Benefits without Compromising Health Benefit Perception. J. Food Sci. 2016, 81, S483–S493. [Google Scholar] [CrossRef]
- Ikeda, K. New Seasonings. Chem. Senses 2002, 27, 847–849. [Google Scholar] [CrossRef] [Green Version]
- Mitsuda, H.; Shikanai, T. Studies on the Utilization of Chlorella for Food: Studies on the Nutritive Values of Cell Free Algal Proteins (Commemoration Issue Dedicated to Professor Sankichi Takei On the Occasion of his Retirement). Bull. Inst. Chem. Res. Kyoto Univ. 1960, 38, 40–58. [Google Scholar]
- Dewi, E.N.; Amalia, U.; Mel, M. The Effect of Different Treatments to the Amino Acid Contents of Micro Algae Spirulina sp. Aquat. Procedia 2016, 7, 59–65. [Google Scholar] [CrossRef]
- Kamenidou, I.; Aggelopoulos, S.; Batzios, A.C. Natural Medical Attributes and Benefits of Spirulina: Segmentation Based on Consumers Knowledge. J. Med. Plants Res. 2011, 5, 3192–3199. [Google Scholar]
- Kamenidou, I.; Priporas, C.-V. Factors Predicting Consumers’ Knowledge of Spirulina Health Benefits. J. Food Agric. Environ. 2010, 8, 16–20. [Google Scholar]
- Lafarga, T.; Rodríguez-Bermúdez, R.; Morillas-España, A.; Villaró, S.; García-Vaquero, M.; Morán, L.; Sánchez-Zurano, A.; González-López, C.V.; Acién-Fernández, F.G. Consumer knowledge and attitudes towards microalgae as food: The case of Spain. Algal Res. 2021, 54, 102174. [Google Scholar] [CrossRef]
- Rzymski, P.; Jaśkiewicz, M. Microalgal food supplements from the perspective of Polish consumers: Patterns of use, adverse events, and beneficial effects. J. Appl. Phycol. 2017, 29, 1841–1850. [Google Scholar] [CrossRef] [Green Version]
- Moons, I.; De Pelsmacker, P.; Barbarossa, C. The Drivers of the Usage Intention of Spirulina Algae in Food in Different Market Segments. In Proceedings of the 16th International Marketing Trends Conference, Madrid, Spain, 26–28 January 2017; pp. 1–22. [Google Scholar]
- Moons, I.; Barbarossa, C.; De Pelsmacker, P. The Determinants of the Adoption Intention of Eco-friendly Functional Food in Different Market Segments. Ecol. Econ. 2018, 151, 151–161. [Google Scholar] [CrossRef]
- Weinrich, R.; Elshiewy, O. Preference and willingness to pay for meat substitutes based on micro-algae. Appetite 2019, 142, 104353. [Google Scholar] [CrossRef]
- Thomas, C.; Symoneaux, R.; Pantin-Sohier, G.; Picouet, P.; Maître, I. Perceptions of Spirulina from French Consumers of Organic Products; hal-02615769; HAL Open Science: Lyon, France, 2020. [Google Scholar]
- Roßmann, M.; Rösch, C. Key-Narratives of Microalgae Nutrition: Exploring futures through a public Delphi survey in Germany. Sci. Public Policy 2020, 47, 137–147. [Google Scholar] [CrossRef]
- Ricci, E.C.; Banterle, A.; Stranieri, S. Trust to Go Green: An Exploration of Consumer Intentions for Eco-friendly Convenience Food. Ecol. Econ. 2018, 148, 54–65. [Google Scholar] [CrossRef] [Green Version]
- Sachdeva, S.; Jordan, J.; Mazar, N. Green consumerism: Moral motivations to a sustainable future. Curr. Opin. Psychol. 2015, 6, 60–65. [Google Scholar] [CrossRef]
- Griskevicius, V.; Tybur, J.M.; Van den Bergh, B. Going green to be seen: Status, reputation, and conspicuous conservation. J. Personal. Soc. Psychol. 2010, 98, 392–404. [Google Scholar] [CrossRef] [Green Version]
- Burdock, G.A.; Carabin, I.G. Generally recognized as safe (GRAS): History and description. Toxicol. Lett. 2004, 150, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Office of Food Additive Safety Determining Regulatory Status Food Ingredientg the Regulatory Status of a Food Ingredient. Available online: https://www.fda.gov/food/food-ingredients-packaging/determining-regulatory-status-food-ingredient (accessed on 20 April 2021).
- FDA Generally Recognized as Safe (GRAS). Available online: https://www.fda.gov/food/food-ingredients-packaging/generally-recognized-safe-gras (accessed on 20 April 2021).
- (Government of Canada) About Novel and Genetically-Modified (GM) Foods. Available online: https://www.canada.ca/en/health-canada/services/food-nutrition/genetically-modified-foods-other-novel-foods.html (accessed on 20 April 2021).
- (Health Canada) Natural Health Products Ingredients Database. Available online: http://webprod.hc-sc.gc.ca/nhpid-bdipsn/search-rechercheReq.do?lang=eng (accessed on 20 April 2021).
- Standard 1.5.1; (Federal Register of Legislation) Australia New Zealand Food Standards Code—Standard 1.5.1—Novel Foods. Available online: https://www.legislation.gov.au/Details/F2017C00324 (accessed on 20 April 2021).
- Shen, R. China Regulations on New Food Raw Materials. Available online: https://food.chemlinked.com/foodpedia/china-regulations-new-food-raw-materials#C6 (accessed on 20 April 2021).
- China, S.C. of the N.P.C.—Food Safety Law of the People’s Republic of China. Available online: http://www.lawinfochina.com/Display.aspx?LookType=3&Lib=law&Cgid=113981&Id=7344&SearchKeyword=&SearchCKeyword=&paycode=# (accessed on 20 April 2021).
- Sun, J. The Regulation of “Novel Food” in China: The Tendency of Deregulation. Eur. Food Feed Law Rev. 2015, 10, 442–448. [Google Scholar]
- (Instrumentalities of the State Council) China Administrative Measures for the Safety Review of New Food Raw Materials. Available online: http://www.lawinfochina.com/display.aspx?lib=law&id=15358&CGid&EncodingName=big5 (accessed on 20 April 2021).
- Dos Santos Fernandes De Araujo, R.; Peteiro, C. Algae as Food and Food Supplements in Europe; Publications Office of the European Union: Luxembourg, 2021; ISBN 978-92-76-40548-1. [Google Scholar]
- Pulz, O.; Gross, W. Valuable products from biotechnology of microalgae. Appl. Microbiol. Biotechnol. 2004, 65, 635–648. [Google Scholar] [CrossRef]
- European Commission. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions. In A Blueprint to Safeguard Europe’s Water Resources; European Commission: Brussels, Belgium, 2012. [Google Scholar]
- Ruiz, J.; Olivieri, G.; de Vree, J.; Bosma, R.; Willems, P.; Reith, J.H.; Eppink, M.H.M.; Kleinegris, D.M.M.; Wijffels, R.H.; Barbosa, M.J. Towards industrial products from microalgae. Energy Environ. Sci. 2016, 9, 3036–3043. [Google Scholar] [CrossRef] [Green Version]
- EU Open Data Portal. The Official Portal for European Data. Available online: https://data.europa.eu/en (accessed on 10 April 2021).
- Schulze, P.S.C.; Carvalho, C.F.M.; Pereira, H.; Gangadhar, K.N.; Schüler, L.M.; Santos, T.F.; Varela, J.C.S.; Barreira, L. Urban wastewater treatment by Tetraselmis sp. CTP4 (Chlorophyta). Bioresour. Technol. 2017, 223, 175–183. [Google Scholar] [CrossRef]
- Mazarrasa, I.; Olsen, Y.S.; Mayol, E.; Marbà, N.; Duarte, C.M. Global Unbalance in Seaweed Production, Research Effort and Biotechnology Markets. Biotechnol Adv 2014, 32, 1028–1036. [Google Scholar] [CrossRef]
- Buschmann, A.H.; Camus, C.; Infante, J.; Neori, A.; Israel, Á.; Hernández-González, M.C.; Pereda, S.V.; Gomez-Pinchetti, J.L.; Golberg, A.; Tadmor-Shalev, N.; et al. Seaweed production: Overview of the global state of exploitation, farming and emerging research activity. Eur. J. Phycol. 2017, 52, 391–406. [Google Scholar] [CrossRef]
- Mazarrasa, I.; Olsen, Y.S.; Mayol, E.; Marbà, N.; Duarte, C.M. Rapid growth of seaweed biotechnology provides opportunities for developing nations. Nat. Biotechnol. 2013, 31, 591–592. [Google Scholar] [CrossRef] [Green Version]
- Duncan, T.V. Applications of Nanotechnology in Food Packaging and Food Safety: Barrier Materials, Antimicrobials and Sensors. J. Colloid Interface Sci. 2011, 363, 1–24. [Google Scholar] [CrossRef]
- Koynde, A.K.; Chew, K.W.; Manickam, S.; Chang, J.S.; Show, P.L. Emerging Algal Nanotechnology for High-Value Compounds: A Direction to Future Food Production. Trends Food Sci. Technol. 2021, 116, 290–302. [Google Scholar] [CrossRef]
- Carvalho, A.P.; Meireles, L.A.; Malcata, F.X. Microalgal Reactors: A Review of Enclosed System Designs and Performances. Biotechnol. Prog. 2006, 22, 1490–1506. [Google Scholar] [CrossRef] [PubMed]
- Cuaresma, M.; Janssen, M.; van den End, E.J.; Vílchez, C.; Wijffels, R.H. Luminostat operation: A tool to maximize microalgae photosynthetic efficiency in photobioreactors during the daily light cycle? Bioresour. Technol. 2011, 102, 7871–7878. [Google Scholar] [CrossRef] [PubMed]
- SCHOTT Algae cultivation in flat panel reactors compared to tubular glass PBRs; 2017. Available online: https://www.schott.com/en-gb/applications/photobioreactors (accessed on 1 March 2021).
- Sierra, E.; Acién, F.G.; Fernández, J.M.; García, J.L.; González, C.; Molina, E. Characterization of a flat plate photobioreactor for the production of microalgae. Chem. Eng. J. 2008, 138, 136–147. [Google Scholar] [CrossRef]
- Ministério da Marinha Portaria no 22.559; Diário da República: Lisbon, Portugal, 1967; p. 264.
- D’Oliveira, M.L. Sobre a utilização de algumas algas marinhas da nossa costa. Brotéria Ciências Nat. 1947, 16, 77–82. [Google Scholar]
- Viana, C.E. Argaço; AO NORTE: Viana do Castelo, Portugal, 2012; Available online: http://www.ao-norte.com/argaco.php (accessed on 1 March 2022).
- Un Océano de Algas Multiuso. Available online: https://elpais.com/diario/2008/03/21/tendencias/1206054003_850215.html (accessed on 1 March 2022).
- Varatojo, F. Alimentação Macrobiótica Padrão. Available online: https://www.institutomacrobiotico.com/pt-pt/imp/artigos/alimentacao-macrobiotica-padrao (accessed on 1 March 2021).
- Rahikainen, M.; Yang, B. Macroalgae as food and feed ingredients in the Baltic Sea region—Regulation by the European Union. 2020, pp. 1–20. Available online: https://www.submariner-network.eu/images/grass/GRASS_O3.4a_EU_regulation_of_seaweed_food_and_feed.pdf (accessed on 19 February 2021).
- (Provida) Provida, Portugal. Available online: https://www.provida.pt/produtos/#!/pesquisa/alga/?search=prods&query=alga (accessed on 19 February 2021).
- Nutrialga, Portugal. Available online: https://www.nutrialga.com/ (accessed on 6 February 2021).
- Loja das Algas, Portugal. Available online: https://www.lojadasalgas.pt/ (accessed on 6 February 2021).
- Drennan, F. Seaweed Bushcraft guide: Seaweed in Season. Bushcr. J. 2016. [Google Scholar]
- Rhatigan, P. Prannie Rhatigan’s Irish Seaweed Christmas Kitchen: Christmastide by the Coast; Inishmurray Ink Publishing: London, UK, 2018; ISBN 1916493602. [Google Scholar]
- Oliveira, J. 1o Encontro Nacional sobre Macroalgas Marinhas; LNETI INIP: Lisbon, Portugal, 1990. [Google Scholar]
- Chapman, V.J.; Chapman, D.J. Seaweeds and their Uses; Springer: Dordrecht, The Netherlands, 1980; ISBN 978-94-009-5808-1. [Google Scholar]
- (Ocean Rainforest) Ocean Rainforest Products (Sustainable Nordic Seaweed). Available online: https://www.oceanrainforest.com/full-range (accessed on 1 March 2022).
- McKenna, S. Extreme Greens: Understanding Seaweeds: Cooking, Foraging, Cosmetic, 1st ed.; McKenna, J., Ed.; Estragon Press: Cork, Ireland, 2013; ISBN 1906927197. [Google Scholar]
- Quéva, R.; Le Joncour, C. Algues gourmandes; Flammarion: Paris, France, 2017; ISBN 978-2-08-140848-7. [Google Scholar]
- (CBI) The European Market Potential for Edible Seaweed. Available online: https://www.cbi.eu/market-information/natural-ingredients-health-products/seaweed/market-potential (accessed on 10 April 2021).
- (DGRM) Algas: Denominação Comercial para as Diferentes Macroalgas e Microalgas que Podem ser Comercializadas em Portugal. 27 November 2019. Available online: https://www.dgrm.mm.gov.pt/documents/20143/56136/ALGAS+27.Nov.2019.pdf/32aefb8d-6fc3-54b8-d4dd-89e465390495 (accessed on 10 April 2021).
- Guiry, M.D. Use of seaweed as food in Ireland. Available online: https://www.seaweed.ie/uses_ireland/irishseaweedfood.php (accessed on 1 February 2021).
- (AlgAran) Organic Seaveg & Seaweed Supplements. Available online: https://www.seaweedproducts.ie/product-category/organic-seaveg-seaweed-supplements/ (accessed on 14 March 2022).
- (Seaweed Solutions) Seaweed Farming—The Future of Cultivation is at Sea. Available online: https://seaweedsolutions.com (accessed on 1 March 2022).
- (PORTO-MUIÑOS) Las verduras del mar. Available online: https://www.portomuinos.com (accessed on 1 March 2022).
- (AlgAran) Algaran Organic Vegan Kelp Supplements. Available online: https://www.seaweedproducts.ie/product/algaran-organic-vegan-kelp-supplements-70-days/ (accessed on 14 March 2022).
- Williams, M. Edible Seaweed Foraging—Galloway Wild Foods, Ireland. Available online: https://gallowaywildfoods.com/category/edible-wild-seaweeds/ (accessed on 11 February 2021).
- Pires, P. Receitas UBQ Madeira, Portugal. Available online: https://www.ubqmadeira.com/receitas/ (accessed on 21 February 2021).
- Ornelas, B. Receitas: Omelete de Espinafres com a Alga Asparagopsis taxiformis. Available online: https://www.ubqmadeira.com/receitas/ (accessed on 21 February 2021).
- Alga SeaExpert, Portugal. Available online: https://seaexpert-azores.com/ (accessed on 17 February 2021).
- Milinovic, J.; Campos, B.; Mata, P.; Diniz, M.; Noronha, J.P. Umami free amino acids in edible green, red, and brown seaweeds from the Portuguese seashore. J. Appl. Phycol. 2020, 32, 3331–3339. [Google Scholar] [CrossRef]
- (El Bulli) El Bulli Restaurant menu (2006–2009). Available online: http://www.elbulli.com/catalogo/catalogo/index.php?lang=en (accessed on 7 February 2021).
- Ziino, G.; Nibali, V.; Panebianco, A. Bacteriological investigation on “Mauro” sold in Catania. Vet. Res. Commun. 2010, 34, 157–161. [Google Scholar] [CrossRef] [Green Version]
- Araujo, G.S.; Cotas, J.; Morais, T.; Leandro, A.; García-Poza, S.; Gonçalves, A.M.M.M.; Pereira, L. Calliblepharis jubata cultivation potential—A comparative study between controlled and semi-controlled aquaculture. Appl. Sci. 2020, 10, 7553. [Google Scholar] [CrossRef]
- Albe, M. Mauru: La ricetta dell’insalta con l’alga siciliana. Available online: https://www.greenme.it/mangiare/altri-alimenti/mauru-ricetta-alga/ (accessed on 11 February 2021).
- Black, W.A.P. Seaweeds and their value in foodstuffs. Proc. Nutr. Soc. 1953, 12, 32–39. [Google Scholar] [CrossRef] [Green Version]
- Zemke-White, W.L.; Ohno, M. World seaweed utilisation: An end-of-century summary. J. Appl. Phycol. 1999, 11, 369–376. [Google Scholar] [CrossRef]
- CEVA Réglementation Algues Alimentaires Synthèse CEVA au 10/02/2014. 2014. Available online: https://www.foodplanet.fr/app/download/5806453856/R3A9glementation+algues+alimentaires+2014.pdf (accessed on 11 February 2021).
- García Tasende, M.; Peteiro, C. Explotación de las macroalgas marinas: Galicia como caso de estudio hacia una gestión sostenible de los recursos. Ambienta 2015, 111, 116–132. [Google Scholar]
- MacLean, R. Eat Your Greens: An Examination of the Potential Diet Available in Ireland during the Mesolithic. Ulster J. Archaeol. 1993, 56, 1–8. [Google Scholar]
- (ALGAplus) Tok de Mar Products by ALGAplus. Available online: https://algaplus.lojasonlinectt.pt/catalog (accessed on 10 April 2021).
- (DanskTANG) Seaweed from Denmark. Available online: https://www.danish-seaweed.com/seaweed-types/ (accessed on 14 March 2022).
- Rhatigan, P. Irish Seaweed Kitchen (The comprehensive Guide to Healthy Everyday Cooking with Seaweeds); Booklink: Northampton, MA, USA, 2009; ISBN 1906886229. [Google Scholar]
- Weinberger, F.; Paalme, T.; Wikström, S.A. Seaweed resources of the Baltic Sea, Kattegat and German and Danish North Sea coasts. Bot. Mar. 2020, 63, 61–72. [Google Scholar] [CrossRef] [Green Version]
- WoRMS Taxon Details: Coccotylus truncatus. Available online: https://www.marinespecies.org/aphia.php?p=taxdetails&id=145654#links (accessed on 17 March 2022).
- Hairon, D. Edible seaweeds in Jersey. Available online: https://jerseywalkadventures.co.uk/press-office/edible-seaweeds-in-jersey/ (accessed on 17 February 2021).
- Algamar, Portugal. Available online: https://www.algamar.com/pt-pt/quem-somos/ (accessed on 6 February 2021).
- ALGA4FOOD Gastronomia: O Alimento que vem do mar. Available online: https://alga4food.wixsite.com/page/gastronomia (accessed on 11 February 2021).
- Commission Implementing Regulation (EU). Commission Implementing Regulation (EU) 2018/460 of 20 March 2018 authorising the placing on the market of Ecklonia cava phlorotannins as a novel food under Regulation (EU) 2015/2283 of the European Parliament and of the Council and amending Commission Im. Off. J. Eur. Union. 2018, 78, 2–6. [Google Scholar]
- Gabriel, P. (ALGA4FOOD) Algas, Onde o mar Começa. Available online: https://dd4d232f-da72-4e83-b936-ede1885a4984.filesusr.com/ugd/baccf3_06402b38772549dd88c708818d5b0e32.pdf (accessed on 11 April 2021).
- Dias, N. My Name is Best, José Besteiro! 50 anos, 50 Receitas; 1st ed.; Âncora Editora: Lisbon, Portugal, 2016; ISBN 9789727805778. [Google Scholar]
- Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; Kuhnle, G.G.; et al. Re-evaluation of carrageenan (E 407) and processed Eucheuma seaweed (E 407a) as food additives. EFSA J. 2018, 16. [Google Scholar] [CrossRef]
- Pereira, J. A Treatise on Food and Diet with Observation on the Dietetic Regimen suited for Disordered States of the Digestive Organs.; Fowler & Wells Publisher: New York, NY, USA, 1843; ISBN 1247549038. [Google Scholar]
- Patarra, R.F.; Buschmann, A.H.; Abreu, M.H.; Neto, A.I. Cultivo de macroalgas nos Açores… Oportunidades e desafios. In Boletim de Biotecnologia; Sociedade Portuguesa de Biotecnologia: Braga, Portugal, 2014; Volume 2. [Google Scholar]
- (Celeiro) Celeiro, Portugal. Available online: https://www.celeiro.pt/ (accessed on 19 February 2021).
- EC Commission implementing Regulation (EU) 2017/2470 of 20 December 2017 establishing the Union list of novel foods in accordance with Regulation (EU) 2015/2283 of the European Parliament and of the Council on novel foods. Off. J. Eur. Union 2017, 351, 1–188.
- Fralick, R.A.; Baldwin, H.P.; Neto, A.I.; Hehre, E.J. Physiological responses of Pterocladia and Gelidium (Gelidiales, Rhodophyta) from the Azores, Portugal. Hydrobiologia 1990, 204–205, 479–482. [Google Scholar] [CrossRef] [Green Version]
- Fralick, R. Algas agarófitas nos Açores. Relatório Final (R/5/77); Centro de Documentação Tradução e. Autónoma dos Açores: Açores, Portugal, 1977. [Google Scholar]
- Abreu, H. Exploração de Macroalgas: Regulamentação; Aveiro, Portugal, 2014. [Google Scholar]
- Rodrigues, D.; Freitas, A.C.; Pereira, L.; Rocha-Santos, T.A.P.; Vasconcelos, M.W.; Roriz, M.; Rodríguez-Alcalá, L.M.; Gomes, A.M.P.; Duarte, A.C. Chemical composition of red, brown and green macroalgae from Buarcos bay in Central West Coast of Portugal. Food Chem. 2015, 183, 197–207. [Google Scholar] [CrossRef]
- Largo, D.; Critchley, A.; Hurtado, A.; Paul, N.; Pereira, L.; Cornish, M. Seaweed resources of the world: A 2020 vision. Part 4. Bot. Mar. 2020, 63, 299–301. [Google Scholar] [CrossRef]
- Denis, C.; Morançais, M.; Li, M.; Deniaud, E.; Gaudin, P.; Wielgosz-Collin, G.; Barnathan, G.; Jaouen, P.; Fleurence, J. Study of the chemical composition of edible red macroalgae Grateloupia turuturu from Brittany (France). Food Chem. 2010, 119, 913–917. [Google Scholar] [CrossRef]
- Munier, M.; Dumay, J.; Morançais, M.; Jaouen, P.; Fleurence, J. Variation in the Biochemical Composition of the Edible Seaweed Grateloupia turuturu Yamada Harvested from Two Sampling Sites on the Brittany Coast (France): The Influence of Storage Method on the Extraction of the Seaweed Pigment R-Phycoerythrin. J. Chem. 2013, 2013, 568548. [Google Scholar] [CrossRef] [Green Version]
- Pereira, L. University of C. Algas à mesa. Workshop Sobre Algas Comestíveis. IMAR-CMA/Universidade de Coimbra; Museu do Traje: Lisbon, Portugal, 2010. [Google Scholar]
- Palminha, F. As algas marinhas nos costumes faialenses. 1958; pp. 113–116.
- Pateira, M.L. Algas—Recursos e Investigação em Portugal. 1992.
- Rodrigues, F. “Legumes Do Mar” Saltam para as Suas Receitas. Available online: http://achadosmaracores.blogspot.com/2014/03/legumes-do-mar-saltam-para-as-suas.html (accessed on 3 February 2021).
- Pimenta, I. Macroalgas na Alimentação Humana—A Congelação como Processo de Conservação. 2010, pp. 1–110. Available online: http://hdl.handle.net/20.500.11960/2075 (accessed on 19 February 2021).
- Neves, B.; Real, H.; Carvalho, T. Algas a gosto: Considerações nutricionais e de saúde; Craveiro, C., Ed.; Multitema: Porto, Portugal, 2019; ISBN 978-989-8631-41-1. [Google Scholar]
- Rey, F.; Cartaxana, P.; Melo, T.; Calado, R.; Pereira, R.; Abreu, H.; Domingues, P.; Cruz, S.; Domingues, M.R. Domesticated Populations of Codium tomentosum Display Lipid Extracts with Lower Seasonal Shifts than Conspecifics from the Wild—Relevance for Biotechnological Applications of this Green Seaweed. Mar. Drugs 2020, 18, 188. [Google Scholar] [CrossRef] [Green Version]
- C-, C.; Gmbh, N.; Nordrhein-westfalen, L.; German, T.; Drink, S.; Land, T.; Rhine-westphalia, N.; Bundesverwaltungsgericht, T.; Court, F.A. Court of Justice of the European Union PRESS RELEASE No 69/21 EU Law Prohibits the Addition of the alga Lithothamnium Calcareum in the Processing of Organic Foodstuffs Such as rice- and Soya-Based Organic Drinks for the Purpose of Their Enrichment with Calcium; Court of Justice of the European Union: Luxembourg, 2021; Volume 2018. [Google Scholar]
- (Violey) Natumi Organic Rice Drink Calcium from seaweed Lithoathamnium. Available online: https://www.violey.com/en/natumi-organic-rice-drink-calcium_p_24785.html (accessed on 4 August 2021).
- (Vecteur Santé) Lithothamne de mer de Norvège 500 mg. Available online: https://www.naturitas.fr/p/complements/algues/lithothamne-de-mer-de-norvege-500mg-80-capsules-vecteur-sante (accessed on 1 March 2022).
- Nauticorvo Erva do Calhau—Corvo Açores 2008. Available online: https://youtu.be/fbXqBwoWtxU (accessed on 1 February 2021).
- (Expolab Centro Ciência Viva) Workshop “Alimentação do Oceano: Algas Como Fonte Alternativa de Proteína”. Available online: https://www.youtube.com/watch?v=wvMHcSjKHPc&ab_channel=ExpolabCentroCiênciaViva (accessed on 1 February 2021).
- Matos, S. Designing Food Cultures: Propagating the Consumption of Seaweed in the Azores Islands Through Recipes. Iridescent 2012, 2, 24–33. [Google Scholar] [CrossRef]
- Patarra, A.R.F. Fatty Acids of Selected Azorean Seaweeds. Pesquisa de Ácidos Gordos em Macroalgas Marinhas do litoral dos Açores. Master Thesis, University of Porto, Porto, Portugal, 2008. [Google Scholar]
- Algas comercializadas Flying Fish Azores. Available online: https://www.flyingfishazores.com/algas-pt (accessed on 17 March 2022).
- European Commission Commission Decision 2009/778/EC concerning the extension of uses of algal oil from the micro-algae Schizochytrium sp. as a novel food ingredient under Regulation (EC) No 258/97 of the European Parliament and of the Council. Off. J. Eur. Union 2009, L 278, 56–57.
- Dias, C. “Erva Patinha” tem Elevado teor de Proteínas e Fibras e o seu Consumo Regular Reduziria o Número de Acidentes Cardiovasculares. Available online: http://correiodosacores.pt/NewsDetail/ArtMID/383/ArticleID/25396/“Erva-patinha”-tem-elevado-teor-de-prote237nas-e-fibras-e-o-seu-consumo-regular-reduziria-o-n250mero-de-acidentes-cardiovasculares (accessed on 21 February 2021).
- Agenda Açores Top Azores: 19 Sabores açorianos que tens mesmo de provar. Available online: https://agendacores.pt/19-sabores-acorianos-que-tens-mesmo-de-provar/ (accessed on 16 February 2021).
- Lahaye, M.; Gomez-Pinchetti, J.-L.; del Rio, M.J.; Garcia-Reina, G. Natural decoloration, composition and increase in dietary fibre content of an edible marine algae, Ulva rigida (Chlorophyta), grown under different nitrogen conditions. J. Sci. Food Agric. 1995, 68, 99–104. [Google Scholar] [CrossRef]





| Challenges | Description |
|---|---|
| Production constraints | Scale-up system Contaminants |
| High costs | Capital and operational costs at upstream and downstream production processes |
| Environmental concerns | Resources (energy, water, and fertilizer) demand Not yet a priority of consumers |
| Health safety | Algae toxicity Contaminants (nonbiological and biological) |
| Legality | Legal approval for consumption and commercialization |
| Consumer’s perception | Colour, odor, flavor, and texture |
| Region/Country | Categories | Organizations | Regulations |
|---|---|---|---|
| EU | Foods, food supplements, and food additives | Scientific Committee on Food; European Food Safety Authority; European Committee for Standardization | General Food Law Regulation (EU) No 178/2002; Novel Food Regulation (EU) 2015/2283; Directive 2002/46/EC; SANCO/2006/E4/018 report; SEC (2008) 2976; SEC (2008) 2977; (EC) 1333/2008 and (EU) 231/2012 legislations |
| USA | Foods and food ingredients | Food and Drug Administration | Generally Recognized As Safe status |
| Canada | Novel foods | Health Canada | Guidelines for the Safety Assessment of Novel Foods |
| Australia and New Zealand | Novel foods and novel food ingredients | Food Standards Australia New Zealand | Food Standards Code |
| China | New food raw materials | National Health Commission | Food Safety Law; Chinese Administrative Measures for Safety Review of New Food Raw Materials |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendes, M.C.; Navalho, S.; Ferreira, A.; Paulino, C.; Figueiredo, D.; Silva, D.; Gao, F.; Gama, F.; Bombo, G.; Jacinto, R.; et al. Algae as Food in Europe: An Overview of Species Diversity and Their Application. Foods 2022, 11, 1871. https://doi.org/10.3390/foods11131871
Mendes MC, Navalho S, Ferreira A, Paulino C, Figueiredo D, Silva D, Gao F, Gama F, Bombo G, Jacinto R, et al. Algae as Food in Europe: An Overview of Species Diversity and Their Application. Foods. 2022; 11(13):1871. https://doi.org/10.3390/foods11131871
Chicago/Turabian StyleMendes, Madalena Caria, Sofia Navalho, Alice Ferreira, Cristina Paulino, Daniel Figueiredo, Daniel Silva, Fengzheng Gao, Florinda Gama, Gabriel Bombo, Rita Jacinto, and et al. 2022. "Algae as Food in Europe: An Overview of Species Diversity and Their Application" Foods 11, no. 13: 1871. https://doi.org/10.3390/foods11131871