In Vivo Assessment of the Calcium Salt-Forming Ability of a New Calcium Silicate-Based Intracanal Medicament: Bio-C Temp
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Materials
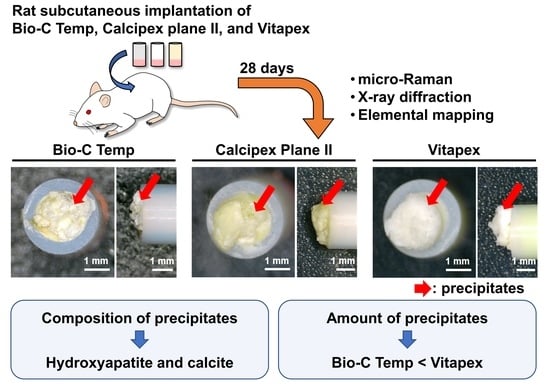
2.3. Rat Subcutaneous Implantation
2.4. Stereomicroscopy
2.5. Micro-Raman Spectrometry
2.6. XRD
2.7. Elemental Mapping
2.8. Statistical Analysis
3. Results
3.1. Stereomicroscopy
3.2. Micro-Raman Spectrometry
3.3. XRD
3.4. Elemental Mapping
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rafter, M. Apexification: A review. Dent. Traumatol. 2005, 21, 1–8. [Google Scholar] [CrossRef]
- Lin, J.C.; Lu, J.X.; Zeng, Q.; Zhao, W.; Li, W.Q.; Ling, J.Q. Comparison of mineral trioxide aggregate and calcium hydroxide for apexification of immature permanent teeth: A systematic review and meta-analysis. J. Formos. Med. Assoc. 2016, 115, 523–530. [Google Scholar] [CrossRef] [Green Version]
- Guerreiro, J.C.M.; Ochoa-Rodrígez, V.M.; Rodrigues, E.M.; Chavez-Andrade, G.M.; Tanomaru-Filho, M.; Guerreiro-Tanomaru, J.M.; Faria, G. Antibacterial activity, cytocompatibility and effect of Bio-C Temp bioceramic intracanal medicament on osteoblast biology. Int. Endod. J. 2021, 54, 1155–1165. [Google Scholar] [CrossRef]
- Oliveira, L.V.; Da Silva, G.R.; Souza, G.L.; Magalhães, T.E.A.; Barbosa, G.L.R.; Turrioni, A.P.; Moura, C.C.G. A laboratory evaluation of cell viability, radiopacity and tooth discoloration induced by regenerative endodontic materials. Int. Endod. J. 2020, 53, 1140–1152. [Google Scholar] [CrossRef]
- Villa, N.; Dos Santos, V.V.; Da Costa, U.M.; Mendes, A.T.; Duarte, P.H.M.; Da Rosa, R.A.; Pereira, J.R.; Só, M.V.R. A new calcium silicate-based root canal dressing: Physical and chemical properties, cytotoxicity and dentinal tubule penetration. Braz. Dent. J. 2020, 31, 598–604. [Google Scholar] [CrossRef]
- Lopes, C.S.; Delfino, M.M.; Tanomaru-Filho, M.; Sasso-Cerri, E.; Guerreiro-Tanomaru, J.M.; Cerri, P.S. Hepatic enzymes and immunoinflammatory response to Bio-C Temp bioceramic intracanal medication implanted into the subcutaneous tissue of rats. Sci. Rep. 2022, 12, 2788. [Google Scholar] [CrossRef]
- Oliveira, L.V.; Souza, G.L.; Silva, G.R.; Magalhães, T.E.A.; Freitas, G.A.N.; Turrioni, A.P.; Barbosa, G.L.R.; Moura, C.C.G. Biological parameters, discolouration and radiopacity of calcium silicate-based materials in a simulated model of partial pulpotomy. Int. Endod. J. 2021, 54, 2133–2144. [Google Scholar] [CrossRef]
- Holland, R.; de Mello, W.; Nery, M.J.; Bernabe, P.F.; de Souza, V. Reaction of human periapical tissue to pulp extirpation and immediate root canal filling with calcium hydroxide. J. Endod. 1977, 3, 63–67. [Google Scholar] [CrossRef]
- Higashi, T.; Okamoto, H. Characteristics and effects of calcified degenerative zones on the formation of hard tissue barriers in amputated canine dental pulp. J. Endod. 1996, 22, 168–172. [Google Scholar] [CrossRef]
- Seux, D.; Couble, M.L.; Hartmann, D.J.; Gauthier, J.P.; Magloire, H. Odontoblast-like cytodifferentiation of human dental pulp cells in vitro in the presence of a calcium hydroxide-containing cement. Arch. Oral Biol. 1991, 36, 117–128. [Google Scholar] [CrossRef]
- Tziafas, D.; Economides, N. Formation of crystals on the surface of calcium hydroxide-containing materials in vitro. J. Endod. 1999, 25, 539–542. [Google Scholar] [CrossRef]
- Tziafas, D.; Panagiotakopoulos, N.; Komnenou, A. Immunolocalization of fibronectin during the early response of dog dental pulp to demineralized dentine or calcium hydroxide-containing cement. Arch. Oral Biol. 1995, 40, 23–31. [Google Scholar] [CrossRef]
- Yoshiba, K.; Yoshiba, N.; Nakamura, H.; Iwaku, M.; Ozawa, H. Immunolocalization of fibronectin during reparative dentinogenesis in human teeth after pulp capping with calcium hydroxide. J. Dent. Res. 1996, 75, 1590–1597. [Google Scholar] [CrossRef]
- Boskey, A.L.; Maresca, M.; Ullrich, W.; Doty, S.B.; Butler, W.T.; Prince, C.W. Osteopontin-hydroxyapatite interactions in vitro: Inhibition of hydroxyapatite formation and growth in a gelatin-gel. Bone Miner. 1993, 22, 147–159. [Google Scholar] [CrossRef]
- Tartaix, P.H.; Doulaverakis, M.; George, A.; Fisher, L.W.; Butler, W.T.; Qin, C.; Salih, E.; Tan, M.; Fujimoto, Y.; Spevak, L.; et al. In vitro effects of dentin matrix protein-1 on hydroxyapatite formation provide insights into in vivo functions. J. Biol. Chem. 2004, 279, 18115–18120. [Google Scholar] [CrossRef] [Green Version]
- Ibn Belal, R.S.; Edanami, N.; Yoshiba, K.; Yoshiba, N.; Ohkura, N.; Takenaka, S.; Noiri, Y. Comparison of calcium and hydroxyl ion release ability and in vivo apatite-forming ability of three bioceramic-containing root canal sealers. Clin. Oral Investig. 2022, 26, 1443–1451. [Google Scholar] [CrossRef]
- Reyes-Carmona, J.F.; Santos, A.S.; Figueiredo, C.P.; Baggio, C.H.; Felippe, M.C.; Felippe, W.T.; Cordeiro, M.M. Host-mineral trioxide aggregate inflammatory molecular signaling and biomineralization ability. J. Endod. 2010, 36, 1347–1353. [Google Scholar] [CrossRef]
- Stammeier, J.A.; Purgstaller, B.; Hippler, D.; Mavromatis, V.; Dietzel, M. In-situ Raman spectroscopy of amorphous calcium phosphate to crystalline hydroxyapatite transformation. MethodsX 2018, 5, 1241–1250. [Google Scholar] [CrossRef]
- Donnelly, F.C.; Purcell-Milton, F.; Framont, V.; Cleary, O.; Dunne, P.W.; Gun’ko, Y.K. Synthesis of CaCO3 nano- and micro-particles by dry ice carbonation. Chem. Commun. 2017, 53, 6657–6660. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.L.; Chen, D.M.; Wang, D.; Phillips, D.L. Time-resolved resonance Raman and density functional theory investigation of iodocyclopropanation and addition reactions with alkenes after ultraviolet photolysis of iodoform. J. Org. Chem. 2002, 67, 4228–4235. [Google Scholar] [CrossRef]
- Fava, L.R.; Saunders, W.P. Calcium hydroxide pastes: Classification and clinical indications. Int. Endod. J. 1999, 32, 257–282. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, S.; Mazumdar, D.; Ray, P.K.; Bhattacharya, B. Comparative evaluation of different forms of calcium hydroxide in apexification. Contemp. Clin. Dent. 2014, 5, 6–12. [Google Scholar] [CrossRef]
- Hinata, G.; Yoshiba, K.; Han, L.; Edanami, N.; Yoshiba, N.; Okiji, T. Bioactivity and biomineralization ability of calcium silicate-based pulp-capping materials after subcutaneous implantation. Int. Endod. J. 2017, 50 (Suppl. 2), e40–e51. [Google Scholar] [CrossRef] [Green Version]
- Silva, E.C.A.; Tanomaru-Filho, M.; Silva, G.F.; Lopes, C.S.; Cerri, P.S.; Guerreiro Tanomaru, J.M. Evaluation of the biological properties of two experimental calcium silicate sealers: An in vivo study in rats. Int. Endod. J. 2021, 54, 100–111. [Google Scholar] [CrossRef]
- Alves Silva, E.C.; Tanomaru-Filho, M.; da Silva, G.F.; Delfino, M.M.; Cerri, P.S.; Guerreiro-Tanomaru, J.M. Biocompatibility and bioactive potential of new calcium silicate-based endodontic sealers: Bio-C sealer and Sealer Plus BC. J. Endod. 2020, 46, 1470–1477. [Google Scholar] [CrossRef]
- Lanfranco, A.M.; Schofield, P.F.; Murphy, P.J.; Hodson, M.E.; Mosselmans, J.F.W.; Valsami-Jones, E. Characterization and identification of mixed-metal phosphates in soils: The application of Raman spectroscopy. Min. Mag. 2003, 67, 1299–1316. [Google Scholar] [CrossRef]
- Yang, X.; Tian, J.; Li, M.; Chen, W.; Liu, H.; Wang, Z.; Haapasalo, M.; Shen, Y.; Wei, X. Biocompatibility of a new calcium silicate-based root canal sealer mediated via the modulation of macrophage polarization in a rat model. Materials 2022, 15, 1962. [Google Scholar] [CrossRef]
- Siboni, F.; Taddei, P.; Prati, C.; Gandolfi, M.G. Properties of NeoMTA Plus and MTA Plus cements for endodontics. Int. Endod. J. 2017, 50 (Suppl. S2), e83–e94. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.; Leng, Y. Theoretical analysis of calcium phosphate precipitation in simulated body fluid. Biomaterials 2005, 26, 1097–1108. [Google Scholar] [CrossRef]
- Nekoofar, M.H.; Namazikhah, M.S.; Sheykhrezae, M.S.; Mohammadi, M.M.; Kazemi, A.; Aseeley, Z.; Dummer, P. pH of pus collected from periapical abscesses. Int. Endod. J. 2009, 42, 534–538. [Google Scholar] [CrossRef]
- Miyaji, F.; Iwai, M.; Kokubo, T.; Nakamura, T. Bonelike apatite formation on alkali-treated silicone. Phosphorus Res. Bull. 1996, 6, 119–122. [Google Scholar] [CrossRef] [Green Version]
- Muramatsu, T.; Kashiwagi, S.; Ishizuka, H.; Matsuura, Y.; Furusawa, M.; Kimura, M.; Shibukawa, Y. Alkaline extracellular conditions promote the proliferation and mineralization of a human cementoblast cell line. Int. Endod. J. 2019, 52, 639–645. [Google Scholar] [CrossRef]
- Maeda, H.; Nakano, T.; Tomokiyo, A.; Fujii, S.; Wada, N.; Monnouchi, S.; Hori, K.; Akamine, A. Mineral trioxide aggregate induces bone morphogenetic protein-2 expression and calcification in human periodontal ligament cells. J. Endod. 2010, 36, 647–652. [Google Scholar] [CrossRef]
- Fujita, Y.; Yamamuro, T.; Nakamura, T.; Kotani, S.; Ohtsuki, C.; Kokubo, T. The bonding behavior of calcite to bone. J. Biomed. Mater. Res. 1991, 25, 991–1003. [Google Scholar] [CrossRef]
- Takata, T.; Katauchi, K.; Akagawa, Y.; Nikai, H. New connective tissue attachment formation on various biomaterials implanted in roots. Int. J. Oral Maxillofac. Implant. 1994, 9, 77–84. [Google Scholar]
- Edanami, N.; Ibn Belal, R.S.; Yoshiba, K.; Yoshiba, N.; Ohkura, N.; Takenaka, S.; Noiri, Y. Effect of a resin-modified calcium silicate cement on inflammatory cell infiltration and reparative dentin formation after pulpotomy in rat molars. Aust. Endod. J. 2022, 48, 297–304. [Google Scholar] [CrossRef]
- Kawai, T.; Takemoto, M.; Fujibayashi, S.; Akiyama, H.; Tanaka, M.; Yamaguchi, S.; Pattanayak, D.K.; Doi, K.; Matsushita, T.; Nakamura, T.; et al. Osteoinduction on acid and heat treated porous Ti metal samples in canine muscle. PLoS ONE 2014, 9, e88366. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi, Z.; Shalavi, S.; Yazdizadeh, M. Antimicrobial activity of calcium hydroxide in endodontics: A review. Chonnam Med. J. 2012, 48, 133–140. [Google Scholar] [CrossRef] [Green Version]
- McKee, M.D.; Hoac, B.; Addison, W.N.; Barros, N.M.; Millán, J.L.; Chaussain, C. Extracellular matrix mineralization in periodontal tissues: Noncollagenous matrix proteins, enzymes, and relationship to hypophosphatasia and X-linked hypophosphatemia. Periodontol. 2000 2013, 63, 102–122. [Google Scholar] [CrossRef] [Green Version]
- Yoneda, N.; Noiri, Y.; Matsui, S.; Kuremoto, K.; Maezono, H.; Ishimoto, T.; Nakano, T.; Ebisu, S.; Hayashi, M. Development of a root canal treatment model in the rat. Sci. Rep. 2017, 7, 3315. [Google Scholar] [CrossRef]
- Edanami, N.; Yoshiba, K.; Shirakashi, M.; Ibn Belal, R.S.; Yoshiba, N.; Ohkura, N.; Tohma, A.; Takeuchi, R.; Okiji, T.; Noiri, Y. Impact of remnant healthy pulp and apical tissue on outcomes after simulated regenerative endodontic procedure in rat molars. Sci. Rep. 2020, 10, 20967. [Google Scholar] [CrossRef]
- Kohout, G.D.; He, J.; Primus, C.M.; Opperman, L.A.; Woodmansey, K.F. Comparison of Quick-Set and mineral trioxide aggregate root-end fillings for the regeneration of apical tissues in dogs. J. Endod. 2015, 41, 248–252. [Google Scholar] [CrossRef]




| Material | Manufacturer | Composition |
|---|---|---|
| Bio-C Temp | Angelus, Londrina, PR, Brazil | Tricalcium silicate, dicalcium silicate, tricalcium aluminate, calcium oxide, base resin, calcium tungstate, polyethylene glycol, and titanium oxide |
| Calcipex Plane II | Nishika, Shimonoseki, Japan | Calcium hydroxide (48%), purified water, and others |
| Vitapex | Neo Dental Chemical Products, Tokyo, Japan | Iodoform (40.4%), calcium hydroxide (30.3%), silicone oil (22.4%), and others |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Edanami, N.; Belal, R.S.I.; Takenaka, S.; Yoshiba, K.; Gutierrez, R.E.B.; Takahara, S.; Yoshiba, N.; Ohkura, N.; Noiri, Y. In Vivo Assessment of the Calcium Salt-Forming Ability of a New Calcium Silicate-Based Intracanal Medicament: Bio-C Temp. Dent. J. 2023, 11, 91. https://doi.org/10.3390/dj11040091
Edanami N, Belal RSI, Takenaka S, Yoshiba K, Gutierrez REB, Takahara S, Yoshiba N, Ohkura N, Noiri Y. In Vivo Assessment of the Calcium Salt-Forming Ability of a New Calcium Silicate-Based Intracanal Medicament: Bio-C Temp. Dentistry Journal. 2023; 11(4):91. https://doi.org/10.3390/dj11040091
Chicago/Turabian StyleEdanami, Naoki, Razi Saifullah Ibn Belal, Shoji Takenaka, Kunihiko Yoshiba, Rosa Edith Baldeon Gutierrez, Shintaro Takahara, Nagako Yoshiba, Naoto Ohkura, and Yuichiro Noiri. 2023. "In Vivo Assessment of the Calcium Salt-Forming Ability of a New Calcium Silicate-Based Intracanal Medicament: Bio-C Temp" Dentistry Journal 11, no. 4: 91. https://doi.org/10.3390/dj11040091