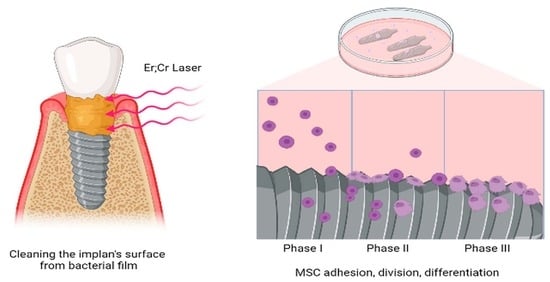
Laser Cleaning Improves Stem Cell Adhesion on the Dental Implant Surface during Peri-Implantitis Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Implants Preparation
2.2. Evaluation of the Ability of MSCs to Attach to the Implant Surface In Vitro
2.3. Scanning Electron Microscopy
2.4. Obtaining Primary Cultures of Mesenchymal Cells from Mouse Bone Marrow
2.5. Laser Scanning and Fluorescence Microscopy
2.6. Determination of the Presence of Cells on the Surfaces of Implants by Flow Cytometry
2.7. Cell Differentiation Assay
2.8. Statistical Analyses
3. Results
3.1. Scanning Electron Microscopy
3.2. Mesenchymal Stem Cells Adhesion on the Surfaces of the Differently Treated Implants
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heitz-Mayfield, L.J.A.; Salvi, G.E. Peri-Implant Mucositis. J. Periodontol. 2018, 89, S257–S266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verardi, S.; Valente, N.A. Peri-Implantitis: Application of a Protocol for the Regeneration of Deep Osseous Defects. A Retrospective Case Series. Int. J. Environ. Res. Public. Health 2021, 18, 12658. [Google Scholar] [CrossRef] [PubMed]
- Poli, P.P.; Cicciu, M.; Beretta, M.; Maiorana, C. Peri-Implant Mucositis and Peri-Implantitis: A Current Understanding of Their Diagnosis, Clinical Implications, and a Report of Treatment Using a Combined Therapy Approach. J. Oral Implantol. 2017, 43, 45–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, R.; Li, M.; Gregory, R.L. Bacterial interactions in dental biofilm. Virulence 2014, 2, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Butera, A.; Pascadopoli, M.; Pellegrini, M.; Gallo, S.; Zampetti, P.; Scribante, A. Oral Microbiota in Patients with Peri-Implant Disease: A Narrative Review. Appl. Sci. 2022, 12, 3250. [Google Scholar] [CrossRef]
- Belibasakis, G.N.; Charalampakis, G.; Bostanci, N.; Stadlinger, B. Peri-implant infections of oral biofilm etiology. Adv. Exp. Med. Biol. 2015, 830, 69–84. [Google Scholar] [CrossRef]
- Hu, Z.; Wu, D.; Zhao, Y.; Chen, S.; Li, Y. Inflammatory cytokine profiles in the crevicular fluid around clinically healthy dental implants compared to the healthy contralateral side during the early stages of implant function. Arch. Oral Biol. 2019, 108, 104509. [Google Scholar] [CrossRef]
- Pokrowiecki, R.; Mielczarek, A.; Zaręba, T.; Tyski, S. Oral Microbiome and Peri-Implant Diseases: Where Are We Now? Ther. Clin. Risk Manag. 2017, 13, 1529–1542. [Google Scholar] [CrossRef] [Green Version]
- Ramesh, D.; Sridhar, S.; Siddiqui, D.A.; Valderrama, P.; Rodrigues, D.C. Detoxification of Titanium Implant Surfaces: Evaluation of Surface Morphology and Bone-Forming Cell Compatibility. J. Bio- Tribo-Corros. 2017, 3, 50. [Google Scholar] [CrossRef]
- Elsreti, M.; Smeo, K.; Gutknecht, N. The Effectiveness of Diode Lasers in Detoxification of Exposed Implant Surfaces in Comparison with Mechanical and Chemical Measures in the Treatment of Peri-Implantitis: A Literature Review. Lasers Dent. Sci. 2022, 6, 1–14. [Google Scholar] [CrossRef]
- Takasaki, A.A.; Aoki, A.; Mizutani, K.; Kikuchi, S.; Oda, S.; Ishikawa, I. Er:YAG Laser Therapy for Peri-Implant Infection: A Histological Study. Lasers Med. Sci. 2007, 22, 143–157. [Google Scholar] [CrossRef] [PubMed]
- de Val, J.E.M.S.; Gómez-Moreno, G.; Ruiz-Linares, M.; Prados-Frutos, J.C.; Gehrke, S.A.; Calvo-Guirado, J.L. Effects of Surface Treatment Modification and Implant Design in Implants Placed Crestal and Subcrestally Applying Delayed Loading Protocol. J. Craniofac. Surg. 2017, 28, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Renvert, S.; Lindahl, C.; Roos Jansåker, A.-M.; Persson, G.R. Treatment of Peri-Implantitis Using an Er:YAG Laser or an Air-Abrasive Device: A Randomized Clinical Trial: Non-Surgical Treatment of Peri-Implantitis. J. Clin. Periodontol. 2011, 38, 65–73. [Google Scholar] [CrossRef]
- Dominiak, M.; Matys, J. Assessment of Pain When Uncovering Implants with Er:YAG Laser or Scalpel for Second Stage Surgery. Adv. Clin. Exp. Med. 2016, 25, 1179–1184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guler, B.; Uraz, A.; Yalım, M.; Bozkaya, S. The Comparison of Porous Titanium Granule and Xenograft in the Surgical Treatment of Peri-Implantitis: A Prospective Clinical Study: Regenerative Theraphy of Peri-Implantitis. Clin. Implant Dent. Relat. Res. 2017, 19, 316–327. [Google Scholar] [CrossRef]
- Bertoldi, C.; Lusuardi, D.; Battarra, F.; Sassatelli, P.; Spinato, S.; Zaffe, D. The Maintenance of Inserted Titanium Implants: In-Vitro Evaluation of Exposed Surfaces Cleaned with Three Different Instruments. Clin. Oral Implants Res. 2017, 28, 57–63. [Google Scholar] [CrossRef]
- Ozkan Karaca, E.; Tunar, O.L. In Vitro Evaluation of Surface Cleaning Methods in Two Different Implant Defect Angulations: A Pilot Study. Biotechnol. Biotechnol. Equip. 2021, 35, 560–566. [Google Scholar] [CrossRef]
- Matsubara, V.H.; Leong, B.W.; Leong, M.J.L.; Lawrence, Z.; Becker, T.; Quaranta, A. Cleaning Potential of Different Air Abrasive Powders and Their Impact on Implant Surface Roughness. Clin. Implant Dent. Relat. Res. 2020, 22, 96–104. [Google Scholar] [CrossRef]
- Wang, C.W.; Ashnagar, S.; Di Gianfilippo, R.; Arnett, M.; Kinney, J.; Wang, H.L. Laser-assisted regenerative surgical therapy for peri-implantitis: A randomized controlled clinical trial. J. Periodontol. 2021, 92, 378–388. [Google Scholar] [CrossRef]
- Geisinger, M.L.; Holmes, C.M.; Vassilopoulos, P.J.; Geurs, N.C.; Reddy, M.S. Is Laser Disinfection an Effective Adjunctive Treatment to Bone Augmentation for Peri-Implantitis? A Review of Current Evidence. Clin. Adv. Periodontics 2014, 4, 274–279. [Google Scholar] [CrossRef]
- Jepsen, K.; Jepsen, S.; Laine, M.L.; Anssari Moin, D.; Pilloni, A.; Zeza, B.; Sanz, M.; Ortiz-Vigon, A.; Roos-Jansåker, A.M.; Renvert, S. Reconstruction of Peri-Implant Osseous Defects: A Multicenter Randomized Trial. J. Dent. Res. 2016, 95, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Furtsev, T.V.; Zeer, G.M. Comparative Research of Implants with Three Types of Surface Processing (TiUnite, SLA, RBM), Control, with Periimplantitis and Processed by 2780 Nm Er;Cr;YSGG Laser. Stomatologiya 2019, 98, 52. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Bohner, L.; Hanisch, M.; Kleinheinz, J.; Sielker, S. Influence of Implant Material and Surface on Mode and Strength of Cell/Matrix Attachment of Human Adipose Derived Stromal Cell. Int. J. Mol. Sci. 2020, 21, 4110. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Song, B.; Pu, J.; Wada, T.; Reid, B.; Tai, G.; Wang, F.; Guo, A.; Walczysko, P.; Gu, Y.; et al. Electrical Signals Control Wound Healing through Phosphatidylinositol-3-OH Kinase-Gamma and PTEN. Nature 2006, 442, 457–460. [Google Scholar] [CrossRef]
- Ogle, O.E. Implant surface material, design, and osseointegration. Dent. Clin. N. Am. 2015, 59, 505–520. [Google Scholar] [CrossRef]
- Sul, Y.T.; Johansson, C.B.; Röser, K.; Albrektsson, T. Qualitative and quantitative observations of bone tissue reactions to anodised implants. Biomaterials 2002, 23, 1809–1817. [Google Scholar] [CrossRef]
- Barfeie, A.; Wilson, J.; Rees, J. Implant surface characteristics and their effect on osseointegration. Br. Dent. J. 2015, 218, E9. [Google Scholar] [CrossRef]
- Wennerberg, A.; Albrektsson, T.; Chrcanovic, B. Long-term clinical outcome of implants with different surface modifications. Eur. J. Oral Implantol. 2018, 11 (Suppl. 1), S123–S136. [Google Scholar]
- Karl, M.; Albrektsson, T. Clinical performance of dental implants with a moderately rough (TiUnite) surface: A meta-analysis of prospective clinical studies. Int. J. Oral Maxillofac. Implants 2017, 32, 717–734. [Google Scholar] [CrossRef] [Green Version]
- Glauser, R.; Portmann, M.; Ruhstaller, P.; Lundgren, A.K.; Hämmerle, C.; Gottlow, J. Stability measurements of immediately loaded machined and oxidized implants in the posterior maxilla. A comparative clinical study using resonance frequency analysis. Appl. Osseointegr. Res. 2001, 2, 27–29. [Google Scholar]
- Pachauri, P.; Bathala, L.R.; Sangur, R. Techniques for dental implant nanosurface modifications. J. Adv. Prosthodont. 2014, 6, 498–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matos, G.R.M. Surface Roughness of Dental Implant and Osseointegration. J. Maxillofac. Oral Surg. 2021, 20, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Renvert, S.; Polyzois, I. Risk indicators for peri-implant mucositis: A systematic literature review. J. Clin. Periodontol. 2015, 42 (Suppl. 16), S172–S186. [Google Scholar] [CrossRef] [PubMed]
- Salvi, G.E.; Cosgarea, R.; Sculean, A. Prevalence and Mechanisms of Peri-implant Diseases. J. Dent. Res. 2016, 96, 31–37. [Google Scholar] [CrossRef]
- Wennerberg, A.; Sennerby, L.; Kultje, C.; Lekholm, U. Some soft tissue characteristics at implant abutments with different surface topography. A study in humans. J Clin. Periodontol. 2003, 30, 88–94. [Google Scholar] [CrossRef]
- Renvert, S.; Lindahl, C.; Persson, G.R. The incidence of peri-implantitis for two different implant systems over a period of thirteen years. J. Clin. Periodontol. 2012, 39, 1191–1197. [Google Scholar] [CrossRef]
- Khoury, F.; Keeve, P.L.; Ramanauskaite, A.; Schwarz, F.; Koo, K.-T.; Sculean, A.; Romanos, G. Surgical treatment of peri-implantitis—Consensus report of working group 4. Int. Dent. J. 2019, 69 (Suppl. 2), 18–22. [Google Scholar] [CrossRef] [Green Version]
- Deppe, H.; Horch, H.H.; Henke, J.; Donath, K. Peri-implant care of ailing implants with the carbon dioxide laser. Int. J. Oral Maxillofac. Implants 2001, 16, 659–667. [Google Scholar] [PubMed]
- Scribante, A.; Butera, A.; Alovisi, M. Customized Minimally Invasive Protocols for the Clinical and Microbiological Management of the Oral Microbiota. Microorganisms 2022, 10, 675. [Google Scholar] [CrossRef]
- Cosola, S.; Oldoini, G.; Giammarinaro, E.; Covani, U.; Genovesi, A.; Marconcini, S. The effectiveness of the information-motivation model and domestic brushing with a hypochlorite-based formula on peri-implant mucositis: A randomized clinical study. Clin. Exp. Dent. Res. 2022, 8, 350–358. [Google Scholar] [CrossRef]
- Rapone, B.; Ferrara, E.; Santacroce, L.; Topi, S.; Gnoni, A.; Dipalma, G.; Mancini, A.; Di Domenico, M.; Tartaglia, G.M.; Scarano, A.; et al. The Gaseous Ozone Therapy as a Promising Antiseptic Adjuvant of Periodontal Treatment: A Randomized Controlled Clinical Trial. Int. J. Environ. Res. Public Health 2022, 19, 985. [Google Scholar] [CrossRef] [PubMed]
- Wenzler, J.-S.; Krause, F.; Böcher, S.; Falk, W.; Birkenmaier, A.; Conrads, G.; Braun, A. Antimicrobial Impact of Different Air-Polishing Powders in a Subgingival Biofilm Model. Antibiotics 2021, 10, 1464. [Google Scholar] [CrossRef] [PubMed]
- Baldi, D.; De Giorgis, L.; Menini, M.; Motta, F.; Colombo, J. Efficacy of Instruments for Professional Oral Hygiene on Dental Implants: A Systematic Review. Appl. Sci. 2022, 12, 26. [Google Scholar] [CrossRef]
- Butera, A.; Pascadopoli, M.; Pellegrini, M.; Gallo, S.; Zampetti, P.; Cuggia, G.; Scribante, A. Domiciliary Use of Chlorhexidine vs. Postbiotic Gels in Patients with Peri-Implant Mucositis: A Split-Mouth Randomized Clinical Trial. Appl. Sci. 2022, 12, 2800. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furtsev, T.V.; Koshmanova, A.A.; Zeer, G.M.; Nikolaeva, E.D.; Lapin, I.N.; Zamay, T.N.; Kichkailo, A.S. Laser Cleaning Improves Stem Cell Adhesion on the Dental Implant Surface during Peri-Implantitis Treatment. Dent. J. 2023, 11, 30. https://doi.org/10.3390/dj11020030
Furtsev TV, Koshmanova AA, Zeer GM, Nikolaeva ED, Lapin IN, Zamay TN, Kichkailo AS. Laser Cleaning Improves Stem Cell Adhesion on the Dental Implant Surface during Peri-Implantitis Treatment. Dentistry Journal. 2023; 11(2):30. https://doi.org/10.3390/dj11020030
Chicago/Turabian StyleFurtsev, Taras V., Anastasia A. Koshmanova, Galina M. Zeer, Elena D. Nikolaeva, Ivan N. Lapin, Tatiana N. Zamay, and Anna S. Kichkailo. 2023. "Laser Cleaning Improves Stem Cell Adhesion on the Dental Implant Surface during Peri-Implantitis Treatment" Dentistry Journal 11, no. 2: 30. https://doi.org/10.3390/dj11020030