Improved Oxide Ion Conductivity of Hexagonal Perovskite-Related Oxides Ba3W1+xV1−xO8.5+x/2
Abstract
:1. Introduction
2. Results and Discussion
2.1. Formation of Ba3W1+xV1−xO8.5+x/2 Solid Solutions and Their Electrical Conductivities
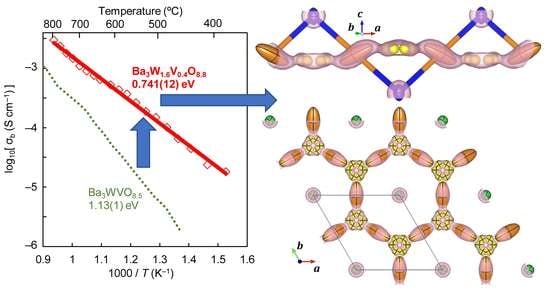
2.2. Oxide Ion Conduction of Ba3W1.6V0.4O8.8
2.3. Crystal Structure Analyses of Ba3W1.6V0.4O8.8 and Ba3WVO8.5
2.4. Neutron Scattering Length Density Analyses of Ba3W1.6V0.4O8.8 and Ba3WVO8.5
2.5. Structural Origins of High Oxide Ion Conductivity in Ba3W1.6V0.4O8.8
3. Materials and Methods
3.1. Synthesis and Characterization of Ba3W1+xV1−xO8.5+x/2 (x = −0.1, −0.05, 0, 0.05, 0.1, 0.25, 0.4, 0.5, 0.6, and 0.75)
3.2. Electrical Properties of Ba3W1.6V0.4O8.8
3.3. Structural and MEM Neutron Scattering Length Density Analyses Using Neutron Diffraction Data of Ba3WVO8.5 and Ba3W1.6V0.4O8.8
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nakayama, S.; Kageyama, T.; Aono, H.; Sadaoka, Y. Ionic Conductivity of Lanthanoid Silicates, Ln10(SiO4)6O3 (Ln=La, Nd, Sm, Gd, Dy, Y, Ho, Er and Yb). J. Mater. Chem. 1995, 5, 1801–1805. [Google Scholar] [CrossRef]
- Brett, D.J.L.; Atkinson, A.; Brandon, N.P.; Skinner, S.J. Intermediate Temperature Solid Oxide Fuel Cells. Chem. Soc. Rev. 2008, 37, 1568–1578. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhao, H.; Liang, J.; Luo, Y.; Chen, G.; Shi, X.; Lu, S.; Gao, S.; Hu, J.; Liu, Q.; et al. A-site perovskite oxides: An emerging functional material for electrocatalysis and photocatalysis. J. Mater. Chem. A 2021, 9, 6650–6670. [Google Scholar] [CrossRef]
- Jacobson, A.J. Materials for Solid Oxide Fuel Cells. Chem. Mater. 2010, 22, 660–674. [Google Scholar] [CrossRef]
- Liao, Y.W.; Kawabata, S.; Yabutsuka, T.; Chen, W.J.; Okumura, H.; Takai, S. Low Temperature Phase Transition Phenomena in Ba- and Pb-Substituted La2Mo2O9 Oxide Ion Conductors. Solid State Ionics 2020, 354, 115405. [Google Scholar] [CrossRef]
- Kharton, V.V.; Tsipis, E.V.; Kolotygin, V.A.; Avdeev, M.; Kennedy, B.J. Ionic Conductivity and Thermal Expansion of Anion-Deficient Sr11Mo4O23 Perovskite. J. Solid State Electrochem. 2020, 24, 2943–2951. [Google Scholar] [CrossRef]
- Gazda, M.; Miruszewski, T.; Jaworski, D.; Mielewczyk-Gryń, A.; Skubida, W.; Wachowski, S.; Winiarz, P.; Dzierzgowski, K.; Łapiński, M.; Szpunar, I.; et al. Novel Class of Proton Conducting Materials—High Entropy Oxides. ACS Mater. Lett. 2020, 2, 1315–1321. [Google Scholar] [CrossRef]
- Istomin, S.Y.; Lyskov, N.V.; Mazo, G.N.; Antipov, E.V. Electrode Materials Based on Complex D-Metal Oxides for Symmetrical Solid Oxide Fuel Cells. Russ. Chem. Rev. 2021, 90, 644–676. [Google Scholar] [CrossRef]
- Tarasova, N.A.; Animitsa, I.E.; Galisheva, A.O.; Medvedev, D.A. Layered and Hexagonal Perovskites as Novel Classes of Proton-Conducting Solid Electrolytes. A Focus Review. Electrochem. Mater. Technol. 2022, 1, 20221004. [Google Scholar] [CrossRef]
- Coduri, M.; Karlsson, M.; Malavasi, L. Structure–Property Correlation in Oxide-Ion and Proton Conductors for Clean Energy Applications: Recent Experimental and Computational Advancements. J. Mater. Chem. A 2022, 10, 5082–5110. [Google Scholar] [CrossRef]
- Zhang, W.; Yashima, M. Recent Developments in Oxide Ion Conductors: Focusing on Dion–Jacobson Phases. Chem. Commun. 2023, 59, 134–152. [Google Scholar] [CrossRef] [PubMed]
- Yasui, Y.; Tansho, M.; Fujii, K.; Sakuda, Y.; Goto, A.; Ohki, S.; Mogami, Y.; Iijima, T.; Kobayashi, S.; Kawaguchi, S.; et al. Hidden Chemical Order in Disordered Ba7Nb4MoO20 Revealed by Resonant X-Ray Diffraction and Solid-State NMR. Nat. Commun. 2023, 14, 2337. [Google Scholar] [CrossRef] [PubMed]
- Andreev, R.D.; Korona, D.V.; Anokhina, I.A.; Animitsa, I.E. Novel Nb5+-doped hexagonal perovskite Ba5In2Al2ZrO13 (structure, hydration, electrical conductivity). Chim. Techno Acta 2022, 9, 20229414. [Google Scholar] [CrossRef]
- Kluczny, M.; Song, J.T.; Akbay, T.; Niwa, E.; Takagaki, A.; Ishihara, T. Sillén–Aurivillius Phase Bismuth Niobium Oxychloride, Bi4NbO8Cl, as a New Oxide-Ion Conductor. J. Mater. Chem. A 2022, 10, 2550–2558. [Google Scholar] [CrossRef]
- Huang, K.; Feng, M.; Goodenough, J.B. Synthesis and Electrical Properties of Dense Ce0.9Gd0.1O1.95 Ceramics. J. Am. Ceram. Soc. 2005, 81, 357–362. [Google Scholar] [CrossRef]
- Ishihara, T.; Matsuda, H.; Takita, Y. Doped LaGaO3 Perovskite Type Oxide as a New Oxide Ionic Conductor. J. Am. Chem. Soc. 1994, 116, 3801–3803. [Google Scholar] [CrossRef]
- Kwon, O.H.; Choi, G.M. Electrical Conductivity of Thick Film YSZ. Solid State Ionics 2006, 177, 3057–3062. [Google Scholar] [CrossRef]
- Yashima, M.; Tsujiguchi, T.; Sakuda, Y.; Yasui, Y.; Zhou, Y.; Fujii, K.; Torii, S.; Kamiyama, T.; Skinner, S.J. High Oxide-Ion Conductivity through the Interstitial Oxygen Site in Ba7Nb4MoO20-Based Hexagonal Perovskite Related Oxides. Nat. Commun. 2021, 12, 556. [Google Scholar] [CrossRef]
- Yashima, M.; Tsujiguchi, T.; Fujii, K.; Niwa, E.; Nishioka, S.; Hester, J.R.; Maeda, K. Direct Evidence for Two-Dimensional Oxide-Ion Diffusion in the Hexagonal Perovskite-Related Oxide Ba3MoNbO8.5−δ. J. Mater. Chem. A 2019, 7, 13910–13916. [Google Scholar] [CrossRef]
- McCombie, K.S.; Wildman, E.J.; Ritter, C.; Smith, R.I.; Skakle, J.M.S.; Mclaughlin, A.C. Relationship between the Crystal Structure and Electrical Properties of Oxide Ion Conducting Ba3W1.2Nb0.8O8.6. Inorg. Chem. 2018, 57, 11942–11947. [Google Scholar] [CrossRef]
- Gilane, A.; Fop, S.; Sher, F.; Smith, R.I.; Mclaughlin, A.C. The Relationship between Oxide-Ion Conductivity and Cation Vacancy Order in the Hybrid Hexagonal Perovskite Ba3VWO8.5. J. Mater. Chem. A 2020, 8, 16506–16514. [Google Scholar] [CrossRef]
- Gilane, A.; Fop, S.; Tawse, D.N.; Ritter, C.; Mclaughlin, A.C. Variable Temperature Neutron Diffraction Study of the Oxide Ion Conductor Ba3VWO8.5. Inorg. Chem. 2022, 61, 1597–1602. [Google Scholar] [CrossRef] [PubMed]
- Kuang, X.; Allix, M.; Ibberson, R.M.; Claridge, J.B.; Niu, H.; Rosseinsky, M.J. Oxygen Vacancy Ordering Phenomena in the Mixed-Conducting Hexagonal Perovskite Ba7Y2Mn3Ti2O20. Chem. Mater. 2007, 19, 2884–2893. [Google Scholar] [CrossRef]
- Fop, S.; Skakle, J.M.S.; McLaughlin, A.C.; Connor, P.A.; Irvine, J.T.S.; Smith, R.I.; Wildman, E.J. Oxide Ion Conductivity in the Hexagonal Perovskite Derivative Ba3MoNbO8.5. J. Am. Chem. Soc. 2016, 138, 16764–16769. [Google Scholar] [CrossRef]
- Bernasconi, A.; Tealdi, C.; Malavasi, L. High-Temperature Structural Evolution in the Ba3Mo(1–x)WxNbO8.5 System and Correlation with Ionic Transport Properties. Inorg. Chem. 2018, 57, 6746–6752. [Google Scholar] [CrossRef]
- Murakami, T.; Shibata, T.; Yasui, Y.; Fujii, K.; Hester, J.R.; Yashima, M. High Oxide-Ion Conductivity in a Hexagonal Perovskite-Related Oxide Ba7Ta3.7Mo1.3O20.15 with Cation Site Preference and Interstitial Oxide Ions. Small 2022, 18, 2106785. [Google Scholar] [CrossRef]
- Yasui, Y.; Tsujiguchi, T.; Sakuda, Y.; Hester, J.R.; Yashima, M. Oxide-Ion Occupational Disorder, Diffusion Path, and Conductivity in Hexagonal Perovskite Derivatives Ba3WNbO8.5 and Ba3MoNbO8.5. J. Phys. Chem. C 2022, 126, 2383–2393. [Google Scholar] [CrossRef]
- El Khal, H.; Cordier, A.; Batis, N.; Siebert, E.; Georges, S.; Steil, M.C. Effect of Porosity on the Electrical Conductivity of LAMOX Materials. Solid State Ionics 2017, 304, 75–84. [Google Scholar] [CrossRef]
- Yashima, M.; Yamada, H.; Nuansaeng, S.; Ishihara, T. Role of Ga3+ and Cu2+ in the High Interstitial Oxide-Ion Diffusivity of Pr2NiO4-Based Oxides: Design Concept of Interstitial Ion Conductors through the Higher-Valence d10 Dopant and Jahn–Teller Effect. Chem. Mater. 2012, 24, 4100–4113. [Google Scholar] [CrossRef]
- Rodriguez-Carvajal, J. Nouveaux Développements de FullProf Analyse de La Microstructure et Utilisation Du Recuit Simulé Pour La Résolution de Structures. Comm. Powder Diffr. (IUCr) Newsl. 2001, 26, 12–19. [Google Scholar]
- Avdeev, M.; Hester, J.R. ECHIDNA: A Decade of High-Resolution Neutron Powder Diffraction at OPAL. J. Appl. Crystallogr. 2018, 51, 1597–1604. [Google Scholar] [CrossRef]
- Oishi, R.; Yonemura, M.; Nishimaki, Y.; Torii, S.; Hoshikawa, A.; Ishigaki, T.; Morishima, T.; Mori, K.; Kamiyama, T. Rietveld Analysis Software for J-PARC. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2009, 600, 94–96. [Google Scholar] [CrossRef]
- Oishi-Tomiyasu, R.; Yonemura, M.; Morishima, T.; Hoshikawa, A.; Torii, S.; Ishigaki, T.; Kamiyama, T. Application of Matrix Decomposition Algorithms for Singular Matrices to the Pawley Method in Z-Rietveld. J. Appl. Crystallogr. 2012, 45, 299–308. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for Three-Dimensional Visualization of Crystal, Volumetric and Morphology Data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Światowska-Mrowiecka, J.; Maurice, V.; Zanna, S.; Klein, L.; Marcus, P. XPS Study of Li Ion Intercalation in V2O5 Thin Films Prepared by Thermal Oxidation of Vanadium Metal. Electrochim. Acta 2007, 52, 5644–5653. [Google Scholar] [CrossRef]
- Fleisch, T.H.; Mains, G.J. An XPS Study of the UV Reduction and Photochromism of MoO3 and WO3. J. Chem. Phys. 1982, 76, 780–786. [Google Scholar] [CrossRef]
- Khyzhun, O.Y. XPS, XES and XAS Studies of the Electronic Structure of Tungsten Oxides. J. Alloys Compd. 2000, 305, 1–6. [Google Scholar] [CrossRef]
- Adams, S.; Rao, R.P. High Power Lithium Ion Battery Materials by Computational Design. Phys. Status Solidi 2011, 208, 1746–1753. [Google Scholar] [CrossRef]
- Sahmi, A.; Omeiri, S.; Bensadok, K.; Trari, M. Electrochemical properties of the scheelite BaWO4 prepared by co-precipitation: Application to electro-photocatalysis of ibuprofen degradation. Mater. Sci. Semicond. Process. 2019, 91, 108–114. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kikuchi, Y.; Yasui, Y.; Hester, J.R.; Yashima, M. Improved Oxide Ion Conductivity of Hexagonal Perovskite-Related Oxides Ba3W1+xV1−xO8.5+x/2. Inorganics 2023, 11, 238. https://doi.org/10.3390/inorganics11060238
Kikuchi Y, Yasui Y, Hester JR, Yashima M. Improved Oxide Ion Conductivity of Hexagonal Perovskite-Related Oxides Ba3W1+xV1−xO8.5+x/2. Inorganics. 2023; 11(6):238. https://doi.org/10.3390/inorganics11060238
Chicago/Turabian StyleKikuchi, Yugo, Yuta Yasui, James R. Hester, and Masatomo Yashima. 2023. "Improved Oxide Ion Conductivity of Hexagonal Perovskite-Related Oxides Ba3W1+xV1−xO8.5+x/2" Inorganics 11, no. 6: 238. https://doi.org/10.3390/inorganics11060238