1. Introduction
The benzimidazole is a bicyclic molecule composed of benzene ring and imidazole ring. The compound is isostructural with naturally occurring nucleotides [
1]. Its similarity to natural molecules led to the preparation of derivatives that can be utilized in medicinal chemistry. The very broad spectrum of biological activities that it treats include antimicrobial, antibiofilm, antifungal, antiviral, antioxidant, anti-inflammatory, antidiabetic, antiparasitic, anthelmintic, anticoagulant, antiallergic, antiprotozoal, anticonvulsants, anticancer and cytotoxic activities. There are drugs already used in medicine, such as Albendazole, Bendamustine, Omeprazole, Pimonbendane, Benomyl, Carbendazim, Telmisartan, Pantoprazole, Etonitazene, and Thiabendazole (some of them are depicted in
Scheme 1).
Moreover, benzimidazoles can be utilized as optical sensors for bioimaging and in photovoltaics. There are not only many papers but also many reviews on the topic, such as the one on lanthanide complexes by Cruz-Navarro et al. [
1], by Hernández-Romero et al. on first-row transition metal complexes [
2], and Suarez-Moreno et al. on second- and third-row transition metal complexes [
3]. The last two reviews contain information about the anticancer and antitumor activities of benzimidazole complexes.
In this review, we have focused on the preparation of bis(benzimidazoles) and their complexes that show biological activities. These compounds can be utilized as bridges among metal centers, chelating ligands with nitrogen atoms, or oxygen or sulfur coordinating atoms.
2. Bis(benzimidazole) Synthesis and Some Examples of Complex Preparation
There are many ways for benzimidazole and benzimidazole derived ligands. Some examples of the preparations are mentioned hereafter. The work of Matthews et al. described symmetric as well as asymmetric bis(benzimidazoles) [
4]. The general method can be seen in
Scheme 2.
For example, preparation of 4-(2-Benzimidazolyl)-3-thiabutanoic acid and 2-(1H-benzimidazol-2-ylmethylsulfanylmethyl)-1H-benzimidazole is given.
Thiodiacetic acid and o-phenylenediamine in a solution of 4 M HCl are refluxed for a total of 72 h and allowed to cool to room temperature. A green precipitate was filtered and dried. The precipitate was dissolved in distilled water. The solution was stirred and basified with ammonia solution to pH 9. A precipitate of bis(benzimidazole) was filtered and dried. The filtrate was treated with concentrated HCl until pH 7. White precipitate gave 4-(2-Benzimidazolyl)-3-thiabutanoic acid.
Similarly, 4-(2-benzimidazolyl)-3-oxabutanoic acid was prepared. These acids were used for condensation with 4-nitro-o-phenylenediamine, N-methyl-o-phenylenediamine, or 4,5-dimethyl-o-phenylenediamine to form asymmetric bis(benzimidazoles) [
4]. The ligands were used for the preparation of copper(II) complexes. The complexes were prepared by equimolar addition of ligand to the copper bromide or perchlorate dissolved in methanol. The ligands were tridentate chelating.
Caymaz et al. reported synthesis of 2,2′-bis-(imidazo [1,2-
a]pyridine-8-yl)-1
H,1
H′-[5,5′]-bisbenzimidazole [
5]. The syntheses of compound were performed by reacting the imidazo(1,2-
a)pyridine-8-carbaldehyde with 3,3′-diaminobenzidin (see
Scheme 3).
The compound binds to DNA grooves and has peroxide mediated DNA-cleavage properties. It was tested on cell lines HepG2, DLD-1, and MDA-MB-231, and was found to have high cytotoxic activities [
5].
Synthesis of (2-((1
H-benzo[d]imidazol-2-yl)methylthio)-1
H-benzo[
d]imidazol-6-yl)(phenyl)methanone [BIPM] and its Pd complex was reported by Kumar et al. [
6]. In the first step, (2-mercapto-1
H-benzo[
d]imidazol-6-yl)(phenyl)methanone is prepared in methanol in presence of KOH by reaction of carbon disulfide with 3,4-diaminobenzophenone. Then, 2-(chloromethyl)-1
H-benzo[
d]imidazole was prepared by condensing chloroacetic acid and o-phenylenediamine in 4 M hydrochloric acid. Finally, (2-((1
H-benzo[
d]imidazol-2-yl)methylthio)-1
H-benzo[
d]imidazol-6-yl)(phenyl)methanone [BIPM], was obtained by a reaction of above-mentioned components in methanol (see
Scheme 4).
There are many papers on coordination compounds with 2,6-bis(1
H-benzo[
d]imidazol-2-yl)pyridine (BBP) and its derivatives (see
Scheme 5, top left). These ligands are multidentate ligands and can be coordinated to metal atoms in different metal–ligand ratios. Substitution of the N–H bond in the benzimidazole ring by alkyl groups can lead to the formation of hydrogen bonds, and complexes are studied for interesting optical and magnetic properties [
1]. BBP can be obtained by the reaction of pyridine-2,6-dicarboxylic acid and o-phenylenediamine in the presence of polyphosphoric acid (PPA). Higher yields can be obtained with phosphorus oxide or HCl and reaction in microwave oven. Instead of pyridine-2,6-dicarboxylic acid, pyridine-2,6-dicarbaldehyde can be used, but the yields are low [
1].
N-substituted ligands derived from BBP can be obtained by condensing pyridine-2,6-dicarboxylic acid with N-alkyl-o-phenylenediamine derivative. The other possible way is to deprotonate N3 with a base, followed by a reaction with alkyl or aryl halides. Some examples are given below. The 2,6-bis-(6-nitrobenzimidazol-2-yl)pyridine (BNBP) (see
Scheme 5, top right) was prepared by reaction of BBP with concentrated sulfuric acid and nitric acid. The complex [Ru(BBP)Cl
3] was prepared by a reaction of BBP with ruthenium(III) chloride. The nucleophilic substitution of BBP with 3,5-di-
tert-butylbenzyl bromide or 4-
tert-butylbenzyl chloride, in basic heated DMSO solution, led to preparation of derivatives depicted in
Scheme 5 (bottom) [
7].
Scheme 5.
2,6-
bis(1
H-benzo[
d]imidazol-2-yl)pyridine (BBP), BNBP = 2,6-bis-(6-nitrobenzimidazol-2-yl)pyridine), [Ru(BBP)Cl
3] (middle) [
8]. The substituted ligands (bottom) were obtained via the nucleophilic substitution of 2,6-bis(1
H-benzimidazole-2-yl)pyridine [
7] with 3,5-di-
tert-butylbenzyl bromide or 4-
tert-butylbenzyl chloride in the presence of KOH in DMSO solvent at an elevated temperature.
Scheme 5.
2,6-
bis(1
H-benzo[
d]imidazol-2-yl)pyridine (BBP), BNBP = 2,6-bis-(6-nitrobenzimidazol-2-yl)pyridine), [Ru(BBP)Cl
3] (middle) [
8]. The substituted ligands (bottom) were obtained via the nucleophilic substitution of 2,6-bis(1
H-benzimidazole-2-yl)pyridine [
7] with 3,5-di-
tert-butylbenzyl bromide or 4-
tert-butylbenzyl chloride in the presence of KOH in DMSO solvent at an elevated temperature.
Another example of BBP derivative on pyridine ring was recently reported by Orvos et al. The synthetic route of the ligand is outlined in
Scheme 6 [
9]. The 4-azidopyridine derivative was prepared from dimethyl 4-chloropyridine-2,6-dicarboxylate by the basic hydrolysis of with LiOH. Diamide was obtained with
o-phenylenediamine using
O-(benzotriazol-1-yl)-
N,
N,
N′,
N′-tetramethyluronium tetrafluoroborate (TBTU) in DMF. Hydrogenation on Pd/C in MeOH gave an amino derivative. NH
2-bis(benzimidazole)pyridine was obtained by heating in acetic acid. The reaction of nitrosobenzene with the amino group led to the final product.
3. Biologically Active Bis(benzimidazole) Complexes
Deng et al. have prepared ruthenium complexes that have potential applications as sensitizers for use in cancer radiotherapy [
8]. They prepared [Ru(BBP)Cl
3] (1), [Ru(BBP)
2]Cl
2 (2a), and [Ru(BNBP)
2]Cl
2 (2b). Complex 2b was found to be particularly effective in sensitizing human melanoma A375 cells toward radiation. Moreover, it was found that complex 2b is not toxic to normal cells. Mechanism of action is formation of intracellular reactive oxygen species (ROS) with glutathione (GSH) followed by DNA strand breaks. The subsequent DNA damage induces phosphorylation of p53 (p-p53) and upregulates the expression levels of p21, which inhibits the expression of cyclin-B, leading to G2M arrest. Moreover, p-p53 activates caspases-3 and -8, triggering cleavage of poly(ADP-ribose) polymerase (PARP), finally resulting in apoptosis [
8].
BODIPY iridium(III) complexes containing 2,2′-bis(benzimidazole) show selectivity for cancerous cells over normal cells [
10]. The tetranuclear (2 + 2) complexes were prepared through the self-assembly of benzimidazole and BODIPY ligands with dichloro (pentamethylcyclopentadienyl) iridium dimer. Cytotoxicity studies revealed that the complex is highly selective for cervical cancer cells (HeLa) and human glioblastoma (U87) cancer cells [
10].
[MnBr(CO)
3L2] (3, L2 = 2,2′-bisbenzimidazole), [MnBr(CO)
3L3]·CH
3OH (4, L3 = BBP = 2,6-bis(benzimidazole-2′-yl)pyridine), and
fac-[MnBr(CO)
3L4] (5, L4 = 2,4-bis(benzimidazole-2′-yl) pyridine) were prepared by reactions of MnBr(CO)
5 with appropriate ligands L2–L4, respectively, and characterized by single crystal X-ray diffraction, NMR, IR, UV-vis, and fluorescence spectroscopy [
11]. The CO-release properties were investigated using the myoglobin assay and CO detection, and the results show that all of the complexes could release CO rapidly upon exposure to 365 nm UV light. The fluorescence imaging show that the Mn(I) complexes can be taken up by human liver cells (HL-7702) and liver cancer cells (SK-Hep1), and are suitable for bioimaging. A cell viability assay for SK-Hep1 shows that the anticancer activity of 3 is highest in the studied complexes [
11].
Pd(II) complex with (2-((1
H-benzo[
d]imidazol-2-yl)methylthio)-1
H-benzo[
d]imidazol-5-yl)(phenyl)methanone (BIPM) was prepared by reaction of palladium acetate with BIPM in a 1:1 molar ratio [
6]. BIPM is bidentate N,S chelating ligand, and the other two positions are occupied by oxygen atoms from two acetate anions. The in vitro antiproliferative effect of the BIPM and complex were tested against the MCF7, A549, Ehrlich ascites carcinoma (EAC), and Daltons lymphoma ascites (DLA) carcinoma cell lines. The mechanism is the antiangiogenic effect and promotion of apoptosis. The potential photo-induced binding mode on double-stranded calf thymus DNA and protein cleavage activity study on pBR322 DNA of the complex confirmed apoptosis. The molecular docking study proved its interaction with DNA [
6].
Ruthenium mixed ligand complex with 2-(1
H-benzimidazol-2-ylmethylsulfanylmethyl)-1
H-benzimidazole and Schiff base (2-((
E)-1
H-1,2,4-triazol-5-yliminomethyl) phenol) was reported by Sur et al. [
12]. The antibacterial effect of the complex was studied against
Staphylococcus aureus, vancomycin-resistant
Staphylococcus aureus (VRSA), methicillin-resistant
Staphylococcus aureus (MRSA), and
Staphylococcus epidermidis. Very high antibacterial activity was observed on growth curves and by fluorescence imaging. Moreover, in vivo tests on VRSA-infected mice proved better healing of skin wounds.
Mononuclear, binuclear, and multinuclear silver complexes of composition [Ag
2(methacrylate)
2(Etobb)
2]·CH
3CN
1, [Ag(methacrylate)(Bobb)]
2, and [Ag
2(methacrylate)
2(Aobb)]
n 3, where Etobb = 1,3-bis(1-ethylbenzimidazol-2-yl)-2-oxapropane, Bobb = 1,3-bis(1-benzylbenzimidazol-2-yl)-2-oxapropane, Aobb = 1,3-bis(1-allylbenzimidazol-2-yl)-2-oxapropane were prepared by Zhang et al. [
13]. Synthesis of the ligands is in
Scheme 7. The three complexes have been prepared by reaction of silver nitrate with sodium methacrylate and corresponding ligand. Single-crystal X-ray diffraction revealed that complex
1 is binuclear, and the silver atom is coordinated by two N atoms to two Etobb ligands and oxygen of methacrylate. The complex
2 is mononuclear with a chelating ligand, but the oxygen atom of bis(benzimidazole is not involved in coordination. Complex
3 is a metal–organic compound with a diamond-like multinuclear silver center, with each silver atom bridged by two Aobb ligands and two methacrylate ions to form 1-D single-coordination polymer chain structures that extend into 2-D frameworks through π–π interactions. The binding modes of DNA were checked through absorption titration experiments of CT-DNA with complexes at 270 nm. A binding to DNA through intercalation with strong π–π stacking to the DNA base pairs was proved. Hydroxyl radical scavenging activity revealed the inhibitory effect of the complexes on OH˙ radicals.
Rodriguez-Cordero et al. have characterized ZnLBr
2 complexes with bis(benzimidazole) prepared by reaction of citraconic acid with o-phenylenediamine or its derivative obtained by following reaction with benzylbromide in DMF [
14]. Tetrahedral zinc coordination was proven by single crystal X-ray analysis. Zinc is coordinated by two Br atoms and a chelating N-N ligand. UV spectra of ligands and complexes showed decreases in peak intensities when increasing amounts of CT DNA. These spectral changes are consistent with intercalation or partial intercalation of the ligands and complexes into the DNA.
Pan et al. have prepared and proved structures of [Cu(bmbp)(HCOO)(H
2O)](ClO
4)·DMF (1), [Co(bmbp)
2]
2(ClO
4)
4·DMF·H
2O (2) and [Zn(bmbp)
2]
2(ClO
4)
4·DMF·H
2O (3), where bmbp is 4-butyloxy-2,6-bis(1-methyl-2-benzimidazolyl)pyridine, complexes by single crystal X-ray analysis [
15]. Complex 1 has square-pyramidal geometry, and complexes 2 and 3 are distorted octahedral. The complexes and bmbp were tested on a human esophageal cancer cell line (Eca109). Inhibition of the growth was proven, and complex 1 showed that it was the most active (IC50 = 26.09 μM).
Mixed ligand Cu(II) complexes [Cu(BBP)(L)H
2O]SO
4 (where L = 2,2′ bipyridine (bpy), and ethylene diamine (en)), have been prepared, and DNA-binding properties proved by absorption spectroscopy, fluorescence spectroscopy, viscosity measurements and thermal denaturation methods. DNA intercalation mechanism was suggested as well as the cleavage of plasmid pBR322, in the presence of H
2O
2 [
16].
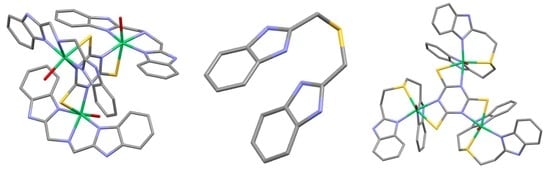
Very interesting ligand synthesis and copper complexes are presented by Suwalsky et al. [
17]. The authors have prepared tetradentate Bis(2-methylbenzimidazolyl)(2-methylthioethyl)amine (L1) (see
Figure 1A), and bis(1-methyl-2-methylbenzimidazolyl)(2-methylthioethyl)amine (L1Me). The first ligand was prepared by refluxing 1-tert-butoxycarbonyl-2-cloromethylbenzimidazole and 2-methylthioethylamine in the presence of K
2CO
3 and NaI in CH
3CN. The second ligand was prepared similarly with 1-methyl-2-chloromethylbenzimidazole. The complexes were prepared by reaction of copper perchlorates with ligands. The effect on the morphology of human erythrocytes and antiproliferative effect was tested on HeLa, REH, A546, and K-562 cells.
Similar Bis(benzimidazole)thio- and selenoether ligands and their nickel(II) complexes were prepared by the same group of authors [
18]. This time, 1-methyl-2-(chloromethyl)benzimidazole reacted with (2-phenylethylthio)ethylamine (for L3Me) or (2-phenylseleno)ethylamine (for L4Me). Mononuclear and binuclear Ni(II) complexes were obtained by a reaction of nickel chloride with the ligands. Their structures can be seen in
Figure 1.
The stability of complexes in aqueous solutions was monitored for 72 h by UV–vis spectroscopy, and these are stable in solutions. The cytotoxicity of complexes was screened against SK-LU-1 (human lung adenocarcinoma), HeLa (human cervical carcinoma), and HEK-293 (non-tumoral human embryonic kidney) cell lines using an MTT. It was found that the complexes are less cytotoxic in comparison with cisplatin, but they are selective to tumor cell lines.
1-(1
H-benzimidazol-2-yl)-
N-(1
H-benzimidazol-2-ylmethyl)methanamine (abb) and 2-(1
H-benzimidazol-2-ylmethylsulfanylmethyl)-1
H-benzimidazole (tbb) have been prepared and characterized [
19]. The trinuclear complex [Ni
3(abb)
3(H
2O)
3(μ-ttc)](ClO
4)
3, was where ttcH
3 = trithiocyanuric acid was prepared and characterized by X-ray (depicted in
Figure 2A1,A2). The complex and ligands were tested on bacteria strains
Staphylococcus aureus,
Escherichia coli, and
Saccharomyces cerevisiae. The complex was more active than ligands. The same complex was used together with another trinuclear complex [Ni
3(tebb)
3(H
2O)
3(µ-ttc)](ClO
4)
3, tebb = 2-[2-[2-(1
H-benzimidazol-2-yl)ethylsulfanyl]ethyl]-1
H-benzimidazole, to study cytotoxicity on breast cell lines T-47D, MCF-7 and non-malignant HBL-100 (complex cation shown in
Figure 2B) [
20].
It was found that complexes are very cytotoxic (24IC50 = 9.5 μM). The complex with abb was encapsulated in ferritin modified with folic acid to overcome toxicity to normal cells and enable transport to cancer cells. For a comparison of cytotoxicity of trinuclear complex with a mononuclear tebb complex, [Ni(tebb)
2](ClO
4)
2 has been prepared (the cation shown in
Figure 2C1,C2) [
21]. The complex is readily uptaken by malignant MDA-MB-231 and CACO-2 cells and is not toxic to Hs27 fibroblasts. The lowest IC50 values were found for MDA-MB-231 cells (5.2 μM). DNA cleavage, DNA fragmentation leads to the formation of reactive oxygen species [
21]. Antibacterial study against
Staphylococcus aureus and
Escherichia coli on [Ni
3(tebb)
3(H
2O)
3(µ-ttc)](ClO
4)
3 was reported by Ashrafi [
22].
4. Conclusions
The goal of the mini review was to perform a literature search of the current state of the art of chemistry of bis(benzimidazole) syntheses and the preparation of complexes with the ligands. There are plenty of papers on the topic and many reviews. We have focused on complexes that were studied for their biological properties in the last 7 years.
Bis(benzimidazoles) were selected from the broad collection of papers dealing with benzimidazole complexes because bis(benzimidazoles) are structurally interesting and offer chelating and as well as bridging modes of coordination to central atoms. Plenty of these complexes have been known for ages, though these were prepared as models mimicking biological systems, so the data on biological activities was often missing. These known compounds can be further studied for biological properties and can find their potential to be used as drugs, for example, in cases where known antibiotics are insufficient to fight against resistant bacteria. From the literature, it is obvious that the complexes were mostly studied as antiproliferative, anticancer, and antitumor agents. From this point of view the studies are concerned on non-platinum metal-based drugs. Except of ruthenium, palladium and iridium complexes were found. There are some recent results on copper, cobalt, and zinc, and nickel, although nickel is mentioned in studies as a toxic metal. There are not many papers on silver complexes, and metals such as gold or iron can be other way to diversify our knowledge on biological properties of coordination compounds [
2,
3,
23]. Very promising are combinations of ligands such as benzimidazoles with other biologically active ligands, for example Schiff bases. Some benzimidazoles contain sulfur or selenium that can increase their biological action or they can contain arms with the atoms to be more strongly coordinated to central atoms [
18]. It should also be of interest to include P ligands to combine them with benzimidazoles to increase bioactivity, for example in the case of 2,6-bis(2-(diphenylphosphanyl)-1
H-imidazol-1-yl)pyridine [
24].
Fewer studies have been performed on bacteria strains. There are some papers on copper, zinc, and nickel bis(benzimidazole) complexes and their antibacterial properties, but the data are not available for all of the already known species. These can be future trends of studies on benzimidazole biological active complexes. Probable toxicity of prepared complexes can be overcome by using transporters on the base of nanoparticles that can deliver the complexes without side effects to healthy cells.