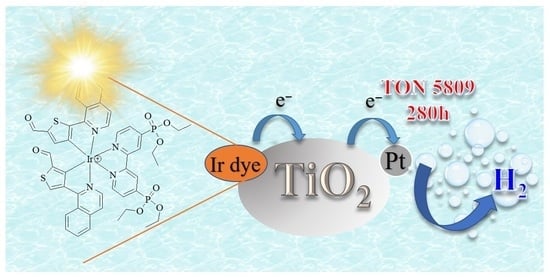
Development of Aldehyde Functionalized Iridium(III) Complexes Photosensitizers with Strong Visible-Light Absorption for Photocatalytic Hydrogen Generation from Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Instrumentation
2.3. Cyclic Voltammetry Measurements
2.4. Computational Details
2.5. Electrochemical Impedance Spectroscopy and Photocurrent Measurements
2.6. Preparation of Platinized TiO2
2.7. Adsorption of Iridium(III) Photosensitizer onto Platinized TiO2
2.8. Light-Driven Photocatalytic H2 Production Studies
2.9. Synthetic Procedure
3. Results and Discussion
3.1. Synthesis of the Materials
3.2. Photophysical Properties
3.3. Electrochemical Properties
3.4. Density Functional Theory Calculation
3.5. Electrochemical Impedance Spectroscopy Measurements for Iridium(III) Dyes
3.6. Photocurrent Measurements for Iridium(III) Dyes
3.7. Light-Driven Hydrogen Generation Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chu, S.; Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef]
- Yang, M.; Yarnell, J.E.; El Roz, K.; Castellano, F.N. A Robust Visible-Light-Harvesting Cyclometalated Ir(III) Diimine Sensitizer for Homogeneous Photocatalytic Hydrogen Production. ACS Appl. Energy Mater. 2020, 3, 1842–1853. [Google Scholar] [CrossRef]
- Watanabe, M. Dye-sensitized photocatalyst for effective water splitting catalyst. Sci. Technol. Adv. Mater. 2017, 18, 705–723. [Google Scholar] [CrossRef] [Green Version]
- Wong, W.-Y.; Ho, C.-L. Heavy metal organometallic electrophosphors derived from multi-component chromophores. Coord. Chem. Rev. 2009, 253, 1709–1758. [Google Scholar] [CrossRef]
- Wong, W.-Y.; Ho, C.-L. Functional metallophosphors for effective charge carrier injection/transport: New robust OLED materials with emerging applications. J. Mater. Chem. 2009, 19, 4457–4482. [Google Scholar] [CrossRef]
- Chi, Y.; Chou, P.T. Transition-metal phosphors with cyclometalating ligands: Fundamentals and applications. Chem. Soc. Rev. 2010, 39, 638–655. [Google Scholar] [CrossRef] [PubMed]
- Vilaça, G.; Jousseaume, B.; Mahieux, C.; Belin, C.; Cachet, H.; Bernard, M.C.; Vivier, V.; Toupance, T. Tin dioxide materials chemically modified with trialkynylorganotins: Functional nanohybrids for photovoltaic applications. Adv. Mater. 2006, 18, 1073–1077. [Google Scholar] [CrossRef]
- Siu, C.-H.; Ho, C.-L.; He, J.; Chen, T.; Cui, X.; Zhao, J.; Wong, W.-Y. Thiocyanate-free ruthenium(II) cyclometalated complexes containing uncommon thiazole and benzothiazole chromophores for dye-sensitized solar cells. J. Organomet. Chem. 2013, 748, 75–83. [Google Scholar] [CrossRef]
- Wang, C.; Chen, Y.; Fu, W.-F. New platinum and ruthenium Schiff base complexes for water splitting reactions. Dalton Trans. 2015, 44, 14483–14493. [Google Scholar] [CrossRef] [PubMed]
- Mehtab, A.; Alshehri, S.M.; Ahmad, T. Photocatalytic and photoelectrocatalytic water splitting by porous g-C3N4 nanosheets for hydrogen generation. ACS Appl. Nano Mater. 2022, 5, 12656–12665. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Dutta, S. A review on H2 production through photocatalytic reactions using TiO2/TiO2-assisted catalysts. Fuel 2018, 220, 607–620. [Google Scholar] [CrossRef]
- Gopinath, M.; Marimuthu, R. A review on solar energy-based indirect water-splitting methods for hydrogen generation. Int. J. Hydrog. Energy 2022, 47, 37742–37759. [Google Scholar] [CrossRef]
- Goldsmith, J.I.; Hudson, W.R.; Lowry, M.S.; Anderson, T.H.; Bernhard, S. Discovery and high-throughput screening of heteroleptic iridium complexes for photoinduced hydrogen production. J. Am. Chem. Soc. 2005, 127, 7502–7510. [Google Scholar] [CrossRef] [PubMed]
- Lowry, M.S.; Bernhard, S. Synthetically tailored excited states: Phosphorescent, cyclometalated iridium(III) complexes and their applications. Chem. Eur. J. 2006, 12, 7970–7977. [Google Scholar] [CrossRef] [PubMed]
- Lowry, M.S.; Goldsmith, J.I.; Slinker, J.D.; Rohl, R.; Pascal, R.A.; Malliaras, G.G.; Bernhard, S. Single-layer electroluminescent devices and photoinduced hydrogen production from an ionic iridium(III) complex. Chem. Mater. 2005, 17, 5712–5719. [Google Scholar] [CrossRef]
- Curtin, P.N.; Tinker, L.L.; Burgess, C.M.; Cline, E.D.; Bernhard, S. Structure-activity correlations among iridium(III) photosensitizers in a robust water-reducing system. Inorg. Chem. 2009, 48, 10498–10506. [Google Scholar] [CrossRef]
- Tinker, L.L.; Mcdaniel, N.D.; Curtin, P.N.; Smith, C.K.; Ireland, M.J.; Bernhard, S. Visible light induced catalytic water reduction without an electron relay. Chem. Eur. J. 2007, 13, 8726–8732. [Google Scholar] [CrossRef]
- Tinker, L.L.; Bernhard, S. Photon-Driven Catalytic Proton Reduction with a Robust Homoleptic Iridium(III) 6-Phenyl-2,2′-bipyridine Complex (Ir(C^N^N)2+). Inorg. Chem. 2009, 48, 10507–10511. [Google Scholar] [CrossRef]
- Metz, S.; Bernhard, S. Robust photocatalytic water reduction with cyclometalated Ir(III) 4-vinyl-2,2′-bipyridine complexes. Chem. Commun. 2010, 46, 7551–7553. [Google Scholar] [CrossRef]
- Bodedla, G.B.; Tritton, D.N.; Chen, X.; Zhao, J.; Guo, Z.; Leung, K.C.-F.; Wong, W.-Y.; Zhu, X. Cocatalyst-free Photocatalytic Hydrogen Evolution with Simple Heteroleptic Iridium(III) Complexes. ACS Appl. Energy Mater. 2021, 4, 3945–3951. [Google Scholar] [CrossRef]
- Deaton, J.C.; Castellano, F.N. Archetypal Iridium(III) compounds for optoelectronic and photonic applications: Photophysical properties and synthetic methods. In Iridium(III) in Optoelectronic and Photonics Applications; Zysman-Colman, E., Ed.; John Wiley & Sons Ltd.: New York, NY, USA, 2017; pp. 1–69. [Google Scholar] [CrossRef] [Green Version]
- Garakyaraghi, S.; McCusker, C.E.; Khan, S.; Koutnik, P.; Bui, A.T.; Castellano, F.N. Enhancing the Visible-Light Absorption and Excited-State Properties of Cu(I) MLCT Excited States. Inorg. Chem. 2018, 57, 2296–2307. [Google Scholar] [CrossRef]
- Peiris, S.; de Silva, H.B.; Ranasinghe, K.N.; Bandara, S.V.; Perera, I.R. Recent development and future prospects of TiO2 photocatalysis. J. Chin. Chem. Soc. 2021, 68, 738–769. [Google Scholar] [CrossRef]
- Huang, H.; Banerjee, S.; Sadler, P.J. Recent Advances in the Design of Targeted Iridium(III) Photosensitizers for Photodynamic Therapy. ChemBioChem 2018, 19, 1574–1589. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-F.; Lei, Y.; Luo, T.; Liu, J.-M. Photocatalytic H2 Production from Water by Metal-free Dye-sensitized TiO2 Semiconductors: The Role and Development Process of Organic Sensitizers. ChemSusChem 2020, 13, 5863–5895. [Google Scholar] [CrossRef]
- Wang, P.; Guo, S.; Wang, H.-J.; Chen, K.-K.; Zhang, N.; Zhang, Z.M.; Lu, T.B. A broadband and strong visible-light-absorbing photosensitizer boosts hydrogen evolution. Nat. Commun. 2019, 10, 3155. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhao, X.; Zhao, Y.; Yang, T.; Liu, X.; Xie, J.; Li, G.; Zhu, D.; Tan, H.; Su, Z. Photosensitizers based on Ir(III) complexes for highly efficient photocatalytic hydrogen generation. Dyes Pigments 2019, 170, 107547. [Google Scholar] [CrossRef]
- Takizawa, S.Y.; Pérez-Bolívar, C.; Anzenbacher, P., Jr.; Murata, S. Cationic Iridium Complexes Coordinated with Coumarin Dyes–Sensitizers for Visible-Light-Driven Hydrogen Generation. Eur. J. Inorg. Chem. 2012, 2012, 3975–3979. [Google Scholar] [CrossRef]
- Zhao, J.; Wu, W.; Sun, J.; Guo, S. Triplet photosensitizers: From molecular design to applications. Chem. Soc. Rev. 2013, 42, 5323–5351. [Google Scholar] [CrossRef]
- Yiu, S.-C.; Ho, P.-Y.; Kwok, Y.-Y.; He, X.; Wang, Y.; Yu, W.H.; Ho, C.-L.; Huang, S. Development of Strong Visible-Light-Absorbing Cyclometalated Iridium(III) Complexes for Robust and Efficient Light-Driven Hydrogen Production. Chem. Eur. J. 2022, 28, e202104575. [Google Scholar] [CrossRef]
- Adeloye, A.O.; Ajibade, P.A. Towards the Development of Functionalized PolypyridineLigands for Ru(II) Complexes as Photosensitizers in Dye-Sensitized Solar Cells (DSSCs). Molecules 2014, 19, 12421–12460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, L.; Araujo, C.M.; Ahlquist, M.S.; Sun, L. Highly efficient and robust molecular ruthenium catalysts for water oxidation. Proc. Natl. Acad. Sci. USA 2012, 109, 15584–15588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Q.; Wu, L.; Chen, M.; Guo, Y.; Xie, T.; Wang, P. Aromatic TpyRu2+ (L)2Cl derivatives as water oxidation catalysts (Tpy = 2,2′:6′,2″-terpyridine, Ru = ruthenium, L = pyridine or isoquinoline). Catal. Commun. 2019, 122, 38–42. [Google Scholar] [CrossRef]
- Joya, K.S.; Joya, Y.F.; Ocakoglu, K.; van de Krol, R. Water-splitting catalysis and solar fuel devices: Artificial leaves on the move. Angew. Chem. Int. Ed. 2013, 52, 10426–10437. [Google Scholar] [CrossRef] [PubMed]
- Lo, K.K.-W.; Chung, C.-K.; Zhu, N. Synthesis, Photophysical and Electrochemical Properties, and Biological Labeling Studies of Cyclometalated Iridium(III) Bis (pyridylbenzaldehyde) Complexes: Novel Luminescent Cross-Linkers for Biomolecules. Chem. Eur. J. 2003, 9, 475–483. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, Q.; Wu, Y.; Li, F.; Yang, H.; Yi, T.; Huang, C. Selective phosphorescence chemosensor for homocysteine based on an iridium(III) complex. Inorg. Chem. 2007, 46, 11075–11081. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Q.; Li, M.-J. Aldehyde group functionalized iridium(III) complexes for the selective sensing of homocysteine. J. Organomet. Chem. 2019, 898, 120874. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, S.; Yang, H.; Wu, Y.; Yang, C.; Liu, X.; Zhao, Q.; Wu, H.; Liang, J.; Li, F.; et al. Water-soluble phosphorescent iridium(III) complexes as multicolor probes for imaging of homocysteine and cysteine in living cells. J. Mater. Chem. 2011, 21, 18974–18982. [Google Scholar] [CrossRef]
- Fukui, M.; Tanaka, A.; Hashimoto, K.; Kominami, H. Visible light-induced heterogeneous Meerwein–Ponndorf–Verley-type reduction of an aldehyde group over an organically modified titanium dioxide photocatalyst. Chem. Commun. 2017, 53, 4215–4218. [Google Scholar] [CrossRef]
- Kobayashi, A.; Watanabe, S.; Yoshida, M.; Kato, M. Importance of the Molecular Orientation of an Iridium(III)-Heteroleptic Photosensitizer Immobilized on TiO2 Nanoparticles. ACS Appl. Energy Mater. 2018, 1, 2882–2890. [Google Scholar] [CrossRef]
- Wahyuono, R.A.; Amthor, S.; Müller, C.; Rau, S.; Dietzek, B. Structure of Diethyl-Phosphonic Acid Anchoring Group Affects the Charge-Separated State on an Iridium(III) Complex Functionalized NiO Surface. ChemPhotoChem 2020, 4, 618–629. [Google Scholar] [CrossRef]
- Murakami, T.N.; Yoshida, E.; Koumura, N. Carbazole dye with phosphonic acid anchoring groups for long-term heat stability of dye-sensitized solar cells. Electrochim. Acta 2014, 131, 174–183. [Google Scholar] [CrossRef]
- Bae, E.; Choi, W.; Park, J.; Shin, H.S.; Kim, S.B.; Lee, J.S. Effects of surface anchoring groups (carboxylate vs. phosphonate) in ruthenium-complex-sensitized TiO2 on visible light reactivity in aqueous suspensions. J. Phys. Chem. B 2004, 108, 14093–14101. [Google Scholar] [CrossRef]
- Tang, X.; Liu, W.; Wu, J.; Lee, C.S.; You, J.; Wang, P. Synthesis, crystal structures, and photophysical properties of triphenylamine-based multicyano derivatives. J. Org. Chem. 2010, 75, 7273–7278. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Zhao, G.; Yang, X.; Jiang, X.; Liu, J.; Tian, H.; Gao, Y.; Liu, X.; Han, K.; Sun, M.; et al. Photoinduced intramolecular charge-transfer state in thiophene-π-conjugated donor–acceptor molecules. J. Mol. Struct. 2008, 876, 102–109. [Google Scholar] [CrossRef]
- Zhang, W.; Li, B.; Ma, H.; Zhang, L.; Guan, Y.; Zhang, Y.; Zhang, X.; Jing, P.; Yue, S. Combining Ruthenium(II) Complexes with Metal-Organic Frameworks to Realize Effective Two-Photon Absorption for Singlet Oxygen Generation. ACS Appl. Mater. Interfaces 2016, 8, 21465–21471. [Google Scholar] [CrossRef] [PubMed]
- Pitre, S.P.; McTiernan, C.D.; Vine, W.; DiPucchio, R.; Grenier, M.; Scaiano, J.C. Visible-light actinometry and intermittent illumination as convenient tools to study Ru (bpy)3Cl2 mediated photoredox transformations. Sci. Rep. 2015, 5, 16397. [Google Scholar] [CrossRef] [Green Version]
- Filevich, O.; Zayat, L.; Baraldo, L.M.; Etchenique, R. Long wavelength phototriggering: Ruthenium-based caged compounds. In Luminescent and Photoactive Transition Metal Complexes as Biomolecular Probes and Cellular Reagents; Lo, K., Ed.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 47–68. [Google Scholar] [CrossRef]
- Li, G.; Hu, K.; Robson, K.C.; Gorelsky, S.I.; Meyer, G.J.; Berlinguette, C.P.; Shatruk, M. Tris-heteroleptic ruthenium-dipyrrinate chromophores in a dye-sensitized solar cell. Chem. Eur. J. 2015, 21, 2173–2181. [Google Scholar] [CrossRef]
- Baglio, J.A.; Calabrese, G.S.; Harrison, D.J.; Kamieniecki, E.; Ricco, A.J.; Wrighton, M.S.; Zoski, G.D. Electrochemical characterization of p-type semiconducting tungsten disulfide photocathodes: Efficient photoreduction processes at semiconductor/liquid electrolyte interfaces. J. Am. Chem. Soc. 1983, 105, 2246–2256. [Google Scholar] [CrossRef]
- Frisch, M.E.; Trucks, G.; Schlegel, H.B.; Scuseria, G.; Robb, M.; Cheeseman, J.; Scalmani, G.; Barone, V.; Petersson, G.; Nakatsuji, H. Gaussian 16; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Tawada, Y.; Tsuneda, T.; Yanagisawa, S.; Yanai, T.; Hirao, K. A long-range-corrected time-dependent density functional theory. J. Chem. Phys. 2004, 120, 8425–8433. [Google Scholar] [CrossRef] [PubMed]
- Andrae, D.; Haeussermann, U.; Dolg, M.; Stoll, H.; Preuss, H. Energy-adjusted ab initio pseudopotentials for the second and third row transition elements. Theor. Chim. Acta 1990, 77, 123–141. [Google Scholar] [CrossRef]
- Mennucci, B.; Tomasi, J. Continuum solvation models: A new approach to the problem of solute’s charge distribution and cavity boundaries. J. Chem. Phys. 1997, 106, 5151–5158. [Google Scholar] [CrossRef]
- Hong, G.B.; Ma, C.M. Photocatalytic degradation of indoor air pollutants by Pt-TiO2. J. Nanomater. 2012, 2012, 405361. [Google Scholar] [CrossRef] [Green Version]
- Cistrone, P.A.; Silvestri, A.P.; Hintzen, J.C.; Dawson, P.E. Rigid peptide macrocycles from on-resin Glaser stapling. ChemBioChem 2018, 19, 1031–1035. [Google Scholar] [CrossRef]
- Yao, X.; Ho, P.-Y.; Yiu, S.-C.; Suramitr, S.; Li, W.-B.; Ho, C.-L.; Hannongbua, S. Development of new thiocyanate-free Ruthenium(II) dyes bearing isoquinoline chromophores for hydrogen production via water splitting. Dyes Pigm. 2022, 205, 110508. [Google Scholar] [CrossRef]
- Ma, D.-L.; Lin, S.; Wang, W.; Yang, C.; Leung, C.-H. Luminescent chemosensors by using cyclometalated iridium(III) complexes and their applications. Chem. Sci. 2017, 8, 878–889. [Google Scholar] [CrossRef] [Green Version]
- Abis, L.; Dalcanale, E.; Du Vosel, A.; Spera, S. Structurally new macrocycles from the resorcinol-aldehyde condensation. Configurational and conformational analyses by means of dynamic NMR, NOE, and T1 experiments. J. Org. Chem. 1988, 53, 5475–5479. [Google Scholar] [CrossRef]
- Yuan, Y.-J.; Zhang, J.-Y.; Yu, Z.-T.; Feng, J.-Y.; Luo, W.-J.; Ye, J.-H.; Zou, Z.G. Impact of ligand modification on hydrogen photogeneration and light-harvesting applications using cyclometalated iridium complexes. Inorg. Chem. 2012, 51, 4123–4133. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-H.; Ling, J.-W.; Lai, S.-H.; Huang, M.-J.; Cheng, C.-H.; Chen, I.-C. Dynamics of the excited states of [Ir(ppy)2bpy]+ with triple phosphorescence. J. Phys. Chem. A 2010, 114, 10339–10344. [Google Scholar] [CrossRef] [PubMed]
- Aggoun, D.; Messasma, Z.; Bouzerafa, B.; Berenguer, R.; Morallon, E.; Ouennoughi, Y.; Ourari, A. Synthesis, Characterization and DFT Investigation of New Metal Complexes of Ni(II), Mn(II) and VO(IV) Containing N,O-donor Schiff Base Ligand. J. Mol. Struct. 2021, 1231, 129923. [Google Scholar] [CrossRef]
- Pal, A.K.; Cordes, D.B.; Pringouri, K.; Anwar, M.U.; Slawin, A.M.; Rawson, J.M.; Zysman-Colman, E. Synthesis and characterization of green-to-yellow emissive Ir(III) complexes of pyridylbenzothiadiazine ligand. J. Coord. Chem. 2016, 69, 1924–1937. [Google Scholar] [CrossRef] [Green Version]
- Ha, M.W.; Park, M.-H.; Hwang, J.Y.; Kim, J.; Kim, D.-H.; Lee, T.-W.; Kim, Y.-H. Synthesis and characterization of homoleptic triply cyclometalated iridium(III) complex containing 6-(pyridin-2-yl)isoquinoline moiety for solution-processable orange-phosphorescent organic light-emitting diodes. Dyes Pigments 2021, 185, 108880. [Google Scholar] [CrossRef]
- Pirzada, B.M.; Dar, A.H.; Shaikh, M.N.; Qurashi, A. Reticular-chemistry-inspired supramolecule design as a tool to achieve efficient photocatalysts for CO2 reduction. ACS Omega 2021, 6, 29291–29324. [Google Scholar] [CrossRef] [PubMed]
- Tritton, D.N.; Tang, F.-K.; Bodedla, G.B.; Lee, F.-W.; Kwan, C.-S.; Leung, K.C.-F.; Zhu, X.; Wong, W.-Y. Development and advancement of iridium(III)-based complexes for photocatalytic hydrogen evolution. Coord. Chem. Rev. 2022, 459, 214390. [Google Scholar] [CrossRef]
- Zakeeruddin, S.M.; Nazeeruddin, M.K.; Pechy, P.; Rotzinger, F.P.; Humphry-Baker, R.; Kalyanasundaram, K.; Gratzel, M.; Shklover, V.; Haibach, T. Molecular Engineering of Photosensitizers for Nanocrystalline Solar Cells: Synthesis and Characterization of Ru Dyes Based on Phosphonated Terpyridines. Inorg. Chem. 1997, 36, 5937–5946. [Google Scholar] [CrossRef] [PubMed]
- Bae, E.; Choi, W. Effect of the anchoring group (carboxylate vs. phosphonate) in Ru-complex-sensitized TiO2 on hydrogen production under visible light. J. Phys. Chem. B 2006, 110, 14792–14799. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Bae, E.; Lee, J.-J.; Park, J.; Choi, W. Effect of the anchoring group in Ru-bipyridyl sensitizers on the photoelectrochemical behavior of dye-sensitized TiO2 electrodes: Carboxylate versus phosphonate linkages. J. Phys. Chem. B 2006, 110, 8740–8749. [Google Scholar] [CrossRef] [PubMed]
- Sen, A.; Groß, A. Promising sensitizers for dye sensitized solar cells: A comparison of Ru(II) with other earth’s scarce and abundant metal polypyridine complexes. Int. J. Quantum Chem. 2019, 119, e25963. [Google Scholar] [CrossRef]
- Suzuki, T.M.; Tanaka, H.; Morikawa, T.; Iwaki, M.; Sato, S.; Saeki, S.; Inoue, M.; Kajino, T.; Motohiro, T. Direct assembly synthesis of metal complex–semiconductor hybrid photocatalysts anchored by phosphonate for highly efficient CO2 reduction. Chem. Commun. 2011, 47, 8673–8675. [Google Scholar] [CrossRef] [Green Version]
- Paul, A.; Das, N.; Halpin, Y.; Vos, J.G.; Pryce, M.T. Carboxy derivatised Ir(III) complexes: Synthesis, electrochemistry, photophysical properties and photocatalytic hydrogen generation. Dalton Trans. 2015, 44, 10423–10430. [Google Scholar] [CrossRef]
- Wu, Y.; Zhu, W. Organic sensitizers from D–π–A to D–A–π–A: Effect of the internal electron-withdrawing units on molecular absorption, energy levels and photovoltaic performances. Chem. Soc. Rev. 2013, 42, 2039–2058. [Google Scholar] [CrossRef] [PubMed]
- Tsuboyama, A.; Iwawaki, H.; Furugori, M.; Mukaide, T.; Kamatani, J.; Igawa, S.; Moriyama, T.; Miura, S.; Takiguchi, T.; Okada, S.; et al. Homoleptic cyclometalated iridium complexes with highly efficient red phosphorescence and application to organic light-emitting diode. J. Am. Chem. Soc. 2003, 125, 12971–12979. [Google Scholar] [CrossRef] [PubMed]
- Bandini, M.; Bianchi, M.; Valenti, G.; Piccinelli, F.; Paolucci, F.; Monari, M.; Umani-Ronchi, A.; Marcaccio, M. Electrochemiluminescent functionalizable cyclometalated thiophene-based iridium(III) complexes. Inorg. Chem. 2010, 49, 1439–1448. [Google Scholar] [CrossRef]
- Sreekala, C.; Jinchu, I.; Sreelatha, K.; Janu, Y.; Prasad, N.; Kumar, M.; Sadh, A.K.; Roy, M. Influence of solvents and surface treatment on photovoltaic response of DSSC based on natural curcumin dye. IEEE J. Photovolt. 2012, 2, 312–319. [Google Scholar] [CrossRef]
- Bisquert, J.; Cendula, P.; Bertoluzzi, L.; Gimenez, S. Energy diagram of semiconductor/electrolyte junctions. J. Phys. Chem. Lett. 2014, 5, 205–207. [Google Scholar] [CrossRef] [PubMed]
- Kavan, L. Conduction band engineering in semiconducting oxides (TiO2, SnO2): Applications in perovskite photovoltaics and beyond. Catal. Today 2019, 328, 50–56. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.; Low, J.; Fang, Y.; Xiao, J.; Chen, X. Engineering heterogeneous semiconductors for solar water splitting. J. Mater. Chem. A 2015, 3, 2485–2534. [Google Scholar] [CrossRef]
- Gillaizeau-Gauthier, I.; Odobel, F.; Alebbi, M.; Argazzi, R.; Costa, E.; Bignozzi, C.A.; Qu, P.; Meyer, G.J. Phosphonate-based bipyridine dyes for stable photovoltaic devices. Inorg. Chem. 2001, 40, 6073–6079. [Google Scholar] [CrossRef]
- Tian, P.; He, X.; Zhao, L.; Li, W.; Fang, W.; Chen, H.; Zhang, F.; Huang, Z.; Wang, H. Enhanced charge transfer for efficient photocatalytic H2 evolution over UiO-66-NH2 with annealed Ti3C2Tx MXenes. Int. J. Hydrog. Energy 2019, 44, 788–800. [Google Scholar] [CrossRef]
- Tian, P.; He, X.; Zhao, L.; Li, W.; Fang, W.; Chen, H.; Zhang, F.; Huang, Z.; Wang, H. Ti3C2 nanosheets modified Zr-MOFs with Schottky junction for boosting photocatalytic HER performance. Sol. Energy 2019, 188, 750–759. [Google Scholar] [CrossRef]
- Wang, H.; Gao, C.; Li, R.; Peng, Z.; Yang, J.; Gao, J.; Yang, Y.; Li, S.; Li, B.; Liu, Z. Ruthenium–Cobalt Nanoalloy Embedded within Hollow Carbon Spheres as a Bifunctionally Robust Catalyst for Hydrogen Generation from Water Splitting and Ammonia Borane Hydrolysis. ACS Sustain. Chem. Eng. 2019, 7, 18744–18752. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, P.; Li, M.; Li, Y.; Zhang, X.; Chen, J.; Fang, X.; Liu, Y.; Yuan, X.; Dai, X.; et al. Interfacial synergy between dispersed Ru sub-nanoclusters and porous NiFe layered double hydroxide on accelerated overall water splitting by intermediate modulation. Nanoscale 2020, 12, 9669–9679. [Google Scholar] [CrossRef] [PubMed]
- Acharya, R.; Naik, B.; Parida, K. Cr(VI) remediation from aqueous environment through modified-TiO2-mediated photocatalytic reduction. Beilstein J. Nanotechnol. 2018, 9, 1448–1470. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Liu, Z.; Guo, C.; Chen, T.; Guo, C.; Lu, Y.; Wang, J. CdS(ZB)/CdS(WZ)/Ni-BTC photocatalytic selective oxidation of benzyl alcohol to benzaldehyde coupled with hydrogen evolution. Appl. Surf. Sci. 2022, 571, 151284. [Google Scholar] [CrossRef]
- Jiang, D.; Wang, W.; Sun, S.; Zhang, L.; Zheng, Y. Equilibrating the plasmonic and catalytic roles of metallic nanostructures in photocatalytic oxidation over Au-modified CeO2. ACS Catal. 2015, 5, 613–621. [Google Scholar] [CrossRef]
- Peerakiatkhajohn, P.; Yun, J.-H.; Wang, S.; Wang, L. Review of recent progress in unassisted photoelectrochemical water splitting: From material modification to configuration design. J. Photonics Energy 2016, 7, 012006. [Google Scholar] [CrossRef]
- Tang, J.-Y.; Guo, R.-T.; Zhou, W.-G.; Huang, C.-Y.; Pan, W.-G. Ball-flower like NiO/g-C3N4 heterojunction for efficient visible light photocatalytic CO2 reduction. Appl. Catal. B 2018, 237, 802–810. [Google Scholar] [CrossRef]
- Cao, S.; Huang, Q.; Zhu, B.; Yu, J. Trace-level phosphorus and sodium co-doping of g-C3N4 for enhanced photocatalytic H2 production. J. Power Sources 2017, 351, 151–159. [Google Scholar] [CrossRef]
- Leng, F.; Liu, H.; Ding, M.; Lin, Q.-P.; Jiang, H.-L. Boosting photocatalytic hydrogen production of porphyrinic MOFs: The metal location in metalloporphyrin matters. ACS Catal. 2018, 8, 4583–4590. [Google Scholar] [CrossRef]
- Duan, L.; Bozoglian, F.; Mandal, S.; Stewart, B.; Privalov, T.; Llobet, A.; Sun, L. A molecular ruthenium catalyst with water-oxidation activity comparable to that of photosystem II. Nat. Chem. 2012, 4, 418–423. [Google Scholar] [CrossRef]
- Sherman, B.D.; Sheridan, M.V.; Wee, K.R.; Marquard, S.L.; Wang, D.; Alibabaei, L.; Ashford, D.L.; Meyer, T.J. A Dye-Sensitized Photoelectrochemical Tandem Cell for Light Driven Hydrogen Production from Water. J. Am. Chem. Soc. 2016, 138, 16745–16753. [Google Scholar] [CrossRef] [PubMed]
- Pellegrin, Y.; Odobel, F. Sacrificial electron donor reagents for solar fuel production. C. R. Chim. 2017, 20, 283–295. [Google Scholar] [CrossRef] [Green Version]
- Ho, P.-Y.; Wang, Y.; Yiu, S.-C.; Yu, W.-H.; Ho, C.-L.; Huang, S. Starburst Triarylamine Donor-Based Metal-Free Photosensitizers for Photocatalytic Hydrogen Production from Water. Org. Lett. 2017, 19, 1048–1051. [Google Scholar] [CrossRef] [PubMed]










| Photosensitizers | λmax/nm (ε/105 M−1 cm−1) | λonset/nm |
|---|---|---|
| Ir1 | 325(2.38), 418(0.69) | 550 |
| Ir2 | 321(2.36), 414(0.67) | 539 |
| Ir3 | 312(2.06), 360(1.78), 452(1.00), 492(0.71), 564(0.32) | 585 |
| Ir4 | 308(2.03), 355(1.77), 447(0.98), 483(0.69), 553(0.31) | 576 |
| [Ir(ppy)2(dcbpy)]+ | 256(0.72), 308(0.32), 379(0.10) | 466 |
| Dye | /V | EHOMO [a]/eV | Eg[b]/eV | Eox*[c]/V | ELUMO [d]/eV |
|---|---|---|---|---|---|
| Ir1 | 0.76 | −5.56 | 2.25 | −1.49 | −3.31 |
| Ir2 | 0.77 | −5.57 | 2.30 | −1.53 | −3.27 |
| Ir3 | 0.73 | −5.53 | 2.12 | −1.38 | −3.41 |
| Ir4 | 0.74 | −5.54 | 2.15 | −1.41 | −3.39 |
| Compounds | HOMO | LUMO |
|---|---|---|
| Ir1 | −5.90 | −3.42 |
| Ir2 | −5.90 | −3.33 |
| Ir3 | −5.97 | −3.48 |
| Ir4 | −5.98 | −3.38 |
| Dye@Pt-TiO2 | Time /h | H2/mL | TON [a] | TOF [b]/h−1 | TOFi [c]/h−1 | Activityi [d] /µmol g−1 h−1 | AQY% | Dye Loading % |
|---|---|---|---|---|---|---|---|---|
| Ir1 | 280 | 6.8 | 4462 | 15.9 | 76.9 | 48,052 | 0.91 | 100% |
| Ir2 | 280 | 7.4 | 4834 | 17.3 | 141.1 | 88,163 | 0.98 | 100% |
| Ir3 | 280 | 8.4 | 5509 | 19.7 | 159.5 | 99,673 | 1.13 | 100% |
| Ir4 | 280 | 8.9 | 5809 | 20.7 | 174.0 | 108,735 | 1.19 | 100% |
| Dye@Pt-TiO2 | Time /h | H2/mL | TON [a] | TOF [b]/h−1 | TOFi [c]/h−1 | Activityi [d] /µmol g−1 h−1 | AQY% | Dye Loading % |
|---|---|---|---|---|---|---|---|---|
| Ir1 | 280 | 2.3 | 1492 | 5.3 | 39.2 | 24,490 | 0.30 | 100% |
| Ir2 | 280 | 4.2 | 2727 | 9.7 | 44.4 | 27,755 | 0.55 | 100% |
| Ir3 | 280 | 4.2 | 2730 | 9.7 | 47.0 | 29,388 | 0.56 | 100% |
| Ir4 | 280 | 4.6 | 3029 | 10.8 | 48.5 | 30,327 | 0.61 | 100% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, X.; Zhang, Q.; Ho, P.-Y.; Yiu, S.-C.; Suramitr, S.; Hannongbua, S.; Ho, C.-L. Development of Aldehyde Functionalized Iridium(III) Complexes Photosensitizers with Strong Visible-Light Absorption for Photocatalytic Hydrogen Generation from Water. Inorganics 2023, 11, 110. https://doi.org/10.3390/inorganics11030110
Yao X, Zhang Q, Ho P-Y, Yiu S-C, Suramitr S, Hannongbua S, Ho C-L. Development of Aldehyde Functionalized Iridium(III) Complexes Photosensitizers with Strong Visible-Light Absorption for Photocatalytic Hydrogen Generation from Water. Inorganics. 2023; 11(3):110. https://doi.org/10.3390/inorganics11030110
Chicago/Turabian StyleYao, Xiao, Qian Zhang, Po-Yu Ho, Sze-Chun Yiu, Songwut Suramitr, Supa Hannongbua, and Cheuk-Lam Ho. 2023. "Development of Aldehyde Functionalized Iridium(III) Complexes Photosensitizers with Strong Visible-Light Absorption for Photocatalytic Hydrogen Generation from Water" Inorganics 11, no. 3: 110. https://doi.org/10.3390/inorganics11030110