Supramolecular Host–Guest Assemblies of [M6Cl14]2–, M = Mo, W, Clusters with γ-Cyclodextrin for the Development of CLUSPOMs
Abstract
:1. Introduction
2. Results and Discussion
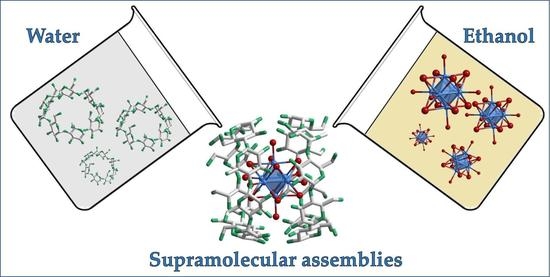
2.1. Inclusion Phenomenon for CLUSPOM Materials
2.2. Synthesis and Structural Characterization of [M6Cli8Cla6]2–/γ-CD Complexes
2.3. Solution Studies
2.4. Physicochemical Properties
3. Materials and Methods
3.1. Materials
3.2. Methods
Single Crystal X-ray Diffraction Analysis
3.3. Synthetic Procedures
3.3.1. Synthesis of (H3O)2{[Mo6Cli8Cla6]@(γ-CD)2}·(γ-CD)·15H2O (Mo6Cl14@2CD·CD)
3.3.2. Synthesis of (H3O)2{[W6Cli8Cla6]@(γ-CD)2}·(γ-CD)·15H2O (W6Cl14@2CD·CD)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Williams, G.T.; Haynes, C.J.E.; Fares, M.; Caltagirone, C.; Hiscock, J.R.; Gale, P.A. Advances in applied supramolecular technologies. Chem. Soc. Rev. 2021, 50, 2737–2763. [Google Scholar] [CrossRef]
- Lehn, J.-M. Towards Complex Matter: Supramolecular Chemistry and Self-organization. Eur. Rev. 2009, 17, 263–280. [Google Scholar] [CrossRef]
- Khan, S.B.; Lee, S.-L. Supramolecular chemistry: Host–guest molecular complexes. Molecules 2021, 26, 3995. [Google Scholar] [CrossRef] [PubMed]
- Teyssandier, J.; De Feyter, S.; Mali, K.S. Host–guest chemistry in two-dimensional supramolecular networks. Chem. Commun. 2016, 52, 11465–11487. [Google Scholar] [CrossRef]
- Kinoshita, T.; Iinuma, F.; Tsuji, A. Microanalysis of proteins and peptides. I. Enhancement of the fluorescence intensity of dansyl amino acids and dansyl proteins in aqueous media and its application to assay of amino acids and proteins. Chem. Pharm. Bull. 1974, 22, 2413–2420. [Google Scholar] [CrossRef]
- Matsui, Y.; Mochida, K. Binding forces contributing to the association of cyclodextrin with alcohol in an aqueous solution. BCSJ 1979, 52, 2808–2814. [Google Scholar] [CrossRef]
- Tucker, E.E.; Christian, S.D. Vapor pressure studies of benzene-cyclodextrin inclusion complexes in aqueous solution. J. Am. Chem. Soc. 1984, 106, 1942–1945. [Google Scholar] [CrossRef]
- Yoshida, N.; Seiyama, A.; Fujimoto, M. Dynamic aspects in host-guest interactions: Mechanism for molecular recognition by.alpha.-cyclodextrin of alkyl-substituted hydroxyphenylazo derivatives of sulfanilic acid. J. Phys. Chem. 1990, 94, 4246–4253. [Google Scholar] [CrossRef]
- Becket, G.; Schep, L.J.; Tan, M.Y. Improvement of the in vitro dissolution of praziquantel by complexation with α-, β- and γ-cyclodextrins. Int. J. Pharm. 1999, 179, 65–71. [Google Scholar] [CrossRef]
- Wimmer, T. Cyclodextrins. In Ullmann’s Encyclopedia of Industrial Chemistry; American Cancer Society: New York, NY, USA, 2003; p. 31. [Google Scholar]
- Dhiman, P.; Bhatia, M. Pharmaceutical applications of cyclodextrins and their derivatives. J. Incl. Phenom. Macrocycl. Chem. 2020, 98, 171–186. [Google Scholar] [CrossRef]
- Pereira, A.G.; Carpena, M.; Oliveira, P.G.; Mejuto, J.C.; Prieto, M.A.; Gandara, J.S. Main applications of cyclodextrins in the food industry as the compounds of choice to form host–guest complexes. Int. J. Mol. Sci. 2021, 22, 1339. [Google Scholar] [CrossRef]
- Szejtli, J. Cyclodextrin Technology; Springer: Dordrecht, The Netherlands, 1988; p. 450. [Google Scholar]
- Falaise, C.; Moussawi, M.A.; Floquet, S.; Abramov, P.A.; Sokolov, M.N.; Haouas, M.; Cadot, E. Probing dynamic library of metal-oxo building blocks with γ-cyclodextrin. J. Am. Chem. Soc. 2018, 140, 11198. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Shi, R.; Wu, Y.-L.; Holcroft, J.M.; Liu, Z.; Frasconi, M.; Wasielewski, M.R.; Li, H.; Stoddart, J.F. Complexation of polyoxometalates with cyclodextrins. J. Am. Chem. Soc. 2015, 137, 4111–4118. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.-G.; Mao, W.-T.; Huang, D.-P.; Wang, Y.; Wang, X.-J.; Zhan, C.-H. A nonconventional host–guest cubic assembly based on γ-cyclodextrin and a Keggin-type polyoxometalate. Nanoscale 2020, 12, 10166–10171. [Google Scholar] [CrossRef]
- Assaf, K.I.; Ural, M.S.; Pan, F.; Georgiev, T.; Simova, S.; Rissanen, K.; Gabel, D.; Nau, W.M. Water structure recovery in chaotropic anion eecognition: High-affinity binding of dodecaborate clusters to γ-cyclodextrin. Angew. Chem. Int. Ed. 2015, 54, 6852–6856. [Google Scholar] [CrossRef] [PubMed]
- El Anwar, S.; Assaf, K.I.; Begaj, B.; Samsonov, M.A.; Růžičková, Z.; Holub, J.; Bavol, D.; Nau, W.M.; Gabel, D.; Grűner, B. Versatile, one-pot introduction of nonahalogenated 2-ammonio-decaborate ions as boron cluster scaffolds into organic molecules; host–guest complexation with γ-cyclodextrin. Chem. Commun. 2019, 55, 13669–13672. [Google Scholar] [CrossRef]
- Falaise, C.; Ivanov, A.A.; Molard, Y.; Amela Cortes, M.; Shestopalov, M.A.; Haouas, M.; Cadot, E.; Cordier, S. From supramolecular to solid state chemistry: Crystal engineering of luminescent materials by trapping molecular clusters in an aluminium-based hosting matrix. Mater. Horiz. 2020, 7, 2399–2406. [Google Scholar] [CrossRef]
- Ivanov, A.A.; Falaise, C.; Abramov, P.A.; Shestopalov, M.A.; Kirakci, K.; Lang, K.; Moussawi, M.A.; Sokolov, M.N.; Naumov, N.G.; Floquet, S.; et al. Host–guest binding hierarchy within redox- and luminescence-responsive supramolecular self-assembly based on chalcogenide clusters and γ-cyclodextrin. Chem. Eur. J. 2018, 24, 13467–13478. [Google Scholar] [CrossRef]
- Ivanov, A.A.; Haouas, M.; Evtushok, D.V.; Pozmogova, T.N.; Golubeva, T.S.; Molard, Y.; Cordier, S.; Falaise, C.; Cadot, E.; Shestopalov, M.A. Stabilization of octahedral metal halide clusters by host−guest complexation with γ-cyclodextrin: Toward nontoxic luminescent compounds. Inorg. Chem. 2022, 61, 14462–14469. [Google Scholar] [CrossRef]
- Moussawi, M.A.; Leclerc-Laronze, N.; Floquet, S.; Abramov, P.A.; Sokolov, M.N.; Cordier, S.; Ponchel, A.; Monflier, E.; Bricout, H.; Landy, D.; et al. Polyoxometalate, cationic cluster, and γ-cyclodextrin: From primary interactions to supramolecular hybrid materials. J. Am. Chem. Soc. 2017, 139, 12793–12803. [Google Scholar] [CrossRef]
- Ivanov, A.A.; Falaise, C.; Landy, D.; Haouas, M.; Mironov, Y.V.; Shestopalov, M.A.; Cadot, E. Tuning the chaotropic effect as an assembly motif through one-electron transfer in a rhenium cluster. Chem. Commun. 2019, 55, 9951–9954. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Falaise, C.; Leclerc, L.; Roch-Marchal, C.; Haouas, M.; Cadot, E. Improvement of the hydrolytic stability of the Keggin molybdo- and tungsto-phosphate anions by cyclodextrins. Inorg. Chem. 2022, 61, 4193–4203. [Google Scholar] [CrossRef] [PubMed]
- Hohenschutz, M.; Bauduin, P.; Lopez, C.G.; Förster, B.; Richtering, W. Superchaotropic nano-ion binding as a gelation motif in cellulose ether solutions. Angew. Chem. Int. Ed. 2023, 62, e202210208. [Google Scholar] [CrossRef]
- Falaise, C.; Khlifi, S.; Bauduin, P.; Schmid, P.; Shepard, W.; Ivanov, A.A.; Sokolov, M.N.; Shestopalov, M.A.; Abramov, P.A.; Cordier, S.; et al. “Host in host” supramolecular core–shell type systems based on giant ring-shaped polyoxometalates. Angew. Chem. Int. Ed. 2021, 60, 14146–14153. [Google Scholar] [CrossRef] [PubMed]
- Hohenschutz, M.; Grillo, I.; Diat, O.; Bauduin, P. How nano-ions act like ionic surfactants. Angew. Chem. Int. Ed. 2020, 59, 8084–8088. [Google Scholar] [CrossRef]
- Assaf, K.I.; Nau, W.M. The chaotropic effect as an assembly motif in chemistry. Angew. Chem. Int. Ed. 2018, 57, 13968–13981. [Google Scholar] [CrossRef]
- Ivanov, A.A.; Falaise, C.; Shmakova, A.A.; Leclerc, N.; Cordier, S.; Molard, Y.; Mironov, Y.V.; Shestopalov, M.A.; Abramov, P.A.; Sokolov, M.N.; et al. Cyclodextrin-assisted hierarchical aggregation of dawson-type polyoxometalate in the presence of {Re6Se8} based clusters. Inorg. Chem. 2020, 59, 11396–11406. [Google Scholar] [CrossRef]
- Tourneur, J.; Fabre, B.; Loget, G.; Vacher, A.; Mériadec, C.; Ababou-Girard, S.; Gouttefangeas, F.; Joanny, L.; Cadot, E.; Haouas, M.; et al. Molecular and material engineering of photocathodes derivatized with polyoxometalate-supported {Mo3S4} HER catalysts. J. Am. Chem. Soc. 2019, 141, 11954–11962. [Google Scholar] [CrossRef]
- Smortsova, Y.; Falaise, C.; Fatima, A.; Ha-Thi, M.-H.; Méallet-Renault, R.; Steenkeste, K.; Al-Bacha, S.; Chaib, T.; Assaud, L.; Lepeltier, M.; et al. Time-resolved spectroscopy and high-efficiency light-driven hydrogen evolution of a {Mo3S4}-containing polyoxometalate-based system. Chem. Eur. J. 2021, 27, 17094–17103. [Google Scholar] [CrossRef]
- Elistratova, J.; Mikhailov, M.; Burilov, V.; Babaev, V.; Rizvanov, I.; Mustafina, A.; Abramov, P.; Sokolov, M.; Konovalov, A.; Fedin, V. Supramolecular assemblies of triblock copolymers with hexanuclear molybdenum clusters for sensing antibiotics in aqueous solutions via energy transfer. RSC Adv. 2014, 4, 27922–27930. [Google Scholar] [CrossRef]
- Harada, A.; Hu, Y.; Yamamoto, S.; Takahashi, S. Preparation and properties of inclusion compounds of ferrocene and its derivatives with cyclodextrins. J. Chem. Soc. Dalton Trans. 1988, 1988, 729–732. [Google Scholar] [CrossRef]
- Imonigie, J.A.; Macartney, D.H. The effects of cyclodextrin inclusion of the ferrocenemonocarboxylate anion on the kinetics of its oxidation by bis(pyridine-2,6-dicarboxylato)cobaltate(III) in aqueous solution. J. Incl. Phenom. Macrocycl. Chem. 1993, 15, 195–203. [Google Scholar] [CrossRef]
- Retna Rej, C.; Ramaraj, R. Electrochemical study of the cyclodextrin encapsulation of a macrocyclic nickel complex. Electrochim. Acta 1999, 44, 2685–2691. [Google Scholar] [CrossRef]
- Patel, P.P.; Welker, M.E. Preparation of (η5-cyclopentadienyl) and (η5-methylcyclopentadienyl) Fe(CO)2Me cyclodextrin inclusion compounds and their subsequent ligand substitution reactions. Attempts at cyclodextrin mediated enantioselective ligand substitution. J. Organomet. Chem. 1997, 547, 103–112. [Google Scholar] [CrossRef]
- Chen, Y.; Luo, L.-b.; Chen, H.-l.; Hu, C.-h.; Chen, J.; Zheng, P.-j. Crystal structure and thermal study of the aqua(butyl)cobaloxime/α-cyclodextrin inclusion complex. Bull. Chem. Soc. Jpn. 2000, 73, 1375–1378. [Google Scholar] [CrossRef]
- Xu, W.; Jain, A.; Betts, B.A.; Demas, J.N.; DeGraff, B.A. Single and multiple binding of β-cyclodextrin and polymeric β-cyclodextrins to luminescent ruthenium(II) α-diimine complexes. J. Phys. Chem. A 2002, 106, 251–257. [Google Scholar] [CrossRef]
- Beck, D.; Brewer, J.; Lee, J.; McGraw, D.; deGraff, B.A.; Demas, J.N. Localizing molecular probes: Inclusion of Re(I) complexes in β-cyclodextrin. Coord. Chem. Rev. 2007, 251, 546–553. [Google Scholar] [CrossRef]
- Lewis, L.N.; Sumpter, C.A.; Davis, M. Platinum-group metal cyclodextrin complexes and their use as command-cure catalysts in silicones. J. Inorg. Organomet. Polym. 1995, 5, 377–390. [Google Scholar] [CrossRef]
- Caron, L.; Canipelle, M.; Tilloy, S.; Bricout, H.; Monflier, E. Unexpected effect of cyclodextrins on water-soluble rhodium complexes. Eur. J. Inorg. Chem. 2003, 2003, 595–599. [Google Scholar] [CrossRef]
- Sieffert, N.; Wipff, G. Importance of interfacial adsorption in the biphasic hydroformylation of higher olefins promoted by cyclodextrins: A molecular dynamics study at the decene/water interface. Chem. Eur. J. 2007, 13, 1978–1990. [Google Scholar] [CrossRef]
- Gerasko, O.A.; Sokolov, M.N.; Fedin, V.P. Mono- and polynuclear aqua complexes and cucurbit[6]uril: Versatile building blocks for supramolecular chemistry. Pure Appl. Chem. 2009, 76, 1633–1646. [Google Scholar] [CrossRef]
- Hernandez-Molina, R.; Sokolov, M.N. Cuboidal cluster aqua complexes with {M3M′Q4} cores (M = Mo, W; M′ = Ni, Pd; Q = S, Se) and their derivatives. Russ. J. Coord. Chem. 2012, 38, 159–166. [Google Scholar] [CrossRef]
- Choi, S.-J.; Brylev, K.A.; Xu, J.-Z.; Mironov, Y.V.; Fedorov, V.E.; Sohn, Y.S.; Kim, S.-J.; Choy, J.-H. Cellular uptake and cytotoxicity of octahedral rhenium cluster complexes. J. Inorg. Biochem. 2008, 102, 1991–1996. [Google Scholar] [CrossRef] [PubMed]
- Sokolov, M.N.; Mihailov, M.A.; Peresypkina, E.V.; Brylev, K.A.; Kitamura, N.; Fedin, V.P. Highly luminescent complexes [Mo6X8(n-C3F7COO)6]2− (X = Br, I). Dalton Trans. 2011, 40, 6375–6377. [Google Scholar] [CrossRef] [PubMed]
- Beltrán, A.; Mikhailov, M.; Sokolov, M.N.; Pérez-Laguna, V.; Rezusta, A.; Revillo, M.J.; Galindo, F. A photobleaching resistant polymer supported hexanuclear molybdenum iodide cluster for photocatalytic oxygenations and photodynamic inactivation of Staphylococcus aureus. J. Mater. Chem. B 2016, 4, 5975–5979. [Google Scholar] [CrossRef]
- Elistratova, J.; Burilov, V.; Mustafina, A.; Mikhailov, M.; Sokolov, M.; Fedin, V.; Konovalov, A. Triblock copolymer-based luminescent organic–inorganic hybrids triggered by heating and fluoroquinolone antibiotics. Polymer 2015, 72, 98–103. [Google Scholar] [CrossRef]
- Ivanov, A.V.; Vasina, T.V.; Nissenbaum, V.D.; Kustov, L.M.; Timofeeva, M.N.; Houzvicka, J.I. Isomerization of n-hexane on the Pt-promoted Keggin and Dawson tungstophosphoric heteropoly acids supported on zirconia. Appl. Catal. A 2004, 259, 65–72. [Google Scholar] [CrossRef]
- Kholdeeva, O.A.; Trubitsina, T.A.; Timofeeva, M.N.; Maksimov, G.M.; Maksimovskaya, R.I.; Rogov, V.A. The role of protons in cyclohexene oxidation with H2O2 catalysed by Ti(IV)-monosubstituted Keggin polyoxometalate. J. Mol. Catal. A Chem. 2005, 232, 173–178. [Google Scholar] [CrossRef]
- Maksimchuk, N.V.; Kovalenko, K.A.; Arzumanov, S.S.; Chesalov, Y.A.; Melgunov, M.S.; Stepanov, A.G.; Fedin, V.P.; Kholdeeva, O.A. Hybrid polyoxotungstate/MIL-101 Materials: Synthesis, characterization, and catalysis of H2O2-based alkene epoxidation. Inorg. Chem. 2010, 49, 2920–2930. [Google Scholar] [CrossRef]
- Yamase, T. Anti-tumor, -viral, and -bacterial activities of polyoxometalates for realizing an inorganic drug. J. Mater. Chem. 2005, 15, 4773–4782. [Google Scholar] [CrossRef]
- Sazani, G.; Pope, M.T. Organotin and organogermanium linkers for simple, direct functionalization of polyoxotungstates. Dalton Trans. 2004, 2004, 1989–1994. [Google Scholar] [CrossRef]
- Hasenknopf, B. Polyoxometalates: Introduction to a class of inorganic compounds and their biomedical applications. Front. Biosci. 2005, 10, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Yue, L.; Wang, S.; Zhou, D.; Zhang, H.; Li, B.; Wu, L. Flexible single-layer ionic organic–inorganic frameworks towards precise nano-size separation. Nat. Commun. 2016, 7, 10742. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, A.A.; Falaise, C.; Laouer, K.; Hache, F.; Changenet, P.; Mironov, Y.V.; Landy, D.; Molard, Y.; Cordier, S.; Shestopalov, M.A.; et al. Size-exclusion mechanism driving host–guest interactions between octahedral rhenium clusters and cyclodextrins. Inorg. Chem. 2019, 58, 13184–13194. [Google Scholar] [CrossRef]
- Costuas, K.; Garreau, A.; Bulou, A.; Fontaine, B.; Cuny, J.; Gautier, R.; Mortier, M.; Molard, Y.; Duvail, J.-L.; Faulques, E.; et al. Combined theoretical and time-resolved photoluminescence investigations of [Mo6Bri8Bra6]2− metal cluster units: Evidence of dual emission. Phys. Chem. Chem. Phys. 2015, 17, 28574–28585. [Google Scholar] [CrossRef] [PubMed]
- Abramov, P.A.; Rogachev, A.V.; Mikhailov, M.A.; Virovets, A.V.; Peresypkina, E.V.; Sokolov, M.N.; Fedin, V.P. Hexanuclear chloride and brimode tungsten clusters and their derivatives. Russ. J. Coord. Chem. 2014, 40, 259–267. [Google Scholar] [CrossRef]
- Kozhomuratova, Z.S.; Mironov, Y.V.; Shestopalov, M.A.; Gaifulin, Y.M.; Kurat’eva, N.V.; Uskov, E.M.; Fedorov, V.E. Cluster compounds [Ca(DMF)6][Mo6Cl14] and [{Ca(OPPh3)4}{Mo6Cl14}]∞: Synthesis, crystal structure, and properties. Russ. J. Coord. Chem. 2007, 33, 1–6. [Google Scholar] [CrossRef]
- Savitzky, A.; Golay, M.J.E. Smoothing and differentiation of data by simplified least squares procedures. Anal. Chem. 1964, 36, 1627–1639. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.a.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Acta Cryst. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Hübschle, C.B.; Sheldrick, G.M.; Dittrich, B. ShelXle: A Qt graphical user interface for SHELXL. J. Appl. Cryst. 2011, 44, 1281–1284. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Compound | λ, nm | τ (A), µs | τ0a, µs | Φ |
|---|---|---|---|---|
| Mo6Cl14 | 750 | τ1 = 30 (0.29) τ2 = 60 (0.71) | 55 | 0.10 |
| Mo6Cl14@2CD·CD | 750 | 61 | 61 | 0.08 |
| W6Cl14 | 760 | τ1 = 12 (0.04) τ2 = 3.5 (0.96) | 4.6 | 0.05 |
| W6Cl14@2CD·CD | – | – | – | – |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanov, A.A.; Abramov, P.A.; Haouas, M.; Molard, Y.; Cordier, S.; Falaise, C.; Cadot, E.; Shestopalov, M.A. Supramolecular Host–Guest Assemblies of [M6Cl14]2–, M = Mo, W, Clusters with γ-Cyclodextrin for the Development of CLUSPOMs. Inorganics 2023, 11, 77. https://doi.org/10.3390/inorganics11020077
Ivanov AA, Abramov PA, Haouas M, Molard Y, Cordier S, Falaise C, Cadot E, Shestopalov MA. Supramolecular Host–Guest Assemblies of [M6Cl14]2–, M = Mo, W, Clusters with γ-Cyclodextrin for the Development of CLUSPOMs. Inorganics. 2023; 11(2):77. https://doi.org/10.3390/inorganics11020077
Chicago/Turabian StyleIvanov, Anton A., Pavel A. Abramov, Mohamed Haouas, Yann Molard, Stéphane Cordier, Clément Falaise, Emmanuel Cadot, and Michael A. Shestopalov. 2023. "Supramolecular Host–Guest Assemblies of [M6Cl14]2–, M = Mo, W, Clusters with γ-Cyclodextrin for the Development of CLUSPOMs" Inorganics 11, no. 2: 77. https://doi.org/10.3390/inorganics11020077