Isolation and Purification of Single Gold Nanoclusters by Alternate Pumping Chromatography
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis
2.3. Chromatography
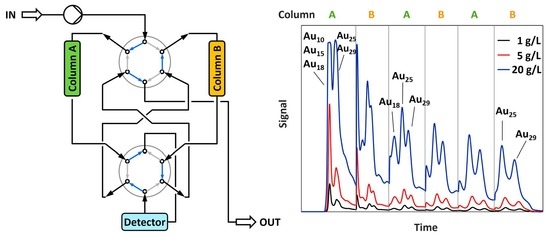
3. Principle and Basic Design of Alternate Pumping
4. Results
4.1. Chromatographic Separation Problem
4.2. Separation by Alternate Pumping
4.2.1. Isolation of Two Larger Clusters
4.2.2. Isolation of a Single Cluster
4.2.3. Isolation of Trace Compounds
4.2.4. Separation of Larger Amounts
5. Summary and Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guiochon, G.; Shirazi, D.G.; Felinger, A.; Katti, A.M. Fundamentals of Preparative and Nonlinear Chromatography, 2nd ed.; Academic Press: Boston, MA, USA, 2006. [Google Scholar]
- Fischer, C.H.; Giersig, M.; Siebrands, T. Analysis of colloidal particles V. Size-exclusion chromatography of colloidal semiconductor particles. J. Chromatogr. A 1994, 670, 89–97. [Google Scholar] [CrossRef]
- Wei, G.T.; Liu, F.K. Separation of nanometer gold particles by size exclusion chromatography. J. Chromatogr. A 1999, 836, 253–260. [Google Scholar] [CrossRef]
- Wei, G.T.; Liu, F.K.; Wang, C.R.C. Shape Separation of Nanometer Gold Particles by Size-Exclusion Chromatography. Anal. Chem. 1999, 71, 2085–2091. [Google Scholar] [CrossRef]
- Süß, S.; Metzger, C.; Damm, C.; Segets, D.; Peukert, W. Quantitative evaluation of nanoparticle classification by size-exclusion chromatography. Powder Technol. 2018, 339, 264–272. [Google Scholar] [CrossRef]
- Gromotka, L.; Uttinger, M.J.; Schlumberger, C.; Thommes, M.; Peukert, W. Classification and characterization of multimodal nanoparticle size distributions by size-exclusion chromatography. Nanoscale 2022, 14, 17354–17364. [Google Scholar] [CrossRef]
- Süß, S.; Bartsch, K.; Wasmus, C.; Damm, C.; Segets, D.; Peukert, W. Chromatographic property classification of narrowly distributed ZnS quantum dots. Nanoscale 2020, 12, 12114–12125. [Google Scholar] [CrossRef]
- Fischer, C.H. Analysis of Colloids. VI. Semiconductor Colloids of High Monodispersity by Preparative Size Exclusion Chromatography. J. Liq. Chromatogr. 1994, 17, 3593–3611. [Google Scholar] [CrossRef]
- Pitkänen, L.; Striegel, A.M. Size-exclusion chromatography of metal nanoparticles and quantum dots. Trends Anal. Chem. 2016, 80, 311–320. [Google Scholar] [CrossRef] [Green Version]
- Peukert, W.; Kaspereit, M.; Hofe, T.; Gromotka, L. Size exclusion chromatography (SEC). In Particle Separation Techniques; Handbooks in Separation Science; Contado, C., Ed.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 409–447. [Google Scholar] [CrossRef]
- Bos, W.; Steggerda, J.J.; Yan, S.; Casalnuovo, J.A.; Mueting, A.M.; Pignolet, L.H. Separation of cationic metal cluster compounds by reversed phase HPLC. Inorg. Chem. 1988, 27, 948–951. [Google Scholar] [CrossRef]
- Liu, F.K.; Chang, Y.C. Using reversed-phase liquid chromatography to monitor the sizes of Au/Pt core/shell nanoparticles. J. Chromatogr. A 2010, 1217, 1647–1653. [Google Scholar] [CrossRef]
- Yang, Y.; Luo, L.; Li, H.P.; Wang, Q.; Yang, Z.G.; Qu, Z.P.; Ding, R. Analysis of metallic nanoparticles and their ionic counterparts in complex matrix by reversed-phase liquid chromatography coupled to ICP-MS. Talanta 2018, 182, 156–163. [Google Scholar] [CrossRef]
- Niihori, Y.; Uchida, C.; Kurashige, W.; Negishi, Y. High-resolution separation of thiolate-protected gold clusters by reversed-phase high-performance liquid chromatography. Phys. Chem. Chem. Phys. 2016, 18, 4251–4265. [Google Scholar] [CrossRef]
- Black, D.M.; Bhattarai, N.; Bach, S.B.H.; Whetten, R.L. Selection and Identification of Molecular Gold Clusters at the Nano(gram) Scale: Reversed Phase HPLC–ESI–MS of a Mixture of Au-Peth MPCs. J. Phys. Chem. Lett. 2016, 7, 3199–3205. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.M.F.; Douglas, A.D.; Murray, R.W. Ion-Pair Chromatographic Separation of Water-Soluble Gold Monolayer-Protected Clusters. Anal. Chem. 2006, 78, 2779–2785. [Google Scholar] [CrossRef] [PubMed]
- Black, D.M.; Alvarez, M.M.; Yan, F.; Griffith, W.P.; Plascencia-Villa, G.; Bach, S.B.H.; Whetten, R.L. Triethylamine Solution for the Intractability of Aqueous Gold–Thiolate Cluster Anions: How Ion Pairing Enhances ESI-MS and HPLC of aq-Aun(pMBA)p. J. Phys. Chem. C 2017, 121, 10851–10857. [Google Scholar] [CrossRef]
- Niihori, Y.; Kikuchi, Y.; Shima, D.; Uchida, C.; Sharma, S.; Hossain, S.; Kurashige, W.; Negishi, Y. Separation of Glutathionate-Protected Gold Clusters by Reversed-Phase Ion-Pair High-Performance Liquid Chromatography. Ind. Eng. Chem. Res. 2017, 56, 1029–1035. [Google Scholar] [CrossRef]
- Niihori, Y.; Shima, D.; Yoshida, K.; Hamada, K.; Nair, L.V.; Hossain, S.; Kurashige, W.; Negishi, Y. High-performance liquid chromatography mass spectrometry of gold and alloy clusters protected by hydrophilic thiolates. Nanoscale 2018, 10, 1641–1649. [Google Scholar] [CrossRef]
- Gromotka, L.; Lübbert, C.; Traoré, N.; Peukert, W. Green and scalable fractionation of gold nanoclusters by anion exchange chromatography. ACS Appl. Nano Mater. 2023. Submitted. [Google Scholar]
- Nicoud, R.M. Chromatographic Processes: Modeling, Simulation, and Design; Cambridge Series in Chemical Engineering; Cambridge University Press: Cambridge, UK, 2015. [Google Scholar] [CrossRef] [Green Version]
- Schmidt-Traub, H.; Schulte, M.; Seidel-Morgenstern, A. (Eds.) Preparative Chromatography, 3rd ed.; Wiley: Hoboken, NJ, USA, 2020. [Google Scholar] [CrossRef]
- Kaspereit, M. Chapter—Advanced operating concepts for Simulated Moving Bed Processes. In Advances in Chromatography; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2009; pp. 165–192. [Google Scholar]
- Satzer, P.; Wellhoefer, M.; Jungbauer, A. Continuous separation of protein loaded nanoparticles by simulated moving bed chromatography. J. Chromatogr. A 2014, 1349, 44–49. [Google Scholar] [CrossRef] [Green Version]
- Arlt, C.R.; Brekel, D.; Neumann, S.; Rafaja, D.; Franzreb, M. Continuous size fractionation of magnetic nanoparticles by using simulated moving bed chromatography. Front. Chem. Sci. Eng. 2021, 15, 1346–1355. [Google Scholar] [CrossRef]
- Porter, R.S.; Johnson, J.F. Circular Gas Chromatograph. Nature 1959, 183, 391–392. [Google Scholar] [CrossRef]
- Bombaugh, K.J.; Levangie, R.F. High Resolution Gel Permeation Chromatography-Using Recycle. Sep. Sci. 1970, 5, 751–763. [Google Scholar] [CrossRef]
- Dingenen, J.; Kinkel, J. Preparative chromatographic resolution of racemates on chiral stationary phases on laboratory and production scales by closed-loop recycling chromatography. J. Chromatogr. A 1994, 666, 627–650. [Google Scholar] [CrossRef]
- Heuer, C.; Seidel-Morgenstern, A.; Hugo, P. Experimental investigation and modelling of closed-loop recycling in preparative chromatography. Chem. Eng. Sci. 1995, 50, 1115–1127. [Google Scholar] [CrossRef]
- Seidel-Morgenstern, A.; Guiochon, G. Theoretical Study of Recycling in Preparative Chromatography. AIChE J. 1993, 39, 809–819. [Google Scholar] [CrossRef]
- Porath, J.O.; Bennich, H. Recycling chromatography. Arch. Biochem. Biophys. 1962, 1, 152–156. [Google Scholar]
- Duvdevani, I.; Biesenberger, J.A.; Tan, M. Recycle gel permeation chromatography. III. Design modifications and some results with polycarbonate. J. Polym. Sci. Part B Polym. Lett. 1971, 9, 429–434. [Google Scholar] [CrossRef]
- Henry, R.A.; Byrne, S.H.; Hudson, D.R. High Speed Recycle Chromatography Using an Alternate Pumping Principle. J. Chromatogr. Sci. 1974, 12, 197–199. [Google Scholar] [CrossRef]
- Liu, Q.; Xiao, J.; Yu, J.; Xie, Y.; Chen, X.; Yang, H. Improved enantioseparation via the twin-column based recycling high performance liquid chromatography. J. Chromatogr. A 2014, 1363, 236–241. [Google Scholar] [CrossRef]
- Gritti, F.; Cormier, S.; Morris, R.; Riley, F.; Yan, Q. Ultrahigh Resolution Semipreparative Liquid Chromatography: Application to Structure Elucidation of Drug Impurities; LC-GC Europe: Chester, UK, 2019; pp. 62–70. [Google Scholar]
- Chen, Y.; Wang, Z.; Zhang, H.; Liu, Y.; Zhang, S.; Meng, Q.; Liu, W. Isolation of High Purity Anthocyanin Monomers from Red Cabbage with Recycling Preparative Liquid Chromatography and Their Photostability. Molecules 2018, 23, 991. [Google Scholar] [CrossRef] [Green Version]
- Gritti, F.; Cormier, S. Performance optimization of ultra high-resolution recycling liquid chromatography. J. Chromatogr. A 2018, 1532, 74–88. [Google Scholar] [CrossRef]
- Wei, F.; Yang, Z.; Zhao, Y.; Wang, Q. A twin-column recycling chromatography with solvent gradient for reinforcing the isolation of minor impurities. AIChE J. 2019, 65, 702–711. [Google Scholar] [CrossRef]
- Wei, F.; Sang, J.; Zhao, Y. Theoretical study of twin-column recycling chromatography with a solvent-gradient for preparative binary separations. J. Chromatogr. A 2021, 1651, 462306. [Google Scholar] [CrossRef]
- Gritti, F. Rebirth of recycling liquid chromatography with modern chromatographic columns: Extension to gradient elution. J. Chromatogr. A 2021, 1653, 462424. [Google Scholar] [CrossRef]
- Al-Somali, A.M.; Krueger, K.M.; Falkner, J.C.; Colvin, V.L. Recycling Size Exclusion Chromatography for the Analysis and Separation of Nanocrystalline Gold. Anal. Chem. 2004, 76, 5903–5910. [Google Scholar] [CrossRef]
- Qian, H.; Zhu, M.; Wu, Z.; Jin, R. Quantum Sized Gold Nanoclusters with Atomic Precision. Acc. Chem. Res. 2012, 45, 1470–1479. [Google Scholar] [CrossRef]
- Zheng, J.; Zhang, J.; Lu, F.; Du, Y.; Cao, D.; Hu, S.; Yang, Y.; Yuan, Z. Visualization of Polymer–Surfactant Interaction by Dual-Emissive Gold Nanocluster Labeling. Biosensors 2022, 12, 686. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, Y.; Liu, Y.; Liu, W.; Lu, F.; Yuan, Z.; Lu, C. Gold Nanocluster-Encapsulated Hyperbranched Polyethyleneimine for Selective and Ratiometric Dopamine Analyses by Enhanced Self-Polymerization. Front. Chem. 2022, 10, 928607. [Google Scholar] [CrossRef]
- Seong, H.; Efremov, V.; Park, G.; Kim, H.; Yoo, J.S.; Lee, D. Atomically Precise Gold Nanoclusters as Model Catalysts for Identifying Active Sites for Electroreduction of CO2. Angew. Chem. Int. Ed. 2021, 60, 14563–14570. [Google Scholar] [CrossRef] [PubMed]
- Sonia, K.; Kukreti, S.; Kaushik, M. Gold nanoclusters: An ultrasmall platform for multifaceted applications. Talanta 2021, 234, 122623. [Google Scholar] [CrossRef]
- Kucera, P.; Manius, G. Recycling liquid chromatography using microbore columns. J. Chromatogr. A 1981, 219, 1–12. [Google Scholar] [CrossRef]
- Gritti, F. Automated High-Resolution Semi-Preparative Gradient Recycling Liquid Chromatography: Principles, Design, and Applications; LC-GC Europe: Chester, UK, 2021; pp. 455–461. [Google Scholar] [CrossRef]











| Peak | Column A | Column B | Difference/% | |||||
|---|---|---|---|---|---|---|---|---|
| Au | 8.37 | 0.613 | 1.701 1.135 | 7.88 | 0.579 | 1.657 1.104 | 5.5 | 2.6 |
| Au | 10.60 | 1.042 | 9.78 | 0.960 | 7.9 | 2.7 | ||
| Au | 11.33 | 1.183 | 10.28 | 0.1060 | 10.4 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Supper, M.; Birner, V.; Gromotka, L.; Peukert, W.; Kaspereit, M. Isolation and Purification of Single Gold Nanoclusters by Alternate Pumping Chromatography. Separations 2023, 10, 214. https://doi.org/10.3390/separations10030214
Supper M, Birner V, Gromotka L, Peukert W, Kaspereit M. Isolation and Purification of Single Gold Nanoclusters by Alternate Pumping Chromatography. Separations. 2023; 10(3):214. https://doi.org/10.3390/separations10030214
Chicago/Turabian StyleSupper, Malvina, Virginia Birner, Lukas Gromotka, Wolfgang Peukert, and Malte Kaspereit. 2023. "Isolation and Purification of Single Gold Nanoclusters by Alternate Pumping Chromatography" Separations 10, no. 3: 214. https://doi.org/10.3390/separations10030214