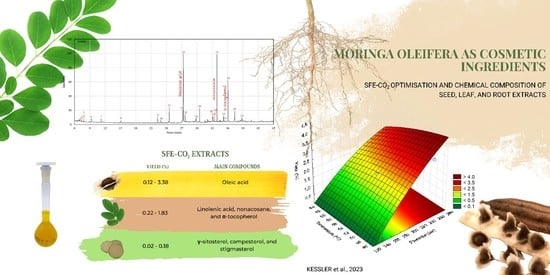
Moringa oleifera L. Screening: SFE-CO2 Optimisation and Chemical Composition of Seed, Leaf, and Root Extracts as Potential Cosmetic Ingredients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material
2.3. Supercritical Fluid Extraction Using Carbon Dioxide
2.4. Design of Experiments and Response Surface Analysis
2.5. Chemical Composition Assays by GC-MS
3. Results and Discussions
3.1. Extraction Yield of Mo SFE-CO2 Extracts
3.2. Optimisation Study of Mo SFE-CO2 Extracts by RSM
3.3. Chemical Profile of Mo SFE-CO2 Extracts Useful for Cosmetic Products
3.3.1. Seed SFE-CO2 Extracts
3.3.2. Leaf SFE-CO2 Extracts
3.3.3. Root SFE-CO2 Extracts
3.4. Selection of the Most Promising Extracts for Further Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rani, N.Z.A.; Husain, K.; Kumolosasi, E. Moringa genus: A review of phytochemistry and pharmacology. Front. Pharmacol. 2018, 9, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dzuvor, C.K.O.; Pan, S.; Amanze, C.; Amuzu, P.; Asakiya, C.; Kubi, F. Bioactive components from Moringa oleifera seeds: Production, functionalities and applications—A critical review. Crit. Rev. Biotechnol. 2021, 42, 271–293. [Google Scholar] [CrossRef] [PubMed]
- Marrufo, T.; Nazzaro, F.; Mancini, E.; Fratianni, F.; Coppola, R.; De Martino, L.; Agostinho, A.B.; De Feo, V. Chemical composition and biological activity of the essential oil from leaves of Moringa oleifera Lam. cultivated in Mozambique. Molecules 2013, 18, 10989–11000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muhammad, A.A.; Pauzi, N.A.S.; Arulselvan, P.; Abas, F.; Fakurazi, S. In Vitro wound healing potential and identification of bioactive compounds from Moringa oleifera Lam. Biomed Res. Int. 2013, 2013, 974580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vergara-Jimenez, M.; Almatrafi, M.M.; Fernandez, M.L. Bioactive components in Moringa Oleifera leaves protect against chronic disease. Antioxidants 2017, 6, 91. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, A.; Bancessi, A.; Pinela, J.; Dias, M.I.; Liberal, A.; Calhelha, R.C.; Ćirić, A.; Soković, M.; Catarino, L.; Ferreira, I.C.F.R.; et al. Nutritional and phytochemical profiles and biological activities of Moringa oleifera Lam. edible parts from Guinea-Bissau (West Africa). Food Chem. 2020, 341, 128229. [Google Scholar]
- Baldisserotto, A.; Buso, P.; Radice, M.; Dissette, V.; Lampronti, I.; Gambari, R.; Manfredini, S.; Vertuani, S. Moringa oleifera leaf extracts as multifunctional ingredients for “natural and organic” sunscreens and photoprotective preparations. Molecules 2018, 23, 664. [Google Scholar] [CrossRef] [Green Version]
- Waterman, C.; Cheng, D.M.; Rojas-Silva, P.; Poulev, A.; Dreifus, J.; Lila, M.A.; Raskin, I. Stable, water extractable isothiocyanates from Moringa oleifera leaves attenuate inflammation in vitro. Phytochemistry 2014, 103, 114–122. [Google Scholar] [CrossRef] [Green Version]
- Ziani, B.E.C.; Rached, W.; Bachari, K.; Alves, M.J.; Calhelha, R.C.; Barros, L.; Ferreira, I.C.F.R. Detailed chemical composition and functional properties of Ammodaucus leucotrichus Cross. & Dur. and Moringa oleifera Lamarck. J. Funct. Foods 2019, 53, 237–247. [Google Scholar]
- Ali, A.; Naveed, A.; Khan, M.S.; Rasool, F.; Iqbal, F.M.; Khan, M.T.; Din, M.U.; Elahi, E. Moisturizing effect of cream containing Moringa oleifera (Sohajana) leaf extract by biophysical techniques: In vivo evaluation. J. Med. Plant Res. 2013, 7, 386–391. [Google Scholar]
- Choi, E.J.; Debnath, T.; Tang, Y.; Ryu, Y.B.; Moon, S.H.; Kim, E.K. Topical application of Moringa oleifera leaf extract ameliorates experimentally induced atopic dermatitis by the regulation of Th1/Th2/Th17 balance. Biomed. Pharmacother. 2016, 84, 870–877. [Google Scholar] [CrossRef]
- Tshabalala, T.; Ndhlala, A.R.; Ncube, B.; Abdelgadir, H.A.; Van Staden, J. Potential substitution of the root with the leaf in the use of Moringa oleifera for antimicrobial, antidiabetic and antioxidant properties. S. Afr. J. Bot. 2020, 129, 106–112. [Google Scholar] [CrossRef]
- Pachauri, S.D.; Khandelwal, K.; Singh, S.P.; Sashidhara, K.V.; Dwivedi, A.K. HPLC method for identification and quantification of two potential anti-inflammatory and analgesic agents-1, 3-dibenzyl urea and aurantiamide acetate in the roots of Moringa oleifera. Med. Chem. Res. 2013, 22, 5284–5289. [Google Scholar] [CrossRef]
- Adeleye, S.A.; Braide, W.; Ibegbulem, C.R.; Nwigwe, V.N.; Ajunwa, O.M.; Korie, M.C. Phytochemistry and antifungal activity of root and seed extracts of Moringa oleifera. Int. J. Adv. Res. Biol. Sci. 2018, 5, 169–176. [Google Scholar]
- Athikomkulchai, S.; Tunit, P.; Tadtong, S.; Jantrawut, P.; Sommano, S.R.; Chittasupho, C. Moringa oleifera seed oil formulation physical stability and chemical constituents for enhancing skin hydration and antioxidant activity. Cosmetics 2021, 8, 2. [Google Scholar] [CrossRef]
- Wang, X.; Wu, J. Modulating effect of fatty acids and sterols on skin aging. J. Funct. Foods 2019, 57, 135–140. [Google Scholar] [CrossRef]
- Zorić, M.; Banožić, M.; Aladić, K.; Vladimir-Knežević, S.; Jokić, S. Supercritical CO2 extracts in cosmetic industry: Current status and future perspectives. Sustain. Chem. Pharm. 2022, 27, 100688. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, Y.G.; Park, J.G.; Lee, J. Supercritical fluid extracts of Moringa oleifera and their unsaturated fatty acid components inhibit biofilm formation by Staphylococcus aureus. Food Control. 2017, 80, 74–82. [Google Scholar] [CrossRef]
- Bhutada, P.R.; Jadhav, A.J.; Pinjari, D.V.; Nemade, P.R.; Jain, R.D. Solvent assisted extraction of oil from Moringa oleifera Lam. seeds. Ind. Crops Prod. 2016, 82, 74–80. [Google Scholar] [CrossRef]
- Khaw, K.Y.; Parat, M.O.; Shaw, P.N.; Falconer, J.R. Solvent Supercritical Fluid Technologies to Extract Bioactive Compounds from Natural Sources: A Review. Molecules 2017, 22, 1186. [Google Scholar] [CrossRef]
- Chemat, F.; Abert, V.M.; Ravi, H.K.; Khadhraoui, B.; Hilali, S.; Perino, S.; Tixier, A.F. Review of alternative solvents for green extraction of food and natural products: Panorama, principles, applications and prospects. Molecules 2019, 24, 3007. [Google Scholar] [CrossRef] [Green Version]
- Matshediso, P.G.; Cukrowska, E.; Chimuka, L. Development of pressurised hot water extraction (PHWE) for essential compounds from Moringa oleifera leaf extracts. Food Chem. 2015, 172, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Pérez, C.; Gilbert-López, B.; Mendiola, J.A.; Quirantes-Piné, R.; Segura-Carretero, A.; Ibáñez, E. Optimization of microwave-assisted extraction and pressurized liquid extraction of phenolic compounds from Moringa oleifera leaves by multiresponse surface methodology. Electrophoresis 2016, 37, 1938–1946. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Wang, Y.; Yang, R.; Liu, X.; Yang, Q.; Qin, X. The application of ultrasound and microwave to increase oil extraction from Moringa oleifera seeds. Ind. Crops Prod. 2018, 120, 1–10. [Google Scholar] [CrossRef]
- da Silva, M.; Trancoso, J.; Tormen, L.; Bombardelli, M.M.; Corazza, M.L.; Bainy, E.M. Extraction of compounds from Moringa oleifera leaves using supercritical CO2 plus ethanol as a cosolvent. J. Food Process. Eng. 2022, 45, e13979. [Google Scholar] [CrossRef]
- Ruttarattanamongkol, K.; Siebenhandl-Ehn, S.; Schreiner, M.; Petrasch, A.M. Pilot-scale supercritical carbon dioxide extraction, physico-chemical properties and profile characterization of Moringa oleifera seed oil in comparison with conventional extraction methods. Ind. Crops Prod. 2014, 58, 68–77. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, D. A parametric study of supercritical carbon dioxide extraction of oil from Moringa oleifera seeds using a response surface methodology. SePurif. Techn. 2013, 113, 9–17. [Google Scholar] [CrossRef]
- Ngamprasertsith, S.; Sukaead, W.; Camy, S.; Condoret, J.S.; Sawangkeaw, R. Recovery of Moringa oleifera oil from seed cake by supercritical carbon dioxide extraction. Eng. J. 2021, 25, 67–74. [Google Scholar] [CrossRef]
- Nguyen, H.N.; Gaspillo, P.D.; Maridable, J.B.; Malaluan, R.M.; Hinode, H.; Salim, C.; Huynh, H.K.P. Extraction of oil from Moringa oleifera kernels using supercritical carbon dioxide with ethanol for pretreatment: Optimization of the extraction process. Chem. Eng. Process. 2011, 50, 1207–1213. [Google Scholar] [CrossRef]
- Rai, A.; Mohanty, B.; Bhargava, R. Experimental modeling and simulation of supercritical fluid extraction of Moringa oleifera seed oil by carbon dioxide. Chem. Eng. Commun. 2017, 204, 957–964. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, D. An experimental investigation into the solubility of Moringa oleifera oil in supercritical carbon dioxide. J. Food Eng. 2014, 138, 1–10. [Google Scholar] [CrossRef]
- Ruttarattanamongkol, K.; Petrasch, A. Oxidative susceptibility and thermal properties of Moringa Oliefera seed oil obtained by pilot-scale subcritical and supercritical carbon dioxide extraction. J. Food Process. Eng. 2016, 39, 226–236. [Google Scholar] [CrossRef]
- Dinesha, B.L.; Udaykumar, N.; Ramachandra, C.T.; Naik, N.; Sankalpa, K.B. Effect of supercritical carbon dioxide conditions on extraction of food phytochemical constituents from Moringa oleifera. Lam seed kernels. Int. J. Food Ferment. Technol. 2016, 6, 8. [Google Scholar] [CrossRef]
- Dinesha, B.L.; Udaykumar, N.; Ramachandra, C.T.; Naik, N.; Hugar, A. Optimization of supercritical extraction process for Moringa (PKM-1) seed kernel oil. Int. J. Agric. Sci. 2015, 5, 95–102. [Google Scholar]
- Rodríguez-Pérez, C.; Mendiola, J.A.; Quirantes-Piné, R.; Ibáñez, E.; Segura-Carretero, A. Green downstream processing using supercritical carbon dioxide, CO2-expanded ethanol and pressurized hot water extractions for recovering bioactive compounds from Moringa oleifera leaves. J. Supercrit. Fluids 2016, 116, 90–100. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, D. Supercritical fluid extraction and characterisation of Moringa oleifera leaves oil. SePurif. Technol. 2013, 118, 497–502. [Google Scholar] [CrossRef]
- Manrique, Y.J.A. Supercritical Fluid Extraction and Fractionation of Bioactive Natural Products from Cork. Ph.D. Dissertation, Chemical Engineering, Faculty of Engineering of the University of Porto, Porto, Portugal, 2017. [Google Scholar]
- NIST—Isothermal Properties for Carbon Dioxide. Available online: https://webbook.nist.gov/ (accessed on 26 June 2022).
- Kessler, J.C.; Vieira, V.; Martins, I.M.; Manrique, Y.A.; Ferreira, P.C.; Calhelha, R.C.; Afonso, A.; Barros, L.; Rodrigues, A.E.; Dias, M.M. Chemical and organoleptic properties of bread enriched with Rosmarinus officinalis L.: The potential of natural extracts obtained through green extraction methodologies as food ingredients. Food Chem. 2022, 384, 132514. [Google Scholar] [CrossRef]
- Meireles, D.; Gomes, J.; Lopes, L.; Hinzmann, M.; Machado, J. A review of properties, nutritional and pharmaceutical applications of Moringa oleifera: Integrative approach on conventional and traditional Asian medicine. Adv. Trad. Med. 2020, 20, 495–515. [Google Scholar] [CrossRef]
- Zeitoun, H.; Michael-Jubeli, R.; El Khoury, R.; Baillet-Guffroy, A.; Tfayli, A.; Salameh, D.; Lteif, R. Skin lightening effect of natural extracts coming from Senegal botanical biodiversity. Int. J. Dermat. 2020, 59, 178–183. [Google Scholar] [CrossRef]
- Chao, C.; Génot, C.; Rodriguez, C.; Magniez, H.; Lacourt, S.; Fievez, A.; Len, C.; Pezron, I.; Luart, D.; van Hecke, E. Emollients for cosmetic formulations: Towards relationships between physico-chemical properties and sensory perceptions. Colloids Surf. A Physicochem. Eng. Asp. 2018, 536, 156–164. [Google Scholar] [CrossRef]
- Puah, C.W.; Choo, Y.M.; Ma, A.N.; Chuah, C.H. Solubility of tocopherol and tocotrienols from palm oil in supercritical carbon dioxide. J. Food Lipids 2007, 14, 377–385. [Google Scholar] [CrossRef]
- Agboke, A.; Attama, A. Bioactive components and antimicrobial activities of n-hexane extract of Moringa oleifera root bark on clinical isolates methicilin resistant Staphylococcus aureus. IJCRCPS 2016, 3, 1–9. [Google Scholar]
- Faizi, S.; Sumbul, S.; Versiani, M.A.; Saleem, R.; Sana, A.; Siddiqui, H. GC/GCMS analysis of the petroleum ether and dichloromethane extracts of Moringa oleifera roots. Asian Pac. J. TroBiomed. 2014, 4, 650–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garmus, T.T.; de Oliveira, G.N.A.; Rammazzina Filho, W.A.; Queiroga, C.L.; Cabral, F.A. Solubility of oleic acid, triacylglycerol and their mixtures in supercritical carbon dioxide and thermodynamic modeling of phase equilibrium. J. Supercrit. Fluids 2019, 143, 275–285. [Google Scholar] [CrossRef]



| Nblock | P (bar) | T (°C) | V [38] (μPa∙s−1) | ρ [38] (kg∙m−3) | Seed | Leaf | Root | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ηext (%) | ηRSM (%) | Error (%) | Solubility (μgext∙gCO2−1) | ηext (%) | ηRSM (%) | Error (%) | Solubility (μgext∙gCO2−1) | ηext (%) | ηRSM (%) | Error (%) | Solubility (μgext∙gCO2−1) | |||||
| E1 1 | 140 (−1) | 45 (−1) | 59 | 720 | 0.18 | 0.71 | 3.02 | 41 | 0.79 | 0.75 | 0.04 | 183 | 0.06 | 0.07 | 0.08 | 15 |
| E2 1 | 140 (−1) | 65 (+1) | 37 | 506 | 0.12 | 0.59 | 3.75 | 29 | 0.39 | 0.35 | 0.11 | 90 | 0.04 | 0.06 | 0.42 | 10 |
| E3 1 | 250 (+1) | 45 (−1) | 83 | 857 | 3.19 | 3.63 | 0.14 | 742 | 1.29 | 1.38 | 0.07 | 300 | 0.16 | 0.18 | 0.12 | 39 |
| E4 1 | 250 (+1) | 65 (+1) | 66 | 762 | 2.10 | 2.48 | 0.18 | 491 | 1.66 | 1.75 | 0.05 | 387 | 0.11 | 0.14 | 0.31 | 25 |
| E5 (CP1) 1 | 195 (0) | 55 (0) | 64 | 747 | 1.94 | 2.15 | 0.11 | 452 | 1.34 | 1.31 | 0.02 | 312 | 0.15 | 0.16 | 0.02 | 35 |
| E6 2 | 117 (−1.41) | 55 (0) | 34 | 480 | 0.10 | 0.12 | 0.18 | 24 | 0.22 | 0.30 | 0.27 | 51 | 0.02 | 0.03 | 0.82 | 04 |
| E7 2 | 273 (+1.41) | 55 (0) | 78 | 830 | 3.38 | 3.53 | 0.04 | 786 | 1.83 | 1.74 | 0.05 | 427 | 0.18 | 0.17 | 0.05 | 41 |
| E8 2 | 195 (0) | 41 (−1.41) | 77 | 830 | 2.29 | 2.33 | 0.02 | 452 | 1.12 | 1.11 | 0.01 | 261 | 0.14 | 0.15 | 0.09 | 32 |
| E9 2 | 195 (0) | 69 (+1.41) | 52 | 656 | 1.31 | 1.44 | 0.10 | 308 | 1.08 | 1.08 | 0.00 | 252 | 0.12 | 0.11 | 0.06 | 28 |
| E10 (CP2) 2 | 195 (0) | 55 (0) | 64 | 747 | 1.81 | 2.15 | 0.18 | 422 | 1.27 | 1.31 | 0.04 | 295 | 0.14 | 0.16 | 0.13 | 32 |
| Mo Plant Parts | Seed | Leaf | Root | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Lack of fit/MS residual | 0.0416062 | 0.0116891 | 0.0004018 | ||||||
| R2 | 0.9905 | 0.9851 | 0.9540 | ||||||
| Regression term | f | F-value | p | f | F-value | p | f | F-value | p |
| Blocks | 1 | 4.3426 | 0.128524 | 1 | 0.0245 | 0.885652 | 1 | 0.71056 | 0.461159 |
| () Pressure (bar) (L) | 1 | 278.3292 | 0.000469 * | 1 | 175.9659 | 0.000926 * | 1 | 48.1694 | 0.006134 * |
| () Pressure (bar) (Q) | 1 | 2.8843 | 0.188009 | 1 | 8.3088 | 0.063400 | 1 | 9.24785 | 0.055822 |
| () Temperature (°C) (L) | 1 | 18.9888 | 0.022334 * | 1 | 0.0657 | 0.814290 | 1 | 3.25902 | 0.168786 |
| ) Temperature (°C) (Q) | 1 | 1.9398 | 0.257957 | 1 | 4.5531 | 0.122569 | 1 | 1.97041 | 0.255011 |
| (L) | 1 | 6.4467 | 0.084751 | 1 | 12.7589 | 0.037500 * | 1 | 0.80103 | 0.436735 |
| Chemical Group | Compound (n°) | CAS [38] | Base Peak | LRI * | RT (min) | Relative Concentration (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| E3 | ± | E5 (CP) | ± | E7 | ± | ||||||
| Aldehydes | |||||||||||
| 7-Tetradecenal (4) | 65128-96-3 | 55, 41, 67 | 1899 | 10.978 | 0.25 | 0.01 | 0.34 | 0.01 | 0.39 | 0.01 | |
| Fatty acids | |||||||||||
| Palmitic acid (5) | 57-10-3 | 43, 73, 60 | 1963 | 11.644 | 1.75 | 0.01 | 2.11 | 0.05 | 1.54 | 0.06 | |
| Methyl oleate (7) | 112-62-9 | 55, 69, 74 | 2098 | 13.025 | 0.294 | 0.001 | 0.32 | 0.01 | 0.29 | 0.01 | |
| Oleic acid (8) | 112-80-1 | 41, 55, 43 | 2146 | 13.495 | 82.04 | 2.08 | 82.02 | 4.74 | 82.02 | 4.08 | |
| Stearic acid (9) | 57-11-4 | 43, 73, 60 | 2164 | 13.677 | 2.42 | 0.01 | 2.43 | 0.08 | 2.33 | 0.12 | |
| Oleic acid chloride (11) | 112-77-6 | 55, 98, 41 | 2421 | 16.055 | 7.33 | 0.08 | 6.30 | 0.06 | 7.13 | 0.11 | |
| Hydrocarbons | |||||||||||
| Hexadecane (1) | 544-76-3 | 57, 43, 71 | 1600 | 8.784 | 0.153 | 0.001 | 0.23 | 0.01 | 0.31 | 0.01 | |
| Heptadecane (2) | 629-78-7 | 57, 43, 71 | 1800 | 10.093 | 0.247 | 0.03 | 0.48 | 0.01 | 0.629 | 0.002 | |
| 8-Hexylpentadecane (6) | 13475-75-7 | 57, 71, 43 | 2000 | 12.025 | 0.48 | 0.01 | 0.72 | 0.02 | 0.808 | 0.001 | |
| Tetracosane (10) | 646-31-1 | 57, 43, 71 | 2397 | 15.842 | 0.68 | 0.02 | 0.87 | 0.04 | 1.04 | 0.02 | |
| Tetratriacontane (15) | 14167-59-0 | 57, 71, 43 | 2897 | 22.288 | 1.11 | 0.04 | 1.53 | 0.01 | 1.12 | 0.03 | |
| Sterols | |||||||||||
| Stigmasta-5,22-dien-3 β-ol, acetate (14) | 4651-48-3 | 43, 55, 81 | 2893 | 22.223 | 0.21 | 0.02 | tr | tr | |||
| Stigmast-5-en-3-ol, oleate (17) | - | 396, 147, 382 | 3076 | 24.188 | 0.61 | 0.01 | 0.470 | 0.004 | 0.54 | 0.02 | |
| Terpenes | |||||||||||
| Phytol (3) | 150-86-7 | 81, 82, 43 | 1834 | 10.396 | 0.23 | 0.01 | 0.275 | 0.001 | 0.25 | 0.02 | |
| Unknown | |||||||||||
| Unknown (12) | 2769 | 20.403 | 1.08 | 0.01 | 1.44 | 0.04 | 1.14 | 0.03 | |||
| Unknown (13) | 2853 | 21.674 | 0.23 | 0.02 | 0.00 | 0.00 | |||||
| Unknown (16) | 2947 | 22.796 | 0.899 | 0.003 | 0.46 | 0.02 | 0.47 | 0.03 | |||
| Identified | 98.92 | 98.10 | 98.39 | ||||||||
| Chemical Group | Compound (n°) | CAS [38] | Base Peak | LRI * | RT (min) | Relative Concentration (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| E4 | ± | E5 (CP) | ± | E7 | ± | ||||||
| Aldehydes | |||||||||||
| 2,4-Nonadienal (1) | 5910-87-2 | 81, 138, 41 | 1004 | 7.101 | 0.146 | 0.001 | 0.13 | 0.01 | 0.081 | 0.002 | |
| Octanal (2) | 124-13-0 | 43, 44, 56 | 1016 | 7.344 | 0.92 | 0.02 | 0.74 | 0.06 | 0.67 | 0.03 | |
| Nonanal (6) | 124-19-6 | 57, 41, 43 | 1107 | 9.285 | 1.56 | 0.06 | 1.29 | 0.22 | 1.076 | 0.005 | |
| cis,cis, cis-7,10,13-Hexadecatrienal (15) | 56797-43-4 | 79, 67, 41 | 2426 | 29.837 | 0.72 | 0.03 | 0.71 | 0.04 | 0.83 | 0.02 | |
| Pentadecanal (20) | 2765-11-9 | 82, 57, 43 | 2838 | 33.436 | 0.75 | 0.01 | 0.50 | 0.02 | 0.81 | 0.04 | |
| Fatty acids | |||||||||||
| Palmitic acid (11) | 57-10-3 | 88, 101, 43 | 1992 | 24.560 | 4.50 | 0.06 | 5.28 | 0.26 | 5.23 | 0.15 | |
| Linoleic acid (12) | 60-33-3 | 67, 81, 95 | 2161 | 27.246 | 3.29 | 0.01 | 3.28 | 0.05 | 3.469 | 0.003 | |
| Linolenic acid (13) | 463-40-1 | 79, 95, 67 | 2167 | 27.324 | 19.40 | 0.01 | 18.85 | 0.05 | 20.17 | 0.15 | |
| Ethyl pentadecanoate (14) | 41114-00-5 | 88, 101, 43 | 2194 | 27.669 | 1.382 | 0.004 | 2.25 | 0.18 | 2.13 | 0.01 | |
| Arachidic acid (18) | 506-32-1 | 88, 43,101 | 2795 | 33.108 | 0.94 | 0.03 | 0.65 | 0.03 | 0.83 | 0.06 | |
| Hydrocarbons | |||||||||||
| Dodecane (7) | 112-40-3 | 57, 43, 71 | 1196 | 11.172 | 1.26 | 0.03 | 1.15 | 0.09 | 0.93 | 0.02 | |
| Tetradecane (8) | 629-59-4 | 57, 43, 71 | 1395 | 15.100 | 1.39 | 0.01 | 1.46 | 0.11 | 1.31 | 0.03 | |
| 8-Hexylpentadecane (16) | 13475-75-7 | 57, 71, 43 | 2500 | 30.525 | 3.74 | 0.03 | 3.55 | 0.14 | 3.75 | 0.12 | |
| Heptacosane (17) | 593-49-7 | 57, 71, 43 | 2701 | 32.436 | 11.73 | 0.04 | 10.04 | 0.19 | 11.23 | 0.38 | |
| Squalane (19) | 111-01-3 | 57, 71, 85 | 2800 | 33.141 | 0.47 | 0.02 | 0.518 | 0.003 | 0.37 | 0.02 | |
| Nonacosane (21) | 630-03-5 | 57, 71, 43 | 2901 | 33.926 | 22.89 | 0.01 | 17.74 | 0.74 | 19.14 | 0.61 | |
| Terpenes | |||||||||||
| α-pinene (3) | 80-56-8 | 93, 91, 92 | 1020 | 7.445 | 0.17 | 0.01 | 0.136 | 0.003 | 0.105 | 0.004 | |
| p-cymene (4) | 99-87-6 | 119, 134, 91 | 1034 | 7.736 | 0.107 | 0.001 | tr | tr | |||
| D-limonene (5) | 5989-27-5 | 68, 93, 67 | 1038 | 7.824 | 0.891 | 0.005 | 0.74 | 0.08 | 0.822 | 0.002 | |
| cis-Phytol (9) | 5492-30-8 | 81, 82, 43 | 1835 | 22.290 | 1.40 | 0.03 | 1.82 | 0.24 | 1.81 | 0.02 | |
| β-amyrone (26) | 638-97-1 | 218, 203, 55 | 3352 | 38.765 | 1.30 | 0.02 | 1.87 | 0.01 | 1.46 | 0.04 | |
| Tocopherols | |||||||||||
| γ-tocopherol (23) | 7616-22-0 | 151, 416, 191 | 3051 | 35.289 | 0.35 | 0.01 | 0.36 | 0.01 | 0.27 | 0.01 | |
| Vitamin E (α-Tocopherol) (24) | 10191-41-0 | 165, 430, 164 | 3129 | 36.081 | 16.41 | 0.17 | 21.51 | 0.79 | 17.49 | 0.37 | |
| Unknown | |||||||||||
| Unknown (10) | 1878 | 22.916 | 0.700 | 0.005 | 0.74 | 0.05 | 0.718 | 0.002 | |||
| Unknown (22) | 3042 | 35.202 | 1.17 | 0.03 | 1.17 | 0.08 | 1.95 | 0.26 | |||
| Unknown (25) | 3245 | 37.409 | 2.42 | 0.05 | 3.52 | 0.07 | 3.35 | 0.01 | |||
| Identified | 95.71 | 94.57 | 93.98 | ||||||||
| Chemical Groups | Compound | CAS [38] | Base Peak | LRI * | RT (min) | Relative Concentration (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| E3 | ± | E5 (CP) | ± | E7 | ± | ||||||
| Fatty acids | |||||||||||
| Ethyl palmitate (1) | 628-97-7 | 88, 101, 43 | 1961 | 11.625 | 0.00 | 3.81 | 0.35 | 8.08 | 0.51 | ||
| Palmitic acid (2) | 57-10-3 | 74, 87, 43 | 1991 | 11.936 | 7.74 | 3.33 | 3.24 | 0.02 | 0.98 | 0.11 | |
| Linolelaidic acid (3) | 2566-97-4 | 67, 41, 81 | 2132 | 13.357 | 0.00 | 3.17 | 0.03 | 8.70 | 0.96 | ||
| Oleic acid (4) | 112-80-1 | 55, 41, 69 | 2137 | 13.408 | 0.00 | 6.57 | 0.34 | 12.30 | 0.25 | ||
| Linoleic acid (5) | 60-33-3 | 67, 81, 95 | 2162 | 13.655 | 2.82 | 0.36 | 1.69 | 0.03 | 0.91 | 0.02 | |
| Stearic acid (6) | 57-11-4 | 43, 73, 60 | 2165 | 13.687 | 4.78 | 0.31 | 3.50 | 0.17 | 3.62 | 0.35 | |
| Ethyl pentadecanoate (8) | 41114-00-5 | 88, 101, 43 | 2191 | 13.944 | 10.36 | 3.09 | 6.30 | 0.39 | 1.53 | 0.24 | |
| Hydrocarbons | |||||||||||
| Hexatriacontane (11) | 630-06-8 | 57, 71, 43 | 2899 | 22.307 | tr | 0.37 | 0.02 | 0.3220 | 0.0001 | ||
| Terpenes | |||||||||||
| 24-Methylenecycloartanol (21) | 1449-09-8 | 55, 95, 41 | 3430 | 29.004 | 2.03 | 0.07 | 2.42 | 0.02 | 2.06 | 0.05 | |
| Sterols | |||||||||||
| 22,23-Dibromostigmasterol acetate (9) | 50633-49-3 | 43, 81, 55 | 2866 | 21.854 | 2.07 | 0.45 | 0.65 | 0.05 | 0.53 | 0.07 | |
| Cholesta-6,22,24-triene, 4,4-dimethyl (10) | - | 55, 43, 83 | 2890 | 22.191 | 1.29 | 0.49 | 0.385 | 0.002 | 0.26 | 0.04 | |
| Cholesteryl bromide (12) | 516-91-6 | 81, 147, 105 | 2921 | 22.776 | 4.43 | 1.31 | 1.59 | 0.01 | 1.14 | 0.13 | |
| 3-Bromocholest-5-ene (13) | 137036-75-0 | 81, 147, 105 | 2942 | 23.217 | 1.86 | 0.20 | 0.83 | 0.03 | 0.48 | 0.08 | |
| Stigmasta-5,22-dien-3β-ol, acetate (14) | 4651-48-3 | 43, 55, 81 | 2956 | 23.516 | 1.33 | 0.11 | 0.540 | 0.002 | 0.28 | 0.03 | |
| Stigmast-5-en-3-ol, oleate (16) | - | 396, 147, 382 | 2987 | 24.195 | 4.49 | 0.73 | 1.99 | 0.03 | 1.16 | 0.09 | |
| Campesterol (18) | 474-62-4 | 43, 41, 55 | 3212 | 25.863 | 17.53 | 0.13 | 19.61 | 0.41 | 17.10 | 0.28 | |
| Stigmasterol (19) | 83-48-7 | 55, 83, 81 | 3238 | 26.216 | 11.24 | 0.17 | 14.08 | 0.25 | 12.52 | 0.23 | |
| γ-Sitosterol (20) | 83-47-6 | 43, 55, 57 | 3300 | 27.049 | 24.63 | 1.48 | 27.68 | 0.56 | 27.08 | 0.39 | |
| Unknown | |||||||||||
| Unknown (7) | 2171 | 13.747 | 0.72 | 0.02 | 0.36 | 0.02 | 0.16 | 0.04 | |||
| Unknown (15) | 2969 | 23.796 | 1.74 | 0.15 | 0.65 | 0.02 | 0.40 | 0.06 | |||
| Unknown (17) | 3153 | 25.122 | 0.95 | 0.04 | 0.58 | 0.01 | 0.40 | 0.01 | |||
| Identified | 96.60 | 98.41 | 99.05 | ||||||||
| Plant Part | Compound | Mass (mgcompound∙gplant part−1) | |||
|---|---|---|---|---|---|
| E3 | E4 | E5 (CP) | E7 | ||
| Seed | Oleic acid | 7.28 a | - | 4.77 b | 8.04 a |
| Leaf | Linolenic acid | - | 2.73 b | 2.24 c | 3.10 a |
| Nonacosane | 0.45 a | 0.33 b | 0.46 a | ||
| α-tocopherol | 0.17 b | 0.20 ab | 0.21 a | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kessler, J.C.; Manrique, Y.A.; Martins, I.M.; Rodrigues, A.E.; Barreiro, M.F.; Dias, M.M. Moringa oleifera L. Screening: SFE-CO2 Optimisation and Chemical Composition of Seed, Leaf, and Root Extracts as Potential Cosmetic Ingredients. Separations 2023, 10, 210. https://doi.org/10.3390/separations10030210
Kessler JC, Manrique YA, Martins IM, Rodrigues AE, Barreiro MF, Dias MM. Moringa oleifera L. Screening: SFE-CO2 Optimisation and Chemical Composition of Seed, Leaf, and Root Extracts as Potential Cosmetic Ingredients. Separations. 2023; 10(3):210. https://doi.org/10.3390/separations10030210
Chicago/Turabian StyleKessler, Júlia C., Yaidelin A. Manrique, Isabel M. Martins, Alírio E. Rodrigues, Maria Filomena Barreiro, and Madalena M. Dias. 2023. "Moringa oleifera L. Screening: SFE-CO2 Optimisation and Chemical Composition of Seed, Leaf, and Root Extracts as Potential Cosmetic Ingredients" Separations 10, no. 3: 210. https://doi.org/10.3390/separations10030210