Digital Twins in Pharmaceutical and Biopharmaceutical Manufacturing: A Literature Review
Abstract
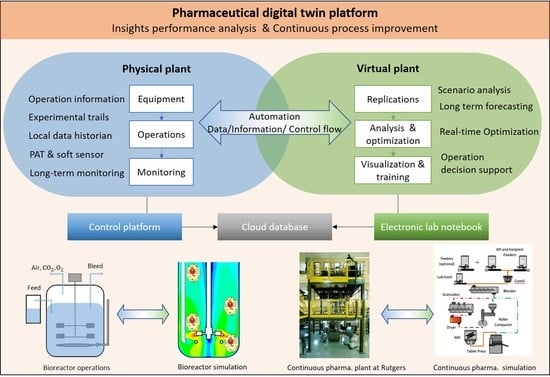
:1. Introduction
2. Digital Twin Framework
2.1. Physical Component
2.2. Virtual Component
2.3. Data Management
2.4. Applications of Digital Twin
2.5. Challenges
3. Digital Twin in Pharmaceutical Manufacturing
3.1. PAT Methods
3.2. Process Modeling
3.3. Data Integration
3.4. Challenges and Opportunities
4. Digital Twin in Biopharmaceutical Manufacturing
4.1. PAT Methods
4.2. Process Modeling
4.3. Data Integration
4.4. Challenges and Opportunities
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Legner, C.; Eymann, T.; Hess, T.; Matt, C.; Böhmann, T.; Drews, P.; Mädche, A.; Urbach, N.; Ahlemann, F. Digitalization: Opportunity and Challenge for the Business and Information Systems Engineering Community. Bus. Inf. Syst. Eng. 2017, 59, 301–308. [Google Scholar] [CrossRef]
- Kritzinger, W.; Karner, M.; Traar, G.; Henjes, J.; Sihn, W. Digital Twin in manufacturing: A categorical literature review and classification. IFAC-PapersOnLine 2018, 51, 1016–1022. [Google Scholar] [CrossRef]
- Oztemel, E.; Gursev, S. Literature review of Industry 4.0 and related technologies. J. Intell. Manuf. 2018, 31, 127–182. [Google Scholar] [CrossRef]
- Tao, F.; Cheng, J.; Qi, Q.; Zhang, M.; Zhang, H.; Sui, F. Digital twin-driven product design, manufacturing and service with big data. Int. J. Adv. Manuf. Technol. 2018, 94, 3563–3576. [Google Scholar] [CrossRef]
- Venkatasubramanian, V. The promise of artificial intelligence in chemical engineering: Is it here, finally? AIChE J. 2019, 65, 466–478. [Google Scholar] [CrossRef]
- Bao, J.; Guo, D.; Li, J.; Zhang, J. The modelling and operations for the digital twin in the context of manufacturing. Enterp. Inf. Syst. 2018, 13, 534–556. [Google Scholar] [CrossRef]
- Tao, F.; Qi, Q.; Wang, L.; Nee, A.Y.C. Digital Twins and Cyber–Physical Systems toward Smart Manufacturing and Industry 4.0: Correlation and Comparison. Engineering 2019, 5, 653–661. [Google Scholar] [CrossRef]
- Haag, S.; Anderl, R. Digital twin—Proof of concept. Manuf. Lett. 2018, 15, 64–66. [Google Scholar] [CrossRef]
- Litster, J.; Bogle, I.D.L. Smart Process. Manufacturing for Formulated Products. Engineering 2019, 5, 1003–1009. [Google Scholar] [CrossRef]
- Tourlomousis, F.; Chang, R.C. Dimensional Metrology of Cell-matrix Interactions in 3D Microscale Fibrous Substrates. Procedia CIRP 2017, 65, 32–37. [Google Scholar] [CrossRef]
- Khan, M.; Wu, X.; Xu, X.; Dou, W. Big data challenges and opportunities in the hype of Industry 4.0. In Proceedings of the 2017 IEEE International Conference on Communications (ICC), Paris, France, 21–25 May 2017. [Google Scholar]
- Li, X.; Li, D.; Wan, J.; Vasilakos, A.V.; Lai, C.-F.; Wang, S. A review of industrial wireless networks in the context of Industry 4.0. Wirel. Netw. 2015, 23, 23–41. [Google Scholar] [CrossRef]
- Uhlemann, T.H.J.; Schock, C.; Lehmann, C.; Freiberger, S.; Steinhilper, R. The Digital Twin: Demonstrating the Potential of Real Time Data Acquisition in Production Systems. Procedia Manuf. 2017, 9, 113–120. [Google Scholar] [CrossRef]
- Belanger, J.; Venne, P.; Paquin, J.-N. The What, Where and Why of Real-Time Simulation. Planet RT. 2010, 1, 37–49. [Google Scholar]
- Roman-Ospino, A.D.; Singh, R.; Ierapetritou, M.; Ramachandran, R.; Mendez, R.; Ortega-Zuniga, C.; Muzzio, F.J.; Romanach, R.J. Near infrared spectroscopic calibration models for real time monitoring of powder density. Int. J. Pharm. 2016, 512, 61–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damiani, L.; Demartini, M.; Guizzi, G.; Revetria, R.; Tonelli, F. Augmented and virtual reality applications in industrial systems: A qualitative review towards the industry 4.0 era. IFAC-PapersOnLine 2018, 51, 624–630. [Google Scholar] [CrossRef]
- Zühlke, D.; Gorecky, D.; Schmitt, M.; Loskyll, M. Human-machine-interaction in the industry 4.0 era. In Proceedings of the 2014 12th IEEE International Conference on Industrial Informatics (INDIN), Porto Alegre, Brazil, 27–30 July 2014. [Google Scholar]
- Zhuang, C.; Liu, J.; Xiong, H. Digital twin-based smart production management and control framework for the complex product assembly shop-floor. Int. J. Adv. Manuf. Technol. 2018, 96, 1149–1163. [Google Scholar] [CrossRef]
- Leng, J.; Zhang, H.; Yan, D.; Liu, Q.; Chen, X.; Zhang, D. Digital twin-driven manufacturing cyber-physical system for parallel controlling of smart workshop. J. Ambient Intell. Humaniz. Comput. 2018, 10, 1155–1166. [Google Scholar] [CrossRef]
- Rosen, R.; von Wichert, G.; Lo, G.; Bettenhausen, K.D. About The Importance of Autonomy and Digital Twins for the Future of Manufacturing. IFAC-PapersOnLine 2015, 48, 567–572. [Google Scholar] [CrossRef]
- Mayani, M.G.; Svendsen, M.; Oedegaard, S.I. Drilling Digital Twin Success Stories the Last 10 Years. In Proceedings of the SPE Norway One Day Seminar, Bergen, Norway, 18 April 2018. [Google Scholar]
- Schleich, B.; Anwer, N.; Mathieu, L.; Wartzack, S. Shaping the digital twin for design and production engineering. CIRP Ann. 2017, 66, 141–144. [Google Scholar] [CrossRef] [Green Version]
- Grieves, M. Digital Twin: Manufacturing Excellence through Virtual Factory Replication. White Paper 2014, 1, 1–7. [Google Scholar]
- Grieves, M.; Vickers, J. Digital Twin: Mitigating Unpredictable, Undesirable Emergent Behavior in Complex Systems. In Transdisciplinary Perspectives on Complex Systems; Springer: Cham, Switzerland, 2017; pp. 85–113. [Google Scholar]
- Stark, R.; Fresemann, C.; Lindow, K. Development and operation of Digital Twins for technical systems and services. CIRP Ann. 2019, 68, 129–132. [Google Scholar] [CrossRef]
- Glaessgen, E.H.; Stargel, D.S. The Digital Twin Paradigm for Future NASA and U.S. Air Force Vehicles. In Proceedings of the 53rd AIAA/ASME/ASCE/AHS/ASC Structures, Structural Dynamics and Materials Conference—Special Session on the Digital Twin, Honolulu, HI, USA, 23–26 April 2012. [Google Scholar]
- O’Connor, T.F.; Yu, L.X.; Lee, S.L. Emerging technology: A key enabler for modernizing pharmaceutical manufacturing and advancing product quality. Int. J. Pharm. 2016, 509, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Ding, B. Pharma Industry 4.0: Literature review and research opportunities in sustainable pharmaceutical supply chains. Process Saf. Environ. Prot. 2018, 119, 115–130. [Google Scholar] [CrossRef]
- Barenji, R.V.; Akdag, Y.; Yet, B.; Oner, L. Cyber-physical-based PAT (CPbPAT) framework for Pharma 4.0. Int. J. Pharm. 2019, 567, 118445. [Google Scholar] [CrossRef] [PubMed]
- Steinwandter, V.; Borchert, D.; Herwig, C. Data science tools and applications on the way to Pharma 4.0. Drug Discov. Today 2019, 24, 1795–1805. [Google Scholar] [CrossRef]
- Lopes, M.R.; Costigliola, A.; Pinto, R.; Vieira, S.; Sousa, J.M.C. Pharmaceutical quality control laboratory digital twin—A novel governance model for resource planning and scheduling. Int. J. Prod. Res. 2019, 1–15. [Google Scholar] [CrossRef]
- Kumar, S.; Talasila, D.; Gowrav, M.; Gangadharappa, H. Adaptations of Pharma 4.0 from Industry 4.0. Drug Invent. Today 2020, 14, 405–415. [Google Scholar]
- Reinhardt, I.C.; Oliveira, D.J.C.; Ring, D.D.T. Current Perspectives on the Development of Industry 4.0 in the Pharmaceutical Sector. J. Ind. Inf. Integr. 2020, 18, 100131. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, W.; Liu, J.; Liu, Z.; Zhou, Z.; Pham, D.T. A Reconfigurable Modeling Approach for Digital Twin-based Manufacturing System. Procedia CIRP 2019, 83, 118–125. [Google Scholar] [CrossRef]
- Kabugo, J.C.; Jämsä-Jounela, S.-L.; Schiemann, R.; Binder, C. Industry 4.0 based process data analytics platform: A waste-to-energy plant case study. Int. J. Electr. Power Energy Syst. 2020, 115, 105508. [Google Scholar] [CrossRef]
- González, I.; Calderón, A.J.; Figueiredo, J.; Sousa, J.M.C. A Literature Survey on Open Platform Communications (OPC) Applied to Advanced Industrial Environments. Electronics 2019, 8, 510. [Google Scholar] [CrossRef] [Green Version]
- O’Donovan, P.; Leahy, K.; Bruton, K.; O’Sullivan, D.T.J. An industrial big data pipeline for data-driven analytics maintenance applications in large-scale smart manufacturing facilities. J. Big Data 2015, 2, 1–26. [Google Scholar]
- Mandenius, C.-F.; Gustavsson, R. Mini-review: Soft sensors as means for PAT in the manufacture of bio-therapeutics. J. Chem. Technol. Biotechnol. 2015, 90, 215–227. [Google Scholar] [CrossRef]
- Bosca, S.; Barresi, A.; Fissore, D. Use of a soft sensor for the fast estimation of dried cake resistance during a freeze-drying cycle. Int. J. Pharm. 2013, 451, 23–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, J.-G.; Qu, L.-L.; Hu, X.-L.; Liu, X.-H. Application of Temperature Inference Method Based on Soft Sensor Technique to Plate Production Process. J. Iron Steel Res. Int. 2011, 18, 24–27. [Google Scholar] [CrossRef]
- Rogina, A.; Šiško, I.; Mohler, I.; Ujević, Ž.; Bolf, N. Soft sensor for continuous product quality estimation (in crude distillation unit). Chem. Eng. Res. Des. 2011, 89, 2070–2077. [Google Scholar] [CrossRef]
- Kadlec, P.; Gabrys, B.; Strandt, S. Data-driven Soft Sensors in the process industry. Comput. Chem. Eng. 2009, 33, 795–814. [Google Scholar] [CrossRef] [Green Version]
- Khalfe, N.; Lahiri, S.; Sawke, S. Soft sensor for better control of carbon dioxide removal process in ethylene glycol plant. Chem. Ind. Chem. Eng. Q. 2011, 17, 17–24. [Google Scholar] [CrossRef]
- Teixeira, B.O.; Castro, W.S.; Teixeira, A.F.; Aguirre, L.A. Data-driven soft sensor of downhole pressure for a gas-lift oil well. Control Eng. Pract. 2014, 22, 34–43. [Google Scholar] [CrossRef]
- Qin, S.J.; Yue, H.; Dunia, R. Self-validating inferential sensors with application to air emission monitoring. Ind. Eng. Chem. Res. 1997, 36, 1675–1685. [Google Scholar] [CrossRef]
- Cao, H.; Mushnoori, S.; Higgins, B.; Kollipara, C.; Fermier, A.; Hausner, D.; Jha, S.; Singh, R.; Ierapetritou, M.; Ramachandran, R. A Systematic Framework for Data Management and Integration in a Continuous Pharmaceutical Manufacturing Processing Line. Processes 2018, 6, 53. [Google Scholar] [CrossRef] [Green Version]
- Muñoz, E.; Capón-García, E.; Espuña, A.; Puigjaner, L. Ontological framework for enterprise-wide integrated decision-making at operational level. Comput. Chem. Eng. 2012, 42, 217–234. [Google Scholar] [CrossRef]
- Židek, K.; Piteľ, J.; Adámek, M.; Lazorík, P.; Hošovský, A. Digital Twin of Experimental Smart Manufacturing Assembly System for Industry 4.0 Concept. Sustainability 2020, 12, 3658. [Google Scholar] [CrossRef]
- Roblek, V.; Meško, M.; Krapež, A. A complex view of industry 4.0. Sage Open 2016, 6, 2158244016653987. [Google Scholar] [CrossRef] [Green Version]
- Sanders, A.; Elangeswaran, C.; Wulfsberg, J. Industry 4.0 implies lean manufacturing: Research activities in industry 4.0 function as enablers for lean manufacturing. J. Ind. Eng. Manag. (JIEM) 2016, 9, 811–833. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Wan, J.; Zhang, D.; Li, D.; Zhang, C. Towards smart factory for industry 4.0: A self-organized multi-agent system with big data based feedback and coordination. Comput. Netw. 2016, 101, 158–168. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Escotet-Espinoza, M.S.; Ierapetritou, M. Process analysis and optimization of continuous pharmaceutical manufacturing using flowsheet models. Comput. Chem. Eng. 2017, 107, 77–91. [Google Scholar] [CrossRef]
- Grossmann, I.E.; Calfa, B.A.; Garcia-Herreros, P. Evolution of concepts and models for quantifying resiliency and flexibility of chemical processes. Comput. Chem. Eng. 2014, 70, 22–34. [Google Scholar] [CrossRef]
- Eugene, E.A.; Gao, X.; Dowling, A.W. Learning and Optimization with Bayesian Hybrid Models. arXiv 2019, arXiv:1912.06269. Available online: https://arxiv.org/abs/1912.06269 (accessed on 22 June 2020).
- Von Stosch, M.; Oliveira, R.; Peres, J.; Feyo de Azevedo, S. Hybrid semi-parametric modeling in process systems engineering: Past, present and future. Comput. Chem. Eng. 2014, 60, 86–101. [Google Scholar] [CrossRef] [Green Version]
- Bhosekar, A.; Ierapetritou, M. Advances in surrogate based modeling, feasibility analysis, and optimization: A review. Comput. Chem. Eng. 2018, 108, 250–267. [Google Scholar] [CrossRef]
- Zendehboudi, S.; Rezaei, N.; Lohi, A. Applications of hybrid models in chemical, petroleum, and energy systems: A systematic review. Appl. Energy 2018, 228, 2539–2566. [Google Scholar] [CrossRef]
- Bohlin, T. Practical Grey-box Process Identification: Theory and Applications, 1st ed.; Springer: London, UK, 2006. [Google Scholar]
- Azarpour, A.; Borhani, T.N.; Alwi, S.R.W.; Manan, Z.A.; Mutalib, M.I.A. A generic hybrid model development for process analysis of industrial fixed-bed catalytic reactors. Chem. Eng. Res. Des. 2017, 117, 149–167. [Google Scholar] [CrossRef]
- Laursen, S.Ö.; Webb, D.; Ramirez, W.F. Dynamic hybrid neural network model of an industrial fed-batch fermentation process to produce foreign protein. Comput. Chem. Eng. 2007, 31, 163–170. [Google Scholar] [CrossRef]
- Schäfer, P.; Caspari, A.; Mhamdi, A.; Mitsos, A. Economic nonlinear model predictive control using hybrid mechanistic data-driven models for optimal operation in real-time electricity markets: In-silico application to air separation processes. J. Process Control 2019, 84, 171–181. [Google Scholar] [CrossRef]
- Bikmukhametov, T.; Jäschke, J. Combining machine learning and process engineering physics towards enhanced accuracy and explainability of data-driven models. Comput. Chem. Eng. 2020, 138, 106834. [Google Scholar] [CrossRef]
- Liu, H.; Wang, K.; Chen, Z.; Jordan, K.E.; Luo, J.; Deng, H. A Parallel Framework for Reservoir Simulators on Distributed-memory Supercomputers. In Proceedings of the SPE/IATMI Asia Pacific Oil & Gas. Conference and Exhibition, Society of Petroleum Engineers, Bali, Indonesia, 20–22 October 2015. [Google Scholar]
- Prakash, A.V.; Chaudhury, A.; Barrasso, D.; Ramachandran, R. Simulation of population balance model-based particulate processes via parallel and distributed computing. Chem. Eng. Res. Des. 2013, 91, 1259–1271. [Google Scholar] [CrossRef]
- Sampat, C.; Bettencourt, F.; Baranwal, Y.; Paraskevakos, I.; Chaturbedi, A.; Karkala, S.; Jha, S.; Ramachandran, R.; Ierapetritou, M. A parallel unidirectional coupled DEM-PBM model for the efficient simulation of computationally intensive particulate process systems. Comput. Chem. Eng. 2018, 119, 128–142. [Google Scholar] [CrossRef]
- MathWorks. Simulation and Model-Based Design. 2020. Available online: https://www.mathworks.com/products/simulink.html (accessed on 19 June 2020).
- COMSOL. Understand, Predict, and Optimize Physics-Based Designs and Processes with COMSOL Multiphysics. 2020. Available online: https://www.comsol.com/comsol-multiphysics (accessed on 19 June 2020).
- PSE. gPROMS FormulatedProducts. 2020. Available online: https://www.psenterprise.com/products/gproms/formulatedproducts (accessed on 19 June 2020).
- Aspentech. aspenONE Product Portfolio. 2020. Available online: https://www.aspentech.com/en/products/full-product-listing (accessed on 19 June 2020).
- Siemens. Engineer Innovation with CFD- Focused Multiphysics Simulation. Available online: https://www.plm.automation.siemens.com/global/en/products/simcenter/STAR-CCM.html (accessed on 19 June 2020).
- Pantelides, C. Digital Design, Digital Operations—The central role of modeling in digital world. In Proceedings of the PSE Advanced Process Modeling Forum, Tarrytown, NY, USA, 10–12 September 2019. [Google Scholar]
- Siemens. Siemens PLM Software. MindSphere: The Cloud-Based, Open IoT Operating System for Digital Transformation. 2017. Available online: https://www.plm.automation.siemens.com/media/global/en/Siemens_MindSphere_Whitepaper (accessed on 27 May 2020).
- GE Digital. Industrial Cloud Based Platform (PaaS). Available online: https://www.ge.com/digital/iiot-platform (accessed on 27 May 2020).
- SEEQ. SEEQ Product Overview. Available online: https://www.seeq.com/product/overview (accessed on 19 June 2020).
- TrendMiner. TrendMiner Self-Service Industrial Analytics. 2020. Available online: https://www.trendminer.com/software/ (accessed on 19 June 2020).
- TIBCO. TIBCO Cloud: Connected Intelligence, Delivered. 2020. Available online: https://cloud.tibco.com/ (accessed on 19 June 2020).
- Amazon. Start Building on AWS Today. 2020. Available online: https://aws.amazon.com/ (accessed on 19 June 2020).
- Microsoft. Create Solutions Today that Adapt for Tomorrow. Invent with Purpose. 2020. Available online: https://azure.microsoft.com/en-us/ (accessed on 19 June 2020).
- Google. Solve more with Google Cloud. 2020. Available online: https://cloud.google.com/ (accessed on 19 June 2020).
- IBM. IBM Watson Products and Solutions. 2020. Available online: https://www.ibm.com/watson/products-services (accessed on 19 June 2020).
- Subramanian, B. The disruptive influence of cloud computing and its implications for adoption in the pharmaceutical and life sciences industry. J. Med. Mark. Device Diagn. Pharm. Mark. 2012, 12, 192–203. [Google Scholar] [CrossRef]
- Leukert, B.; Kubach, T.; Eckert, C.; Tsutsumi, K.; Crawford, M.; Vayssiere, N. IoT 2020: Smart and secure IoT platform. IEC White Pap. 2016, pp. 1–181. Available online: https://www.iec.ch/whitepaper/iotplatform/ (accessed on 25 July 2020).
- Botta, A.; de Donato, W.; Persico, V.; Pescapé, A. Integration of Cloud computing and Internet of Things: A survey. Future Gener. Comput. Syst. 2016, 56, 684–700. [Google Scholar] [CrossRef]
- Venkatasubramanian, V.; Zhao, C.; Joglekar, G.; Jain, A.; Hailemariam, L.; Suresh, P.; Akkisetty, P.; Morris, K.; Reklaitis, G.V. Ontological informatics infrastructure for pharmaceutical product development and manufacturing. Comput. Chem. Eng. 2006, 30, 1482–1496. [Google Scholar] [CrossRef]
- Bray, T.; Paoli, J.; Sperberg-McQueen, C.M.; Eve Maler, F.Y. Extensible Markup Language (XML) 1.0 (Fifth Edition). 2008. Available online: https://www.w3.org/TR/2008/REC-xml-20081126/ (accessed on 28 May 2020).
- Barbosa, D.; Bohannon, P.; Freire, J.; Kanne, C.-C.; Manolescu, I.; Vassalos, V.; Yoshikawa, M. XML Storage. In Encyclopedia of Database Systems; Liu, L., Özsu, M.T., Eds.; Springer: Boston, MA, USA, 2009; pp. 3627–3634. [Google Scholar]
- Michels, J.; Hare, K.; Kulkarni, K.; Zuzarte, C.; Liu, Z.H.; Hammerschmidt, B.; Zemke, F. The New and Improved SQL: 2016 Standard. ACM SIGMOD Rec. 2018, 47, 51–60. [Google Scholar] [CrossRef]
- Agrawal, R.; Ailamaki, A.; Bernstein, P.A.; Brewer, E.A.; Carey, M.J.; Chaudhuri, S.; Doan, A.; Florescu, D.; Franklin, M.J.; Garcia-Molina, H.; et al. The Claremont report on database research. ACM SIGMOD Rec. 2008, 37, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Jones, D.; Snider, C.; Nassehi, A.; Yon, J.; Ben, H. Characterising the Digital Twin: A systematic literature review. CIRP J. Manuf. Sci. Technol. 2020, 29, 36–52. [Google Scholar] [CrossRef]
- Lund, A.M.; Mochel, K.; Lin, J.-W.; Onetto, R.; Srinivasan, J.; Gregg, P.; Bergman, J.E.; Hartling, K.D.; Ahmed, A.; Chotai, S. Digital Twin Interface for Operating Wind Farms; General Electric Co.: Boston, MA, USA, 2015. [Google Scholar]
- Madni, A.; Madni, C.; Lucero, S. Leveraging Digital Twin Technology in Model—Based Systems Engineering. Systems 2019, 7, 7. [Google Scholar] [CrossRef] [Green Version]
- GE Power Digital Solutions, GE Digital Twin—Analytic Engine for the Digital Power Plant. White Pap. 2016. Available online: https://www.ge.com/digital/sites/default/files/download_assets/Digital-Twin-for-the-digital-power-plant-.pdf (accessed on 25 July 2020).
- Tao, F.; Zhang, M. Digital Twin Shop-Floor: A New Shop-Floor Paradigm towards Smart Manufacturing. IEEE Access 2017, 5, 20418–20427. [Google Scholar] [CrossRef]
- Wagner, C.; Grothoff, J.; Epple, U.; Drath, R.; Malakuti, S.; Gruner, S.; Hoffmeister, M.; Zimermann, P. The role of the Industry 4.0 Asset Administration Shell and the Digital Twin during the life cycle of a plant. In Proceedings of the 2017 22nd IEEE International Conference on Emerging Technologies and Factory Automation (ETFA), Limassol, Cyprus, 12–15 September 2017. [Google Scholar]
- Cheng, Y.; Zhang, Y.P.; Ji, P.; Xu, W.J.; Zhou, Z.D.; Tao, F. Cyber-physical integration for moving digital factories forward towards smart manufacturing: A survey. Int. J. Adv. Manuf. Technol. 2018, 97, 1209–1221. [Google Scholar] [CrossRef]
- Siemens Switzerland Ltd. The Digital Twin—Driving Business Value throughout the Building Life Cycle; Siemens Switzerland Ltd.: Zug, Switzerland, September 2018. [Google Scholar]
- Braun, K.; Laupp, G.; Leich, R.; Saur, W.; Scheifele, H.; Schick, J. Method for filling packaging containers by weight. U.S. Patent 4385670A, 31 May 1983. [Google Scholar]
- Guo, F.Y.; Zou, F.; Liu, J.H.; Wang, Z.Q. Working mode in aircraft manufacturing based on digital coordination model. Int. J. Adv. Manuf. Technol. 2018, 98, 1547–1571. [Google Scholar] [CrossRef]
- Tuegel, E.J.; Ingraffea, A.R.; Eason, T.G.; Spottswood, S.M. Reengineering Aircraft Structural Life Prediction Using a Digital Twin. Int. J. Aerosp. Eng. 2011, 2011, 154798. [Google Scholar] [CrossRef] [Green Version]
- Gockel, B.; Tudor, A.; Brandyberry, M.; Penmetsa, R.; Tuegel, E. Challenges with Structural Life Forecasting Using Realistic Mission Profiles. In Proceedings of the 53rd AIAA/ASME/ASCE/AHS/ASC Structures, Structural Dynamics and Materials Conference, Honolulu, HI, USA, 23–26 April 2012. [Google Scholar]
- Glaessgen, E.; Biegel, B.; Chandler, F.; Crichton, D.; LeMoigne, J.; Little, M.; Null, C.; Peters, W.; Ransom, J.; Wang, L. NASA Technology Roadmaps TA11: Modeling, Simulation, Information Technology, and Processing; NASA Office of the Chief Technologist: Washington, DC, USA, 2015. [Google Scholar]
- Qi, Q.; Tao, F.; Hu, T.; Answer, N.; Liu, A.L.; Wei, A.; Wang, L.; Nee, A.Y.C. Enabling technologies and tools for digital twin. J. Manuf. Syst. 2019. [Google Scholar] [CrossRef]
- Toru Ishida, K.I.E. Digital Cities: Technologies, Experiences, and Future Perspectives, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2000. [Google Scholar]
- Parris, C. Meet a Digital Twin. In Minds + Machines; GE Digital: San Francisco, CA, USA, 2017. [Google Scholar]
- Predictive Insights Aid Aircraft Landing Gear Performance | GE Digital. ge.com. 2020. Available online: https://www.ge.com/digital/customers/predictive-insights-aid-aircraft-landing-gear-performance (accessed on 25 July 2020).
- Seshadri, B.R.; Krishnamurthy, T. Structural Health Management of Damaged Aircraft Structures Using the Digital Twin Concept. In Proceedings of the 25th AIAA/AHS Adaptive Structures Conference, Grapevine, TX, USA, 9–13 January 2017. [Google Scholar]
- Damjanovic-Behrendt, V. A digital twin based privacy enhancement mechanism for the automative industry. In Proceedings of the 2018 International Conference on Intelligent Systems (IS), Funchal—Madeira, Portugal, 25–27 September 2018. [Google Scholar]
- Yoo, Y.; Boland, R.J., Jr.; Lyytinen, K.; Majchrzak, A. Organizing for Innovation in the Digitized World. Organ. Sci. 2012, 23, 1398–1408. [Google Scholar] [CrossRef]
- Wannenburg, J.; Malekian, R. Body Sensor Network for Mobile Health Monitoring, a Diagnosis and Anticipating System. IEEE Sens. J. 2015, 15, 6839–6852. [Google Scholar] [CrossRef] [Green Version]
- Bruynseels, K.; de Sio, F.S.; van den Hoven, J. Digital Twins in Health Care: Ethical Implications of an Emerging Engineering Paradigm. Front. Genet. 2018, 9, 31. [Google Scholar] [CrossRef] [PubMed]
- Baillargeon, B.; Rebelo, N.; Fox, D.D.; Taylor, R.L.; Kuhl, E. The Living Heart Project: A robust and integrative simulator for human heart function. Eur. J. Mech. A-Solids 2014, 48, 38–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Francisco, A.; Mohammadi, N.; Taylor, J.E. Smart City Digital Twin-Enabled Energy Management: Toward Real-Time Urban. Building Energy Benchmarking. J. Manag. Eng. 2020, 36, 04019045. [Google Scholar] [CrossRef]
- Silva, B.N.; Khan, M.; Han, K. Towards sustainable smart cities: A review of trends, architectures, components, and open challenges in smart cities. Sustain. Cities Soc. 2018, 38, 697–713. [Google Scholar] [CrossRef]
- Komninos, N. The Architecture of Intelligent Cities. In Proceedings of the 2nd International Conference on Intelligent Environments 2006. Institute of Engineering and Technology, Athens, Greece, 5–6 July 2006. [Google Scholar]
- Datta, S.P.A. Emergence of Digital Twins—Is this the march of reason? J. Innov. Manag. 2017, 5, 14–33. [Google Scholar] [CrossRef] [Green Version]
- Gunes, V.; Peter, S.; Givargis, T.; Vahid, F. A Survey on Concepts, Applications, and Challenges in Cyber-Physical Systems. KSIIS Trans. Internet Inf. Syst. 2014, 8. [Google Scholar] [CrossRef] [Green Version]
- Eckhart, M.; Ekelhart, A. Towards Security-Aware Virtual Environments for Digital Twins. In Proceedings of the 4th ACM Workshop on Cyber-Physical System Security—CPSS ’18, Incheon, Korea, 4–8 June 2018; pp. 61–72. [Google Scholar]
- Rasheed, A.; San, O.; Kvamsdal, T. Digital Twin: Values, Challenges and Enablers from a Modeling Perspective. IEEE Access 2020, 8, 21980–22012. [Google Scholar] [CrossRef]
- Alam, K.M.; El Saddik, A. C2ps: A Digital Twin Architecture Reference Model for the Cloud-Based Cyber-Physical Systems. IEEE Access 2017, 5, 2050–2062. [Google Scholar] [CrossRef]
- Park, H.; Easwaran, A.; Andalam, S. Challenges in Digital Twin Development for Cyber-Physical Production Systems; Springer International Publishing: Cham, Switzerland, 2019; pp. 28–48. [Google Scholar]
- Knapp, E.; Langill, J. Industrial Network Security Securing Critical Infrastructure Networks for Smart Grid, SCADA, and Other Industrial Control. Systems, 2nd ed.; Elsevier: Waltham, MA, USA, 2015. [Google Scholar]
- Elkaseer, A.; Salem, M.; Ali, H.; Scholz, S. Approaches to a Practical Implementation of Industry 4.0. In Proceedings of the 11th International Conference on Advances in Computer-Human Interactions, Rome, Italy, 25–29 March 2018. [Google Scholar]
- Barrasso, D. Developing and applying digital twins for Continuous Drug Product Manufacturing. In Proceedings of the PSE Advanced Process Modeling Forum, Tarrytown, NY, USA, 10–12 September 2019. [Google Scholar]
- Ierapetritou, M.; Muzzio, F.; Reklaitis, G. Perspectives on the continuous manufacturing of powder-based pharmaceutical processes. AIChE J. 2016, 62, 1846–1862. [Google Scholar] [CrossRef]
- Boukouvala, F.; Niotis, V.; Ramachandran, R.; Muzzio, F.J.; Ierapetritou, M.G. An integrated approach for dynamic flowsheet modeling and sensitivity analysis of a continuous tablet manufacturing process. Comput. Chem. Eng. 2012, 42, 30–47. [Google Scholar] [CrossRef]
- Kamble, R.; Sharma, S.; Varghese, V.; Mahadik, K. Process analytical technology (PAT) in pharmaceutical development and its application. Int. J. Pharm. Sci. Rev. Res. 2013, 23, 212–223. [Google Scholar]
- Simon, L.L.; Pataki, H.; Marosi, G.; Meemken, F.; Hungerbühler, K.; Baiker, A.; Tummala, S.; Glennon, B.; Kuentz, M.; Steele, G.; et al. Assessment of Recent Process. Analytical Technology (PAT) Trends: A Multiauthor Review. Org. Process Res. Dev. 2015, 19, 3–62. [Google Scholar] [CrossRef]
- Yu, Z.; Chew, J.; Chow, P.; Tan, R. Recent advances in crystallization control: An industrial perspective. Chem. Eng. Res. Des. 2007, 85, 893–905. [Google Scholar] [CrossRef]
- Sierra-Vega, N.O.; Román-Ospino, A.; Scicolone, J.; Muzzio, F.J.; Romañach, R.J.; Méndez, R. Assessment of blend uniformity in a continuous tablet manufacturing process. Int. J. Pharm. 2019, 560, 322–333. [Google Scholar] [CrossRef]
- Goodwin, D.J.; van den Ban, S.; Denham, M.; Barylski, I. Real time release testing of tablet content and content uniformity. Int. J. Pharm. 2018, 537, 183–192. [Google Scholar] [CrossRef]
- De Beer, T.R.M.; Bodson, C.; Dejaegher, B.; Walczak, B.; Vercruysse, P.; Burggraeve, A.; Lemos, A.; Delattre, L.; Heyden, Y.V.; Remon, J.P.; et al. Raman spectroscopy as a process analytical technology (PAT) tool for the in-line monitoring and understanding of a powder blending process. J. Pharm. Biomed. Anal. 2008, 48, 772–779. [Google Scholar] [CrossRef]
- Singh, R.; Sahay, A.; Muzzio, F.; Ierapetritou, M.; Ramachandran, R. A systematic framework for onsite design and implementation of a control system in a continuous tablet manufacturing process. Comput. Chem. Eng. 2014, 66, 186–200. [Google Scholar] [CrossRef]
- Baranwal, Y.; Román-Ospino, A.D.; Keyvan, G.; Ha, J.M.; Hong, E.P.; Muzzio, F.J.; Ramachandran, R. Prediction of dissolution profiles by non-destructive NIR spectroscopy in bilayer tablets. Int. J. Pharm. 2019, 565, 419–436. [Google Scholar] [CrossRef] [PubMed]
- Shekunov, B.Y.; Chattopadhyay, P.; Tong, H.H.; Chow, A.H. Particle size analysis in pharmaceutics: Principles, methods and applications. Pharm. Res. 2007, 24, 203–227. [Google Scholar] [CrossRef]
- Wu, H.; White, M.; Khan, M. Quality-by-Design (QbD): An integrated process analytical technology (PAT) approach for a dynamic pharmaceutical co-precipitation process characterization and process design space development. Int. J. Pharm. 2011, 405, 63–78. [Google Scholar] [CrossRef]
- Meng, W.; Román-Ospino, A.D.; Panikar, S.S.; O’Callaghan, C.; Gilliam, S.J.; Ramachandran, R.; Muzzio, F.J. Advanced process design and understanding of continuous twin-screw granulation via implementation of in-line process analytical technologies. Adv. Powder Technol. 2019, 30, 879–894. [Google Scholar] [CrossRef]
- Ostergaard, I.; Szilagyi, B.; de Diego, H.L.; Qu, H.; Nagy, Z.K. Polymorphic Control and Scale-up Strategy for Antisolvent Crystallization Using Direct Nucleation Control. Cryst. Growth Des. 2020, 20, 2683–2697. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services, F.D.A. PAT-A Framework for Innovative Pharmaceutical Development, Manufacturing and Quality Assurance; U.S. Department of Health and Human Services, F.D.A.: Rockville, MD, USA, 2004.
- Bakeev, K.A. Process Analytical Technology: Spectroscopic Tools and Implementation Strategies for the Chemical and Pharmaceutical Industries, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- James, M.; Stanfield, C.F.; Bir, G. A Review of Process. Analytical Technology (PAT) in the U.S. Pharmaceutical Industry. Curr. Pharm. Anal. 2006, 2, 405–414. [Google Scholar]
- Nagy, Z.K.; Fevotte, G.; Kramer, H.; Simon, L.L. Recent advances in the monitoring, modelling and control of crystallization systems. Chem. Eng. Res. Des. 2013, 91, 1903–1922. [Google Scholar] [CrossRef]
- Simon, L.L.; Kiss, A.A.; Cornevin, J.; Gani, R. Process engineering advances in pharmaceutical and chemical industries: Digital process design, advanced rectification, and continuous filtration. Curr. Opin. Chem. Eng. 2019, 25, 114–121. [Google Scholar] [CrossRef]
- Papadakis, E.; Woodley, J.M.; Gani, R. Perspective on PSE in pharmaceutical process development and innovation. In Process. Systems Engineering for Pharmaceutical Manufacturing; Elsevier: Amsterdam, The Netherlands, 2018; pp. 597–656. [Google Scholar]
- Pandey, P.; Bharadwaj, R.; Chen, X. Modeling of drug product manufacturing processes in the pharmaceutical industry. In Predictive Modeling of Pharmaceutical Unit Operations; Woodhead Publishing: Sawston, Cambridge, UK, 2017; pp. 1–13. [Google Scholar]
- Escotet-Espinoza, M.S.; Foster, C.J.; Ierapetritou, M. Discrete Element Modeling (DEM) for mixing of cohesive solids in rotating cylinders. Powder Technol. 2018, 335, 124–136. [Google Scholar] [CrossRef]
- Toson, P.; Siegmann, E.; Trogrlic, M.; Kureck, H.; Khinast, J.; Jajcevic, D.; Doshi, P.; Blackwood, D.; Bonnassieux, A.; Daugherity, P.D.; et al. Detailed modeling and process design of an advanced continuous powder mixer. Int. J. Pharm. 2018, 552, 288–300. [Google Scholar] [CrossRef]
- Bhalode, P.; Ierapetritou, M. Discrete element modeling for continuous powder feeding operation: Calibration and system analysis. Int. J. Pharm. 2020, 585, 119427. [Google Scholar] [CrossRef] [PubMed]
- Rantanen, J.; Khinast, J. The Future of Pharmaceutical Manufacturing Sciences. J. Pharm. Sci. 2015, 104, 3612–3638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sajjia, M.; Shirazian, S.; Kelly, C.B.; Albadarin, A.B.; Walker, G. ANN Analysis of a Roller Compaction Process. in the Pharmaceutical Industry. Chem. Eng. Technol. 2017, 40, 487–492. [Google Scholar] [CrossRef]
- Pandey, P.; Katakdaunde, M.; Turton, R. Modeling weight variability in a pan coating process using Monte Carlo simulations. AAPS PharmSciTech 2006, 7, E2–E11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Metta, N.; Verstraeten, M.; Ghijs, M.; Kumar, A.; Schafer, E.; Singh, R.; De Beer, T.; Nopens, I.; Cappuyns, P.; Van Assche, I.; et al. Model development and prediction of particle size distribution, density and friability of a comilling operation in a continuous pharmaceutical manufacturing process. Int. J. Pharm. 2018, 549, 271–282. [Google Scholar] [CrossRef]
- Barrasso, D.; Tamrakar, A.; Ramachandran, R. Model Order Reduction of a Multi-scale PBM-DEM Description of a Wet Granulation Process via ANN. Procedia Eng. 2015, 102, 1295–1304. [Google Scholar] [CrossRef] [Green Version]
- Bostijn, N.; Dhondt, J.; Ryckaert, A.; Szabó, E.; Dhondt, W.; Van Snick, B.; Vanhoorne, V.; Vervaet, C.; De Beer, T. A multivariate approach to predict the volumetric and gravimetric feeding behavior of a low feed rate feeder based on raw material properties. Int. J. Pharm. 2018, 557, 342–353. [Google Scholar] [CrossRef] [Green Version]
- Van Snick, B.; Grymonpre, W.; Dhondt, J.; Pandelaere, K.; Di Pretoro, G.; Remon, J.P.; De Beer, T.; Vervaet, C.; Vanhoorne, V. Impact of blend properties on die filling during tableting. Int. J. Pharm. 2018, 549, 476–488. [Google Scholar] [CrossRef] [Green Version]
- Escotet-Espinoza, M.S.; Moghtadernejad, S.; Oka, S.; Wang, Y.; Roman-Ospino, A.; Schäfer, E.; Cappuyns, P.; Van Assche, I.; Futran, M.; Ierapetritou, M.; et al. Effect of tracer material properties on the residence time distribution (RTD) of continuous powder blending operations. Part. I of II: Experimental evaluation. Powder Technol. 2019, 342, 744–763. [Google Scholar] [CrossRef]
- Escotet-Espinoza, M.S.; Moghtadernejad, S.; Oka, S.; Wang, Z.; Wang, Y.; Roman-Ospino, A.; Schäfer, E.; Cappuyns, P.; Van Assche, I.; Futran, M.; et al. Effect of material properties on the residence time distribution (RTD) characterization of powder blending unit operations. Part. II of II: Application of models. Powder Technol. 2019, 344, 525–544. [Google Scholar] [CrossRef]
- Escotet-Espinoza, M.S.; Vadodaria, S.; Singh, R.; Muzzio, F.J.; Ierapetritou, M.G. Modeling the effects of material properties on tablet compaction: A building block for controlling both batch and continuous pharmaceutical manufacturing processes. Int. J. Pharm. 2018, 543, 274–287. [Google Scholar] [CrossRef]
- Rogers, A.; Hashemi, A.; Ierapetritou, M. Modeling of Particulate Processes for the Continuous Manufacture of Solid-Based Pharmaceutical Dosage Forms. Processes 2013, 1, 67–127. [Google Scholar] [CrossRef] [Green Version]
- Metta, N.; Ghijs, M.; Schäfer, E.; Kumar, A.; Cappuyns, P.; Assche, I.V.; Singh, R.; Ramachandran, R.; Beer, T.D.; Ierapetritou, M.; et al. Dynamic Flowsheet Model Development and Sensitivity Analysis of a Continuous Pharmaceutical Tablet Manufacturing Process Using the Wet Granulation Route. Processes 2019, 7, 234. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Escotet-Espinoza, M.S.; Singh, R.; Ierapetritou, M. Surrogate-based Optimization for Pharmaceutical Manufacturing Processes. In Computer Aided Chemical Engineering; Espuña, A., Graells, M., Puigjaner, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 2797–2802. [Google Scholar]
- U.S. Department of Health and Human Services, F.D.A. Data Integrity and Compliance with Drug CGMP; U.S. Department of Health and Human Services, F.D.A.: Silver Spring, MD, USA, 2018.
- Su, Q.; Bommireddy, Y.; Shah, Y.; Ganesh, S.; Moreno, M.; Liu, J.; Gonzalez, M.; Yazdanpanah, N.; O’Connor, T.; Reklaitis, G.V.; et al. Data reconciliation in the Quality-by-Design (QbD) implementation of pharmaceutical continuous tablet manufacturing. Int. J. Pharm. 2019, 563, 259–272. [Google Scholar] [CrossRef]
- Ganesh, S.; Moreno, M.; Liu, J.; Gonzalez, M.; Nagy, Z.; Reklaitis, G. Sensor Network for Continuous Tablet Manufacturing. In 13th International Symposium on Process. Systems Engineering (PSE 2018); Elsevier: Amsterdam, The Netherlands, 2018; pp. 2149–2154. [Google Scholar]
- Ganesh, S. Continuous Pharmaceutical Manufacturing: Systems Integration for Process Operations Management. Ph.D. Thesis, Purdue University Graduate School, West Lafayette, IN, USA, 2020. [Google Scholar]
- Singh, R.; Sahay, A.; Karry, K.M.; Muzzio, F.; Ierapetritou, M.; Ramachandran, R. Implementation of an advanced hybrid MPC–PID control system using PAT tools into a direct compaction continuous pharmaceutical tablet manufacturing pilot plant. Int. J. Pharm. 2014, 473, 38–54. [Google Scholar] [CrossRef]
- Hailemariam, L.; Venkatasubramanian, V. Purdue Ontology for Pharmaceutical Engineering: Part I. Conceptual Framework. J. Pharm. Innov. 2010, 5, 88–99. [Google Scholar] [CrossRef]
- Hailemariam, L.; Venkatasubramanian, V. Purdue Ontology for Pharmaceutical Engineering: Part II. Applications. J. Pharm. Innov. 2010, 5, 139–146. [Google Scholar] [CrossRef]
- Zhao, C.; Jain, A.; Hailemariam, L.; Suresh, P.; Akkisetty, P.; Joglekar, G.; Venkatasubramanian, V.; Reklaitis, G.V.; Morris, K.; Basu, P. Toward intelligent decision support for pharmaceutical product development. J. Pharm. Innov. 2006, 1, 23–35. [Google Scholar] [CrossRef]
- Muñoz, S.G.; Torres, E.H. Supervised Extended Iterative Optimization Technology for Estimation of Powder Compositions in Pharmaceutical Applications: Method and Lifecycle Management. Ind. Eng. Chem. Res. 2020, 59, 10072–10081. [Google Scholar] [CrossRef]
- Shi, Z.; Hermiller, J.; Muñoz, S.G. Estimation of mass-based composition in powder mixtures using Extended Iterative Optimization Technology (EIOT). AIChE J. 2019, 65, 87–98. [Google Scholar] [CrossRef] [Green Version]
- Kadlec, P.; Grbić, R.; Gabrys, B. Review of adaptation mechanisms for data-driven soft sensors. Comput. Chem. Eng. 2011, 35, 1–24. [Google Scholar] [CrossRef]
- Flåten, G.R. Model Maintenance. In Multivariate Analysis in the Pharmaceutical Industry; Academic Press: Cambridge, MA, USA, 2018; pp. 313–321. [Google Scholar]
- Chan, K.H.; Dozal-Mejorada, E.J.; Cheng, X.; Kephart, R.; Ydstie, B.E. Predictive control with adaptive model maintenance: Application to power plants. Comput. Chem. Eng. 2014, 70, 91–103. [Google Scholar] [CrossRef]
- Chen, K.; Castillo, I.; Chiang, L.H.; Yu, J. Soft Sensor Model Maintenance: A Case Study in Industrial Processes. IFAC-PapersOnLine 2015, 48, 427–432. [Google Scholar] [CrossRef]
- Gama, J.; Žliobaitė, I.; Bifet, A.; Pechenizkiy, M.; Bouchachia, A. A survey on concept drift adaptation. ACM Comput. Surv. 2014, 46, 1–37. [Google Scholar] [CrossRef]
- Janardan, S.M. Concept Drift Streaming Data Classification Algorithms Platforms and Issues. Procedia Comput. Sci. 2017, 122, 804–811. [Google Scholar] [CrossRef]
- Webb, G.I.; Hyde, R.; Cao, H.; Nguyen, H.L.; Petitjean, F. Characterizing concept drift. Data Min. Knowl. Discov. 2016, 30, 964–994. [Google Scholar] [CrossRef] [Green Version]
- Kadwe, Y.; Suryawanshi, V. A Review on Concept Drift. Iosr J. Comput. Eng. 2015, 17, 20–26. [Google Scholar]
- Sun, Y.; Zhang, J.; Xiong, Y.; Zhu, G. Data Security and Privacy in Cloud Computing. Int. J. Distrib. Sens. Netw. 2014, 10, 190903. [Google Scholar] [CrossRef] [Green Version]
- O’Connor, T. Opportunities and Challenges for the Application of Process. Modeling and Simulation for Product Quality Risk Management. In Proceedings of the Advanced Process Modeling Forum, Tarrytown, NY, USA, 10–12 September 2019. [Google Scholar]
- Badr, S.; Sugiyama, H. A PSE perspective for the efficient production of monoclonal antibodies: Integration of process, cell, and product design aspects. Curr. Opin. Chem. Eng. 2020, 27, 121–128. [Google Scholar] [CrossRef]
- Lin-Gibson, S.; Srinivasan, V. Recent Industrial Roadmaps to Enable Smart Manufacturing of Biopharmaceuticals. IEEE Trans. Autom. Sci. Eng. 2019, 2019, 1–8. [Google Scholar] [CrossRef]
- Narayanan, H.; Luna, M.F.; von Stosch, M.; Cruz Bournazou, M.N.; Polotti, G.; Morbidelli, M.; Butte, A.; Sokolov, M. Bioprocessing in the Digital Age: The Role of Process Models. Biotechnol. J. 2020, 15, e1900172. [Google Scholar] [CrossRef] [PubMed]
- Read, E.K.; Park, J.T.; Shah, R.B.; Riley, B.S.; Brorson, K.A.; Rathore, A.S. Process analytical technology (PAT) for biopharmaceutical products: Part I. concepts and applications. Biotechnol. Bioeng. 2010, 105, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Biechele, P.; Busse, C.; Solle, D.; Scheper, T.; Reardon, K. Sensor systems for bioprocess monitoring. Eng. Life Sci. 2015, 15, 469–488. [Google Scholar] [CrossRef]
- Zhao, L.; Fu, H.-Y.; Zhou, W.; Hu, W.-S. Advances in process monitoring tools for cell culture bioprocesses. Eng. Life Sci. 2015, 15, 459–468. [Google Scholar] [CrossRef]
- Roch, P.; Mandenius, C.-F. On-line monitoring of downstream bioprocesses. Curr. Opin. Chem. Eng. 2016, 14, 112–120. [Google Scholar] [CrossRef] [Green Version]
- Guerra, A.; von Stosch, M.; Glassey, J. Toward biotherapeutic product real-time quality monitoring. Crit. Rev. Biotechnol. 2019, 39, 289–305. [Google Scholar] [CrossRef]
- Pais, D.A.M.; Carrondo, M.J.T.; Alves, P.M.; Teixeira, A.P. Towards real-time monitoring of therapeutic protein quality in mammalian cell processes. Curr. Opin. Biotechnol. 2014, 30, 161–167. [Google Scholar] [CrossRef]
- Classen, J.; Aupert, F.; Reardon, K.F.; Solle, D.; Scheper, T. Spectroscopic sensors for in-line bioprocess monitoring in research and pharmaceutical industrial application. Anal. Bioanal. Chem. 2017, 409, 651–666. [Google Scholar] [CrossRef]
- Berry, B.N.; Dobrowsky, T.M.; Timson, R.C.; Kshirsagar, R.; Ryll, T.; Wiltberger, K. Quick generation of Raman spectroscopy based in-process glucose control to influence biopharmaceutical protein product quality during mammalian cell culture. Biotechnol. Prog. 2016, 32, 224–234. [Google Scholar] [CrossRef]
- Mehdizadeh, H.; Lauri, D.; Karry, K.M.; Moshgbar, M.; Procopio-Melino, R.; Drapeau, D. Generic Raman-based calibration models enabling real-time monitoring of cell culture bioreactors. Biotechnol. Prog. 2015, 31, 1004–1013. [Google Scholar] [CrossRef] [PubMed]
- Abu-Absi, N.R.; Martel, R.P.; Lanza, A.M.; Clements, S.J.; Borys, M.C.; Li, Z.J. Application of spectroscopic methods for monitoring of bioprocesses and the implications for the manufacture of biologics. Pharm. Bioprocess. 2014, 2, 267–284. [Google Scholar] [CrossRef]
- Rathore, A.S.; Kateja, N.; Kumar, D. Process integration and control in continuous bioprocessing. Curr. Opin. Chem. Eng. 2018, 22, 18–25. [Google Scholar] [CrossRef]
- Wasalathanthri, D.P.; Rehmann, M.S.; Song, Y.; Gu, Y.; Mi, L.; Shao, C.; Chemmalil, L.; Lee, J.; Ghose, S.; Borys, M.C.; et al. Technology outlook for real-time quality attribute and process parameter monitoring in biopharmaceutical development—A review. Biotechnol. Bioeng. 2020, 117. [Google Scholar] [CrossRef]
- Tang, P.; Xu, J.; Louey, A.; Tan, Z.; Yongky, A.; Liang, S.; Li, Z.J.; Weng, Y.; Liu, S. Kinetic modeling of Chinese hamster ovary cell culture: Factors and principles. Crit. Rev. Biotechnol. 2020, 40, 265–281. [Google Scholar] [CrossRef]
- Farzan, P.; Mistry, B.; Ierapetritou, M.G. Review of the important challenges and opportunities related to modeling of mammalian cell bioreactors. AIChE J. 2017, 63, 398–408. [Google Scholar] [CrossRef]
- Baumann, P.; Hubbuch, J. Downstream process development strategies for effective bioprocesses: Trends, progress, and combinatorial approaches. Eng. Life Sci. 2017, 17, 1142–1158. [Google Scholar] [CrossRef]
- Smiatek, J.; Jung, A.; Bluhmki, E. Towards a Digital Bioprocess. Replica: Computational Approaches in Biopharmaceutical Development and Manufacturing. Trends Biotechnol. 2020. [Google Scholar] [CrossRef]
- Olughu, W.; Deepika, G.; Hewitt, C.; Rielly, C. Insight into the large-scale upstream fermentation environment using scaled-down models. J. Chem. Technol. Biotechnol. 2019, 94, 647–657. [Google Scholar] [CrossRef]
- Li, X.; Scott, K.; Kelly, W.J.; Huang, Z. Development of a Computational Fluid Dynamics Model for Scaling-up Ambr Bioreactors. Biotechnol. Bioprocess Eng. 2018, 23, 710–725. [Google Scholar] [CrossRef]
- Farzan, P.; Ierapetritou, M.G. A Framework for the Development of Integrated and Computationally Feasible Models of Large-Scale Mammalian Cell Bioreactors. Processes 2018, 6, 82. [Google Scholar] [CrossRef] [Green Version]
- Menshutina, N.V.; Guseva, E.V.; Safarov, R.R.; Boudrant, J. Modelling of hollow fiber membrane bioreactor for mammalian cell cultivation using computational hydrodynamics. Bioprocess Biosyst. Eng. 2020, 43, 549–567. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Tang, P.; Yongky, A.; Drew, B.; Borys, M.C.; Liu, S.; Li, Z.J. Systematic development of temperature shift strategies for Chinese hamster ovary cells based on short duration cultures and kinetic modeling. MAbs 2019, 11, 191–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sokolov, M.; Ritscher, J.; MacKinnon, N.; Souquet, J.; Broly, H.; Morbidelli, M.; Butte, A. Enhanced process understanding and multivariate prediction of the relationship between cell culture process and monoclonal antibody quality. Biotechnol. Prog. 2017, 33, 1368–1380. [Google Scholar] [CrossRef] [PubMed]
- Villiger, T.K.; Scibona, E.; Stettler, M.; Broly, H.; Morbidelli, M.; Soos, M. Controlling the time evolution of mAb N-linked glycosylation—Part II: Model–based predictions. Biotechnol. Prog. 2016, 32, 1135–1148. [Google Scholar] [CrossRef] [PubMed]
- Kotidis, P.; Jedrzejewski, P.; Sou, S.N.; Sellick, C.; Polizzi, K.; del Val, I.J.; Kontoravdi, C. Model–based optimization of antibody galactosylation in CHO cell culture. Biotechnol. Bioeng. 2019, 116, 1612–1626. [Google Scholar] [CrossRef]
- Radhakrishnan, D.; Robinson, A.S.; Ogunnaike, B. Controlling the Glycosylation Profile in mAbs Using Time-Dependent Media Supplementation. Antibodies 2017, 7, 1. [Google Scholar] [CrossRef] [Green Version]
- Karst, D.J.; Steinebach, F.; Soos, M.; Morbidelli, M. Process performance and product quality in an integrated continuous antibody production process. Biotechnol. Bioeng. 2017, 114, 298–307. [Google Scholar] [CrossRef]
- Shirahata, H.; Diab, S.; Sugiyama, H.; Gerogiorgis, D.I. Dynamic modelling, simulation and economic evaluation of two CHO cell-based production modes towards developing biopharmaceutical manufacturing processes. Chem. Eng. Res. Des. 2019, 150, 218–233. [Google Scholar] [CrossRef]
- Xing, Z.; Kenty, B.; Koyrakh, I.; Borys, M.; Pan, S.-H.; Li, Z.J. Optimizing amino acid composition of CHO cell culture media for a fusion protein production. Process Biochem. 2011, 46, 1423–1429. [Google Scholar] [CrossRef]
- Spahn, P.N.; Hansen, A.H.; Hansen, H.G.; Arnsdorf, J.; Kildegaard, H.F.; Lewis, N.E. A Markov chain model for N-linked protein glycosylation–towards a low-parameter tool for model-driven glycoengineering. Metab. Eng. 2016, 33, 52–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hutter, S.; Villiger, T.K.; Brühlmann, D.; Stettler, M.; Broly, H.; Soos, M.; Gunawan, R. Glycosylation flux analysis reveals dynamic changes of intracellular glycosylation flux distribution in Chinese hamster ovary fed-batch cultures. Metab. Eng. 2017, 43, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Nolan, R.P.; Lee, K. Dynamic model of CHO cell metabolism. Metab. Eng. 2011, 13, 108–124. [Google Scholar] [CrossRef] [PubMed]
- Bayrak, E.S.; Wang, T.; Cinar, A.; Undey, C. Computational Modeling of Fed-Batch Cell Culture Bioreactor: Hybrid Agent-Based Approach. IFAC-PapersOnLine 2015, 48, 1252–1257. [Google Scholar] [CrossRef]
- Kiparissides, A.; Pistikopoulos, E.N.; Mantalaris, A. On the model-based optimization of secreting mammalian cell (GS-NS0) cultures. Biotechnol. Bioeng. 2015, 112, 536–548. [Google Scholar] [CrossRef]
- Kotidis, P.; Demis, P.; Goey, C.H.; Correa, E.; McIntosh, C.; Trepekli, S.; Shah, N.; Klymenko, O.V.; Kontoravdi, C. Constrained global sensitivity analysis for bioprocess design space identification. Comput. Chem. Eng. 2019, 125, 558–568. [Google Scholar] [CrossRef]
- Narayanan, H.; Sokolov, M.; Morbidelli, M.; Butte, A. A new generation of predictive models: The added value of hybrid models for manufacturing processes of therapeutic proteins. Biotechnol. Bioeng. 2019, 116, 2540–2549. [Google Scholar] [CrossRef]
- Psichogios, D.C.; Ungar, L. A hybrid neural network-first principles approach to process modeling. AIChE J. 1992, 38, 1499–1511. [Google Scholar] [CrossRef]
- Von Stosch, M.; Hamelink, J.-M.; Oliveira, R. Hybrid modeling as a QbD/PAT tool in process development: An industrial E. coli case study. Bioprocess Biosyst. Eng. 2016, 39, 773–784. [Google Scholar] [CrossRef] [Green Version]
- Zalai, D.; Koczka, K.; Párta, L.; Wechselberger, P.; Klein, T.; Herwig, C. Combining mechanistic and data-driven approaches to gain process knowledge on the control of the metabolic shift to lactate uptake in a fed-batch CHO process. Biotechnol. Prog. 2015, 31, 1657–1668. [Google Scholar] [CrossRef]
- Selvarasu, S.; Kim, D.Y.; Karimi, I.A.; Lee, D.-Y. Combined data preprocessing and multivariate statistical analysis characterizes fed-batch culture of mouse hybridoma cells for rational medium design. J. Biotechnol. 2010, 150, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Lienqueo, M.E.; Mahn, A.; Salgado, J.C.; Shene, C. Mathematical Modeling of Protein Chromatograms. Chem. Eng. Technol. 2012, 35, 46–57. [Google Scholar] [CrossRef]
- Shi, C.; Gao, Z.-Y.; Zhang, Q.-L.; Yao, S.-J.; Slater, N.K.H.; Lin, D.-Q. Model-based process development of continuous chromatography for antibody capture: A case study with twin-column system. J. Chromatogr. A 2020, 1619, 460936. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Briskot, T.; Hahn, T.; Baumann, P.; Hubbuch, J. Estimation of adsorption isotherm and mass transfer parameters in protein chromatography using artificial neural networks. J. Chromatogr. A 2017, 1487, 211–217. [Google Scholar] [CrossRef]
- Baur, D.; Angarita, M.; Müller-Späth, T.; Morbidelli, M. Optimal model-based design of the twin-column CaptureSMB process improves capacity utilization and productivity in protein A affinity capture. Biotechnol. J. 2016, 11, 135–145. [Google Scholar] [CrossRef]
- Huter, J.M.; Strube, J. Model-Based Design and Process. Optimization of Continuous Single Pass Tangential Flow Filtration Focusing on Continuous Bioprocessing. Processes 2019, 7, 317. [Google Scholar] [CrossRef] [Green Version]
- Mandenius, C.-F.; Brundin, A. Bioprocess optimization using design-of-experiments methodology. Biotechnol. Prog. 2008, 24, 1191–1203. [Google Scholar] [CrossRef]
- Pleitt, K.; Somasundaram, B.; Johnson, B.; Shave, E.; Lua, L.H.L. Evaluation of process simulation as a decisional tool for biopharmaceutical contract development and manufacturing organizations. Biochem. Eng. J. 2019, 150, 107252. [Google Scholar] [CrossRef]
- Arnold, L.; Lee, K.; Rucker-Pezzini, J.; Lee, J.H. Implementation of Fully Integrated Continuous Antibody Processing: Effects on Productivity and COGm. Biotechnol. J. 2019, 14, 1800061. [Google Scholar] [CrossRef]
- Pollock, J.; Coffman, J.; Ho, S.V.; Farid, S.S. Integrated continuous bioprocessing: Economic, operational, and environmental feasibility for clinical and commercial antibody manufacture. Biotechnol. Prog. 2017, 33, 854–866. [Google Scholar] [CrossRef]
- Yang, O.; Prabhu, S.; Ierapetritou, M. Comparison between Batch and Continuous Monoclonal Antibody Production and Economic Analysis. Ind. Eng. Chem. Res. 2019, 58, 5851–5863. [Google Scholar] [CrossRef]
- Walther, J.; Godawat, R.; Hwang, C.; Abe, Y.; Sinclair, A.; Konstantinov, K. The business impact of an integrated continuous biomanufacturing platform for recombinant protein production. J. Biotechnol. 2015, 213, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Pirrung, S.M.; van der Wielen, L.A.M.; van Beckhoven, R.F.W.C.; van de Sandt, E.J.A.X.; Eppink, M.H.M.; Ottens, M. Optimization of biopharmaceutical downstream processes supported by mechanistic models and artificial neural networks. Biotechnol. Prog. 2017, 33, 696–707. [Google Scholar] [CrossRef] [PubMed]
- Zobel-Roos, S.; Schmidt, A.; Mestmäcker, F.; Mouellef, M.; Huter, M.; Uhlenbrock, L.; Kornecki, M.; Lohmann, L.; Ditz, R.; Strube, J. Accelerating Biologics Manufacturing by Modeling or: Is Approval under the QbD and PAT Approaches Demanded by Authorities Acceptable Without a Digital-Twin? Processes 2019, 7, 94. [Google Scholar] [CrossRef] [Green Version]
- Sencar, J.; Hammerschmidt, N.; Jungbauer, A. Modeling the Residence Time Distribution of Integrated Continuous Bioprocesses. Biotechnol. J. 2020, e2000008. [Google Scholar] [CrossRef]
- Gomis-Fons, J.; Schwarz, H.; Zhang, L.; Andersson, N.; Nilsson, B.; Castan, A.; Solbrand, A.; Stevenson, J.; Chotteau, V. Model–based design and control of a small-scale integrated continuous end-to-end mAb platform. Biotechnol. Prog. 2020, e2995. [Google Scholar] [CrossRef]
- Zahel, T.; Hauer, S.; Mueller, E.M.; Murphy, P.; Abad, S.; Vasilieva, E.; Maurer, D.; Brocard, C.; Reinisch, D.; Sagmeister, P.; et al. Integrated Process Modeling-A Process Validation Life Cycle Companion. Bioengineering 2017, 4, 86. [Google Scholar] [CrossRef] [Green Version]
- Borys, B.S.; Roberts, E.L.; Le, A.; Kallos, M.S. Scale-up of embryonic stem cell aggregate stirred suspension bioreactor culture enabled by computational fluid dynamics modeling. Biochem. Eng. J. 2018, 133, 157–167. [Google Scholar] [CrossRef]
- Sou, S.N.; Jedrzejewski, P.M.; Lee, K.; Sellick, C.; Polizzi, K.M.; Kontoravdi, C. Model–based investigation of intracellular processes determining antibody Fc-glycosylation under mild hypothermia. Biotechnol Bioeng 2017, 114, 1570–1582. [Google Scholar] [CrossRef]
- Agarwal, N.; Mason, A.; Pradhan, R.; Kemper, J.; Bosley, A.; Serfiotis-Mitsa, D.; Wang, J.; Lindo, V.; Ahuja, S.; Hatton, D.; et al. Kinetic modeling as a tool to understand the influence of cell culture process parameters on the glycation of monoclonal antibody biotherapeutics. Biotechnol. Prog. 2019, 35, e2865. [Google Scholar] [CrossRef]
- Sha, S.; Huang, Z.; Wang, Z.; Yoon, S. Mechanistic modeling and applications for CHO cell culture development and production. Curr. Opin. Chem. Eng. 2018, 22, 54–61. [Google Scholar] [CrossRef]
- Zurcher, P.; Sokolov, M.; Bruhlmann, D.; Ducommun, R.; Stettler, M.; Souquet, J.; Jordan, M.; Broly, H.; Morbidelli, M.; Butte, A. Cell culture process metabolomics together with multivariate data analysis tools opens new routes for bioprocess development and glycosylation prediction. Biotechnol. Prog. 2020, e3012. [Google Scholar] [CrossRef] [PubMed]
- Sokolov, M.; Morbidelli, M.; Butte, A.; Souquet, J.; Broly, H. Sequential Multivariate Cell Culture Modeling at Multiple Scales Supports Systematic Shaping of a Monoclonal Antibody toward a Quality Target. Biotechnol. J. 2018, 13, e1700461. [Google Scholar] [CrossRef] [PubMed]
- Behere, K.; Yoon, S. Chromatography bioseparation technologies and in-silico modelings for continuous production of biotherapeutics. J Chromatogr A 2020, 1627, 461376. [Google Scholar] [CrossRef]
- Kumar, V.; Lenhoff, A.M. Mechanistic Modeling of Preparative Column Chromatography for Biotherapeutics. Annu. Rev. Chem. Biomol. Eng. 2020, 11, 235–255. [Google Scholar] [CrossRef]
- Krippl, M.; Dürauer, A.; Duerkop, M. Hybrid modeling of cross-flow filtration: Predicting the flux evolution and duration of ultrafiltration processes. Sep. Purif. Technol. 2020, 248, 117064. [Google Scholar] [CrossRef]
- Lohmann, L.J.; Strube, J. Accelerating Biologics Manufacturing by Modeling: Process Integration of Precipitation in mAb Downstream Processing. Processes 2020, 8, 58. [Google Scholar] [CrossRef] [Green Version]
- Pirrung, S.M.; Berends, C.; Backx, A.H.; van Beckhoven, R.F.W.C.; Eppink, M.H.M.; Ottens, M. Model–based optimization of integrated purification sequences for biopharmaceuticals. Chem. Eng. Sci. X 2019, 3, 100025. [Google Scholar] [CrossRef]
- Sachidananda, M.; Erkoyuncu, J.; Steenstra, D.; Michalska, S. Discrete Event Simulation Modelling for Dynamic Decision Making in Biopharmaceutical Manufacturing. Procedia CIRP 2016, 49, 39–44. [Google Scholar] [CrossRef] [Green Version]
- Petrides, D.; Carmichael, D.; Siletti, C.; Koulouris, A. Biopharmaceutical Process Optimization with Simulation and Scheduling Tools. Bioengineering 2014, 1, 154–187. [Google Scholar] [CrossRef]
- Taras, S.; Woinaroschy, A. Simulation and Multi-objective Optimization of Bioprocesses with Matlab and Superpro Designer Using a Client-server Interface. Chem. Eng. Trans. 2011, 25, 207–212. [Google Scholar]
- Gangadharan, N.; Turner, R.; Field, R.; Oliver, S.G.; Slater, N.; Dikicioglu, D. Metaheuristic approaches in biopharmaceutical process development data analysis. Bioprocess Biosyst. Eng. 2019, 42, 1399–1408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casola, G.; Siegmund, C.; Mattern, M.; Sugiyama, H. Data mining algorithm for pre-processing biopharmaceutical drug product manufacturing records. Comput. Chem. Eng. 2019, 124, 253–269. [Google Scholar] [CrossRef]
- Lee, H.W.; Christie, A.; Xu, J.; Yoon, S. Data fusion-based assessment of raw materials in mammalian cell culture. Biotechnol. Bioeng. 2012, 109, 2819–2828. [Google Scholar] [CrossRef] [PubMed]
- Herold, S.; King, R. Automatic identification of structured process models based on biological phenomena detected in (fed-)batch experiments. Bioprocess Biosyst. Eng. 2014, 37, 1289–1304. [Google Scholar] [CrossRef]
- Luna, M.F.; Martínez, E.C. Iterative modeling and optimization of biomass production using experimental feedback. Comput. Chem. Eng. 2017, 104, 151–163. [Google Scholar] [CrossRef]
- Feidl, F.; Vogg, S.; Wolf, M.; Podobnik, M.; Ruggeri, C.; Ulmer, N.; Wälchli, R.; Souquet, J.; Broly, H.; Butté, A.; et al. Process–wide control and automation of an integrated continuous manufacturing platform for antibodies. Biotechnol. Bioeng. 2020, 117, 1367–1380. [Google Scholar] [CrossRef]
- Fahey, W.; Jeffers, P.; Carroll, P. A business analytics approach to augment six sigma problem solving: A biopharmaceutical manufacturing case study. Comput. Ind. 2020, 116, 103153. [Google Scholar] [CrossRef]
- Portela, R.M.C.; Varsakelis, C.; Richelle, A.; Giannelos, N.; Pence, J.; Dessoy, S.; von Stosch, M. When Is an In Silico Representation a Digital Twin? A Biopharmaceutical Industry Approach to the Digital Twin Concept; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–21. [Google Scholar]




| Areas of Application | Specific Application | Purpose | Component of DT Framework with Software | References |
|---|---|---|---|---|
| Energy production | Steam turbines | Integrates historical data with real-time process to forecast process wear/tear and suggest modifications | Virtual component using Predix | [4,90,91,92] |
| Wind farm | Integrates historical data to enhance process efficiency and predict maintenance strategies | Virtual component based on General Electric (GE) fleet using Predix | [92] | |
| Smart product manufacturing | Factory smart floor map | Redesign manufacturing platforms | Virtual replica of manufacturing floor to optimize location of machinery and sensors | [2,18,93,94,95,96] |
| Digitization of manufacturing of packaging machines | Redesigning product to improve production efficiency and digitize overall process design | Virtual model using Siemens mechatronics concept designer | [96,97] | |
| Aviation industry | DT of next-generation aircrafts | Aircraft structural health management and assessment of potential damage analysis | Virtual replica of airplanes using GE’s Predix software platform | [98,99] |
| Airframe DT simulator (ADT) | Training and engineering solutions | Virtual simulator using GE’s Predix software | [100] | |
| Aerospace industry | DT of outer-space vehicles | Replication of health maintenance problems and monitoring for safety and reliability | Virtual replica of the vehicle’s on-board integrated system | [26,101] |
| Automotive transportation | DT of cars | Prediction and assessment of maintenance issues for improvement of durability of automobile parts | Virtual replica of automobiles | [102] |
| Automated transport vehicles | Vehicle simulations for safe, automated long-distance transportations | Dassault systems using digital control systems | [102] | |
| Healthcare industry | Virtual replica of patients | Surgical operation training and health monitoring using sensors | Virtual component developed using a simulated environment | [4] |
| Living Heart project | 3D model of human heart for analysis of blood circulation and pharmacokinetic/pharmacodynamic (PKPD) testing of medicines | Virtual model using finite element-based modeling environment | [4] | |
| Infrastructure planning | City planning | Construction of smart, sustainable city infrastructure | Virtual digital replica using information communication technology | [103] |
| Features | Discrete-Element Method (DEM)/Computational Fluid Dynamics (CFD)/Finite-Element Method (FEM) | Population Balance Modeling (PBM) | Mechanistic/Mathematical | Semi-Empirical/Hybrid | Data-Driven | Advanced Process Control |
|---|---|---|---|---|---|---|
| Computational complexity | High | Medium | Medium | Low | Low | Low |
| Real-time capability | No | No | Yes | Yes | Yes | Yes |
| Adaptive modeling | No | No | No | Yes | Yes | Yes |
| Reference | Integration Achieved | Tools Used | Limitation |
|---|---|---|---|
| Hailemariam et al. 2010 [166,167] | Presented a data collection ontology to for laboratory data | Extensive Markup Language (XML), Resource Description Framework (RDF), | A limited number of software and processes were connected to the ontology |
| Singh et al. 2014 [132,165] | Physical plant level up to control platform to implement model predictive control (MPC) using sensor data | MATLAB, Process Pulse, DeltaV, SynTQ | Data integration was only achieved till the control platform |
| Cao et al. 2018 [46] | Presented a cloud-based data collection strategy for collecting data from a continuous pharmaceutical manufacturing pilot plant as well as collecting data from analytical equipment | XML, AWS, DeltaV, OSI-PI | A complete integration was presented for data collection, but it lacked its integration with any software for live data prediction |
| Barenji et al. 2019 [29] | Presented a cyber-physical framework for Process Analytical Technology (PAT) tools for pharmaceutical manufacturing | N/A | Data integration was only performed for PAT tools without any integration of analytics |
| Categories | Methods | Platforms | Comments |
|---|---|---|---|
| Upstream Process | |||
| Bioreactor fluid dynamics, system heterogeneity | CFD simulation [201] CFD + PBM simulation [239] CFD + kinetics model [202] | Ansys Fluent, COMSOL Multiphysics | Support to understand operations such as agitation, aeration, nutrients feeding. Guide process scale-up. Computationally expensive. Can reduce the computational time by using a compartment model, hard to be validated. |
| Cell growth, nutrients, and metabolism. Product quality (protein glycosylation) | Kinetic model [204,240,241] | MATLAB, gPROMS, Visual Basic for Applications | Capture and predict the dynamic profile of the cell culture. Correlate critical process parameters (CPPs) and critical quality attributes (CQAs). Require a large amount of data for parameter estimations. |
| Stoichiometric methods [242] | MATLAB, OptFlux etc. | Deal with a large amount of mechanistic reaction, genome-scale simulation. Need to integrate with the kinetic model to capture the dynamic profiles | |
| Multivariate tools [243] | MATLAB | Require a large amount of data. Represent input–output correlations. Do not capture the mechanistic correlations. | |
| Media formulation | Multivariate analysis MFA [211,222] | MATLAB | Identify nutrient correlations, improve productivity and cell viability |
| Product impurities | Regression model and Multivariate analysis [244] | MATLAB | Capture predict titer, aggregation, low molecular weight components, and glycan groups |
| Downstream Process | |||
| Bind-elute/flow-through chromatography | Mechanistic: Plate model, mass balance model, general rate model with their simplifications models [245,246] | MATLAB, CADET, ChromX | Capture moving and stationary phases, obtain breakthrough curves, gradient elution curves. Predict the product concentration and impurities (charge variant, aggregates, host cell proteins) |
| Transport dispersive model—ANN model [225] | MATLAB | ||
| Filtration/ultrafiltration | Mechanistic: Film theory, Osmotic Pressure Model, boundary layer, mass transfer coefficient) [227] | Aspen Custom Modeler | Capture volumetric flow, flux, and pressure across the filtration membrane. Can be used for model predictive control. |
| Hybrid model (ANN-mechanistic film theory) [247] | MATLAB | ||
| Downstream integration (precipitation) | Empirical model (quantitative structure-activity relationship) + Mechanistic model [248] | NA | Physico-chemical process model supported by design of experiment (DoE). Capture CPP and CQA |
| Downstream integration and optimization | Mechanistic model—Artificial Neural Network-Optimization algorithm [249] | MATLAB | Optimize overall process yield and solvent use by adjusting operation parameters such as duration. However, only high molecular weight contamination was considered. |
| Integrated Process | |||
| Residence time distribution | Probability distribution function for each unit operation [236] | Python | Correlate input material operating conditions, design parameters with outlet profile. Easy to update. |
| Activity tracking and decision making | Discrete Event Simulation [250] | Extend Sim, Simul8 | Discrete/dynamic system, track activity, scheduling, and resource utilization |
| Material tracking and decision making | Mechanistic/Empirical model [229,251] | SuperPro Designer, Biosolve | Track material balance and optimize cost-effectiveness. Process debottlenecking, capacity planning |
| Process risk assessment | Implement process model with Monte Carlo analysis [238] | MATLAB | Evaluate parameter sensitivity, impurity purification, and product quality. Hard to apply to computationally expensive model |
| Overall process optimization | Integrate flowsheet model with optimization solvers [252] | SuperPro Designer-VB-Matlab | Optimize environment impact and cost-effectiveness by adjusting 4 operating parameters |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Yang, O.; Sampat, C.; Bhalode, P.; Ramachandran, R.; Ierapetritou, M. Digital Twins in Pharmaceutical and Biopharmaceutical Manufacturing: A Literature Review. Processes 2020, 8, 1088. https://doi.org/10.3390/pr8091088
Chen Y, Yang O, Sampat C, Bhalode P, Ramachandran R, Ierapetritou M. Digital Twins in Pharmaceutical and Biopharmaceutical Manufacturing: A Literature Review. Processes. 2020; 8(9):1088. https://doi.org/10.3390/pr8091088
Chicago/Turabian StyleChen, Yingjie, Ou Yang, Chaitanya Sampat, Pooja Bhalode, Rohit Ramachandran, and Marianthi Ierapetritou. 2020. "Digital Twins in Pharmaceutical and Biopharmaceutical Manufacturing: A Literature Review" Processes 8, no. 9: 1088. https://doi.org/10.3390/pr8091088