1. Introduction
Nanocellulose is a natural cellulose fiber that can be prepared from cellulose; it is represented in plants and animals and produced by bacteria. Nanocellulose is generally contained in a nanosize diameter and is several micrometers in length. According to the types of nanocellulose and based on their morphological characteristics, nanocellulose can be categorized into six groups, including cellulose nanocrystals (CNCs), cellulose nanofibrils (CNFs), amorphous nanocellulose (ANC), cellulose microfibrils (CMFs), microcrystalline cellulose (MCC), and cellulose nanoyarn (CNY) [
1]. Over the past few years, researchers have represented the attractive properties of nanocellulose—e.g., excellent mechanical properties, high surface area for modification, biocompatibility, biodegradability, and low toxicity. Therefore, nanocellulose can be applied in many fields, such as biomedical, pharmaceutical, nanocomposite, cosmetics, environmental, etc. [
2,
3,
4,
5].
Nanocellulose is typically prepared via various approaches such as acid hydrolysis, enzymatic hydrolysis, oxidative, mechanical, and combination methods [
6,
7]. Among these approaches, concentrated strong acid hydrolysis is commonly used for CNC preparation. However, various economic and environmental concerns challenge conventional methods for nanocellulose preparation. Solid acids have shown the advantage of easing acid recovery by crystallization at a low temperature. The current study selected an organic acid, oxalic acid, to hydrolyze cellulose into nanocellulose. Oxalic acid has low water solubility at room temperature but is highly soluble at high temperatures, with results in the effective hydrolysis of cellulose at high concentrations. Li et al. [
8] produced CNCs with a high yield of 80.6% from plant-based acid through oxalic acid hydrolysis. Chen et al. [
9] found that the nanocellulose content (approximately 25%) from eucalyptus obtained by using 50% oxalic acid at 100 °C for 45 min and reducing sugar content was less than 10%. In addition, by combining oxalic acid hydrolysis with mechanical pretreatment, pressured homogenization demonstrated high nanofibrillation yields (76.5%) [
10]. Meanwhile, Bondancia et al. [
11] used another organic acid, citric acid, to produce CNCs. Even though the results revealed a high content of nanocellulose (23.1 ± 0.9%) and high acid recovery for later reuse, the low strength of the green acid resulted in low migration of the acid into the cellulose and high energy consumption (e.g., high temperature, prolonged process).
Moreover, enzymatic hydrolysis is a compromise method, due to its being performed in mild conditions and the fact that it does not produce harmful waste. Enzymatic pretreatment has shown great potential in deconstructing cellulose into nanofibrils via commercial endoglucanase. The pretreatment time and the enzyme dosage were optimized using response surface methodology [
12]. Chen et al. [
13] prepared granular CNCs with diameters of 30 nm from cotton pulp fiber by using a cellulase concentration of 300 U/mL and a hydrolysis time of 5 h. Nevertheless, nanocellulose preparation through enzymatic hydrolysis was operated for a long time and obtained variable types of nanocellulose. Enzymatic hydrolysis is often incorporated with other methods to solve this problem. Ultrasonication with the hydrodynamic forces of ultrasound is a process for the defibrillation of cellulose fiber. Employing acid hydrolysis with ultrasonic treatment improved the production yield and quality of CNCs from palm oil empty fruit bunch pulps. The increase in yield was due to the enhancement of acid accessibility by ultrasonic pretreatment [
14]. Cui et al. [
15] used the green preparation of rod-like CNCs with a lengths of 200–500 nm and widths of 10 nm through ultrasonic-assisted enzymatic hydrolysis. Therefore, the process for intensification with the combination of technologies improved the efficiency of nanocellulose preparation, including increasing production yield, reducing the reaction time, and improving the characteristics of the nanocellulose.
In previous studies, several of them used renewable cellulose sources for nanocellulose preparation, such as sugar bagasse [
16], eucalyptus [
17], pineapple leaf [
18], cotton [
13,
19], and rice straw [
20]. Thailand is recognized as one of the main rice producers in Asia. However, every kilogram of harvested rice can generate a rice straw byproduct of around 1 to 1.5 kg [
21]. Therefore, the abundance of rice straw as agricultural waste was discarded as a leftover in the field, which led to eliminating the rice straw by burning it in open areas to prepare the land for the next crop. Thus, the burning field caused a severe impact on human health. Aquino et al. [
21] reported that the lignocellulosic composition of rice straw was mainly composed of cellulose (32–38%). Thakur et al. [
20] used rice straw to produce nanocellulose by sulfuric acid hydrolysis. The highest yield of nanocellulose (90.28%) was obtained from 75% sulfuric acid at 30 °C and a hydrolysis time for 5 h. The results revealed a rod-like shape with a 5–15 nm diameter. Therefore, rice straw could be considered a suitable source for preparing nanocellulose, because it is low-cost and sustainable.
This study aimed to establish the preparation of nanocellulose from rice straw through enzymatic hydrolysis consisting of ultrasonication-assisted pretreatment and integration with green acid hydrolysis. The rice straw cellulose was prepared via alkaline pretreatment and a bleaching process. A central composite design (CCD) achieved the optimized variable level in the enzymatic hydrolysis. Moreover, this study aimed to improve the efficiency of nanocellulose production by ultrasonication-assisted pretreatment of rice straw cellulose prior to enzymatic hydrolysis and intensified the nanocellulose preparation from rice straw residue using green acid hydrolysis. The different types of nanocellulose were obtained and characterized using scanning electron microscopy (SEM), dynamic light scattering (DLS), zeta potentials, Fourier transform infrared (FTIR), and X-ray diffractometry (XRD).
2. Materials and Methods
2.1. Materials
Rice straw (Oryza sativa) strain DR105 was provided from a local field in San Sai (Chiang Mai, Thailand). After drying at 60 °C in a hot air oven overnight, the rice straw was ground into small particle sizes and sieved through a 24 wire mesh. The ground rice straw was kept in an airtight plastic bag and stored at room temperature. The iKnowZyme® Cellulase (endoglucanase, EC 3.2.1.4) (13,000 IU/mL) is a liquid enzyme from iKnowZyme, Bangkok, Thailand.
2.2. Cellulose Extraction
Rice straw was alkali-pretreated with 5% NaOH solution at a ratio of 1: 20 (
w/
v) under the autoclaving condition for 20 min (modified from Kobkham et al. [
22]). The rice straw was filtrated and washed with distilled water until a neutral pH was obtained and was then dried at 60 °C in a hot air oven overnight. After that, the alkali-pretreated rice straw was bleached with 2% sodium chlorite at 70 °C for 2 h. Rice straw cellulose was obtained by filtration, washed with deionized water until the pH became neutral, and dried in a hot air oven overnight at 60 °C.
2.3. Lignocellulosic Composition
The lignocellulosic composition of rice straw, alkaline-pretreated rice straw, and rice straw cellulose were determined by forage fiber analysis [
23]. This method is a rapid procedure for determining plant cell wall constituents by determining the insoluble fiber in neutral detergent. The sample was exposed to neutral detergent fiber (NDF), acid detergent fiber (ADF), acid detergent lignin (ADL), and acid-insoluble ash (AIA).
The NDF is defined as the cell wall constituents (cellulose, hemicellulose, and lignin) obtained from the yield recovered after refluxing with a neutral detergent fiber reagent for 1 h. After that, the solution was filtrated through a crucible filter, washed with DI water followed by acetone, dried, and weighed.
ADF is an estimate of soluble hemicellulose in an acid detergent solution containing sulfuric acid and cetyl trimethyl ammonium bromide via refluxing of the sample for 1 h. Then, the residue was separated by filtration and washing with DI water and acetone. The solid residue was dried and weighed. The obtained residue comprised cellulose, lignin, and acid-insoluble ash (mainly silica).
The ADL was used to evaluate the cellulose content after hydrolyzing the ADF with the acid detergent solution containing 72% sulfuric acid for 3 h, and the solid residue was filtered, washed, dried, and weighed. The remained residue refers to lignin and acid-insoluble ash.
AIA is designed to determine the amount of insoluble ash in sulfuric acid from the ADL process. The ADL residue was ignited at 500 °C for 3 h in the furnace and weighed.
The percentages of hemicellulose, cellulose, and lignin were then evaluated using the following equation:
Hemicellulose (%) = %NDF − %ADF
Cellulose (%) = %ADF − %ADL
Lignin (%) = %ADL − %AIA
The ground-dried samples were heated with the concentrated nitric acid on a hot plate stirrer until the solution was clear. The hydrogen peroxide was added and heated until the solution was colorless. The digested solution was diluted and analyzed by the ICP-OES.
2.4. Nanocellulose Preparation
2.4.1. Optimization of Nanocellulose Preparation Using Enzymatic Hydrolysis
The two variables, cellulase concentration (10–300 U/mL) and rice straw cellulose concentration (0.05–0.20%,
w/v), were employed to optimize the condition of enzymatic hydrolysis in nanocellulose preparation. A total of 13 runs of the central composite design (CCD) with two variables and five levels were evaluated for the interaction effects on nanocellulose preparation by response surface methodology, as shown in
Table 1. The statistical software Design-Expert
® version 7.0.0 (Stat-Ease Inc., MN, USA) was used to generate an experimental design. According to the designed experiment, the condition was carried out in 25 mL of 50 mM acetate buffer (pH 5.0) and incubated at 30 °C with a shaking rate of 120 rpm for 1 h in a shaking incubator (ThermoStable
TMIS-20, Daihan Scientific (Thailand) Co., Ltd., Seoul, South Korea). The reaction was stopped by boiling for 10 min. After that, the mixture was centrifuged using a J2-MC centrifuge (Beckman, New York, USA) at 10,000 rpm for 15 min, and the supernatant was collected for reducing sugar evaluation. The precipitate was dispersed into the DI water in an ultrasonication bath (Branson, Connecticut, USA) for 10 min. The solid residue of rice straw cellulose was separated by centrifugation at 3500 rpm for 10 min, and the nanocellulose suspension was collected in a glass bottle and stored at 4 °C before further characterization. The solid residue of rice straw cellulose content was also calculated.
Validation of Nanocellulose Preparation in Optimal Condition
The optimized cellulase and rice straw cellulose concentrations were validated by the predicted nanocellulose preparation to ensure quadratic model fitting.
2.4.2. Improvement of Nanocellulose Preparation by Ultrasonic-Assisted Pretreatment
The rice straw cellulose was added to 50 mM acetate buffer (pH 5.0) and treated with a high-intensity ultrasonic processor (VCX 750, Sonics®, Manchester, CT, USA) using 500 W and 20 kHz for 5 min (at the amplitude of 65%, pulses of 2 s on and 1 s off). Afterward, the treated rice straw cellulose was hydrolyzed by enzymatic hydrolysis under optimal condition. The reaction was stopped, and the mixture was separated. The supernatant was then determined for the reducing sugar content. The precipitate was dispersed in DI water and centrifuged to obtain nanocellulose suspension for characterization. The solid residue of rice straw cellulose was then further hydrolyzed.
2.4.3. Intensification of Nanocellulose Preparation by Integrated Oxalic Acid Hydrolysis
After enzymatic hydrolysis was assisted with ultrasonic treatment, the solid residue of rice straw cellulose was hydrolyzed using 50% oxalic acid at 100 °C for 45 min (modified from Chen et al. [
24]). The reaction was terminated by adding 50 mL of hot DI water and quickly filtrated under a vacuum. The oxalic acid in the filtrate was recrystallized. The aliquot was collected, and the precipitate was resuspended with DI water, followed by centrifuging at 10,000 rpm for 10 min until the pH became neutral. The obtained nanocellulose was dispersed using an ultrasonic bath for 10 min and centrifuged at 3500 rpm. The nanocellulose suspension and remaining rice straw cellulose were evaluated and characterized.
2.5. Analysis Methods
Nanocellulose Content
The content of nanocellulose was calculated using the following Equation (1) [
24]
where W is the weight of rice straw cellulose (g), W
1 is the total reducing sugar content (g), and W
2 is the weight of solid residue of rice straw cellulose (g).
The reducing sugar content was determined by the DNS method. The absorbance of the reacted solution was measured at 540 nm.
The solid residue of rice straw cellulose was obtained after enzymatic hydrolysis. The residue was dried in a hot air oven at 60 °C overnight and weighed.
2.6. Characterization of Rice Straw Nanocellulose
2.6.1. Scanning Electron Microscopy
The surface morphology of rice straw cellulose, nanocellulose, and solid residue cellulose was observed using a field emission scanning electron microscope (FE-SEM, JSM-6335 F, JEOL, Tokyo, Japan) at the accelerating voltage of 15 kV. The samples were placed on Cu tape and coated with gold.
2.6.2. Dynamic Light Scattering and Zeta Potential Analysis
The hydrodynamic particle size distribution of nanocellulose solution and zeta potentials were determined by Zetasizer (Zen 3600, UK).
2.6.3. Fourier Transform Infrared Spectroscopy
Fourier transform infrared spectroscopy investigated the chemical functional groups represented in rice straw cellulose and nanocellulose structure. The chemical structure was analyzed using an FTIR spectrometer (Tensor 27, Bruker, Billerica, MA, USA) with a wavenumber between 400–4000 cm−1. The samples were dried at 105 °C for 3–5 h. Then, the samples were mixed with KBr and pressed under a vacuum to form pellets.
2.6.4. X-ray Diffractometry
All dried samples of rice straw cellulose and nanocellulose were prepared on a glass sample holder, and the diffraction intensity was observed using a X-ray diffractometer (Miniflex II, Rigaku, Japan) with angular scanning of 3° to 60°. The crystallinity index (CrI) was calculated with following Equation (2) [
25].
whereas I
c is the diffraction intensity of reflections at 2θ of 22.7
o for the crystal face of cellulose structure, and I
am is the diffraction intensity of the amorphous domain at 2θ of 18.0
o.
4. Discussion
The chemical composition of raw rice straw showed a high cellulose content. Moreover, the selected alkali pretreatment and bleaching process of the rice straw demonstrated a very high content of cellulose of about 87.7%. The pretreatment of rice straw could eliminate the hemicellulose and lignin content by approximately 79% and 91%, respectively. The results related to Kobkham et al. [
22] studied the alkaline pretreatment of rice straw with 30% NaOH under autoclaving conditions and obtained a high cellulose content. Modenbach and Nokes [
26] indicated that the alkaline pretreatment result in cleavage of the ester bond cross-linking between cellulose, hemicellulose, and lignin, thus resulting in the accessibility of the enzymatic hydrolysis. In addition, the bleaching process removed the remaining residues of lignin and hemicellulose by breaking down the conjugated bonds of phenolic compounds and organic matter from oxidation reactions [
27,
28]. Lee et al. [
17] used rice straw as the cellulose to prepare nanocellulose by acid hydrolysis. After alkali pretreatment with NaOH and bleaching by NaClO
2, the results showed cellulose (94.1%). Therefore, these results indicated that the obtained rice straw cellulose could be utilized as a source of cellulose in nanocelulose preparation.
The appearance of the rice straw and rice straw cellulose demonstrated the change in color of the rice straw to white after the pretreatment and bleaching process. The results corresponded to Kampeerapappun [
28], who obtained white corn husk cellulose after an alkali-pretreatment and bleaching process and indicated that almost pure cellulose was obtained. In addition, the morphologies of rice straw cellulose by SEM revealed that the chemical pretreatment by NaOH destroyed the complex lignocellulosic structure or recalcitrance of rice straw under autoclaving conditions and the bleaching process. Therefore, the surface of rice straw cellulose increased the opportunity for hydrolysis in nanocellulose preparation, especially enzymatic hydrolysis [
29].
A five-level cellulase concentration and rice straw cellulose concentration with five replications of center points (as shown in
Table 3) were studied. The design matrix of central composite design (CCD) of 13 runs represented the actual values of variables along with the predicted and actual response of nanocellulose content. Moreover,
Table 4 illustrates the response surface quadratic model fitting for the ANOVA. The second-order regression model was statistically valid when the f-value had a low probability value (
p-value) less than 0.05 at a 95% confidence level. The R
2 (91%) showed more than 80%, thus exhibiting a good fit of the regression model and a coefficient of variation (CV) of less than 10%. A lower CV evaluated the excellent reliability of the experiment. Hence, the regression model was adequate to predict the expected optimization. The positive and negative effects of each variable on the nanocellulose isolation were investigated through the coefficient estimate value. The regression coefficients and their significance of the response surface quadratic model on nanocellulose preparation were displayed.
The results revealed that a high cellulase concentration significantly negatively affected nanocellulose preparation, because rice straw cellulose was hydrolyzed into the reducing sugar with a high conversion rate to result in a decreasing nanocellulose content. The rice straw cellulose concentration demonstrated that a high concentration was applied, thus resulting in a lower nanocellulose content. This was due to the fact that the cellulose can absorb the water and leaves low mass transfer in the solution, thereby inhibiting the cellulose, which corresponded to Aguiar et al. [
29]. Furthermore, the interactive model term of cellulose and rice straw cellulose concentration (
X1X2) had positively affected the nanocellulose preparation.
The regression equation demonstrated a graphic of a three-dimensional response surface plot, as is shown in
Figure 2, and represents the relationship between the experimental level of each cellulase and rice straw cellulose concentration to the response value. The results showed that high substrate concentrations led to more significant enzyme inhibition by the substrate or product. Additionally, the high concentration of enzyme loading caused an increase in hydrolyzing the cellulose chains into glucose. This research revealed that the concentrations of cellulase (107 U/mL) and rice straw cellulose (0.13%
w/v) resulted in the maximum nanocellulose content of 32.4% (
w/w). The results related to Chen et al. [
30], who achieved the highest content (20.17%) of nanocellulose from the pulp using a cellulase concentration of 200 U/Ml.
The nanocellulose preparation from rice straw cellulose was improved by ultrasonication-assisted treatment prior to enzymatic hydrolysis under optimal conditions.
Figure 3 revealed increased nanocellulose content, reducing sugar concentration, and cellulose hydrolysis compared to enzymatic hydrolysis alone. The nanocellulose content increased appropriately by about 61.5%. The results indicated that the ultrasonic probe could distribute a higher intensity of ultrasonication as a cavitation effect. Cavitation is the formation of bubbles in a liquid by ultrasonic waves. The bubbles can collapse the cellulose chain and destroy the hydrogen bonding and Van der Waals between microfibrils, thus resulting in the accessibility of enzymes [
31]. The ultrasonication generates the cavitation energy and breaks down the intermolecular hydrogen bond during treatment, thus allowing the enzyme to penetrate the amorphous region of cellulose [
32]. As a result, ultrasonication increased the nanocellulose yield by releasing and dispersing the nanocellulose in the suspension.
Moreover, nanocellulose preparation from rice straw cellulose by enzymatic hydrolysis was intensified by the integration with oxalic acid hydrolysis. The results demonstrated that the nanocellulose content increased to 65.0 ± 0.16%(
w/w), thereby leading to more than 90% cellulose hydrolysis. The nanocellulose content was very encouraging compared to other biobased approaches, due to the swelling structure of the solid residue of rice straw cellulose that led to hydrolyzing by oxalic acid. Li et al. [
8] produced cellulose nanocrystals from softwood with oxalic acid for 30 and 60 min. The cellulose nanocrystal content was obtained at about 58.3% and 80.6%, respectively. Moreover, Chen et al. [
24] used 60% oxalic acid to hydrolyze bleached kraft eucalyptus pulp fiber.
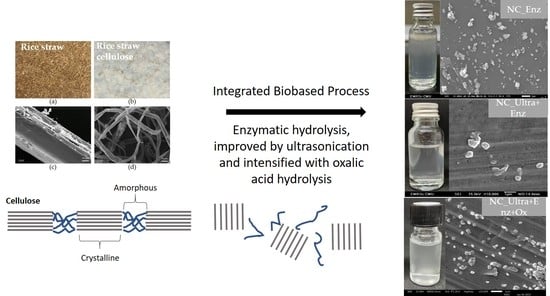
The characteristics of nanocellulose from rice straw cellulose, such as morphology, size distribution, zeta potential, functional group, and crystallinity, were determined. The SEM photographs demonstrated that all nanocellulose was spherical or granular shaped. The appearances of nanocellulose suspension were all turbid and homogenously dispersed in DI water. According to Chen et al. [
30], the results reported that the product obtained from pulp fiber hydrolysis with a cellulose concentration at 200 U/mL were spherical particles with a particle size of about 30 nm, which were mixed with irregular rod-like particles of several hundreds of nanometers in length. Moreover, Chen et al. [
9] obtained granular nanocellulose from cotton by enzymatic hydrolysis at 100 U/mL. They reported that a low cellulase concentration mainly acted on the amorphous regions by endoglucanase and split the cellulose microfibers at the amorphous regions to obtain the nanocellulose. When the cellulase concentration was higher, both amorphous and crystalline regions were hydrolyzed by endoglucanase. Chen et al. [
24] obtained needle-like cellulose nanocrystals along with large particles of fibrous cellulosic solid residue after the hydrolysis of bleached kraft eucalyptus with oxalic acid.
In addition, zeta potential measurement is a crucial characterization technique for investigating the surface charge of nanoparticles in a colloidal solution and the predictability of the nanosuspension stability. The zeta potential of nanoparticles was generally in the range of −30 to +30 mV, thereby indicating colloidal stability. A higher positive or negative than 30 mV of zeta potential values demonstrates that the nanosuspension is moderately stable and provides a long-term nanoparticle dispersion in the solution due to the electrostatic repulsion of the individual particle [
33,
34]. The higher the zeta potential, the more likely the suspension is to be stable, as the charged particles extrude each other, and this force overcomes the natural tendency to aggregate. It can provide valuable information about the behavior of particles in emulsions, foams, and aggregations of nanocellulose, which should be low for good results as a reinforcement agent. All produced nanocellulose suspensions obtained from rice straw hydrolysis by integrated biobased processes resulted in zeta potentials of −34.2 to −38.8 mV. According to Xu and Chen. [
35], they also reported that the zeta potential values of nanocellulose from enzymatic hydrolysis were −31.13 and −31 mV. The nanosuspension was stable for several weeks without agglomeration.
Figure 6 displays the FTIR spectra of rice straw cellulose and nanocellulose. The results indicated that the represented functional groups of prepared nanocelluloses were similar to cellulose. The intensities of the nanocellulose structure were more potent than the rice straw cellulose, especially for the adsorption bands of water, due to an increase in the surface as a nanosize, greater exposure of the -OH groups to the environment, and more adsorbed water molecules on the surface, including the other groups that were be exposed [
13].
In addition, this research indicated that the prepared nanocellulose from enzyme hydrolysis with/without ultrasonic pretreatment and intensified by oxalic acid hydrolysis represented the abundant surface hydroxyl groups to result in high hydrophilicity and availability for modification.
The results revealed that integrated biobased processes affected the crystalline structure of the rice straw cellulose. As a result, the XRD patterns of prepared nanocellulose from rice straw by integrated biobased processes represented the crystallinity change from rice straw cellulose. Furthermore, the
Crl values of obtained nanocellulose were obviously decreased and had a low crystallinity (16.3–30.6%). The results corresponded to Ioelovich [
36], who obtained amorphous nanocellulose with
CrI values of 20–25% from cotton cellulose using sulfuric acid hydrolysis. Meanwhile, the
CrI values of the remaining solid residues of the rice straw cellulose were between 53.2 and 53.9%. The results corresponded to Kalita et al. [
37], who produced microcrystalline cellulose from fodder grass. The
CrI was found to be 57%. In addition, Ternite et al. [
38] reported that the
CrI of microcrystalline cellulose from cotton linter was 65%. The results demonstrated that all nanocelluloses represented the characteristics of amorphous nanocellulose due to a lower
Crl. A low (
CrI < 25%) refers to high amorphous regions that can be classified as amorphous nanocellulose. The characteristic properties of amorphous regions contributed to the flexibility, high sorption ability, hydrophilicity, and accessibility of the reagents [
1]. This research indicated that the integrated processes based on the optimized enzymatic hydrolysis demonstrated a potential approach for producing amorphous granular nanocellulose characteristics when using rice straw as a cellulose source. Furthermore, the prepared granular nanocelluloses could be further used as a thickening agent, thereby making it possible to modify them for immobilizing the bioactive substances in cosmetic, pharmaceutical, and medical applications.