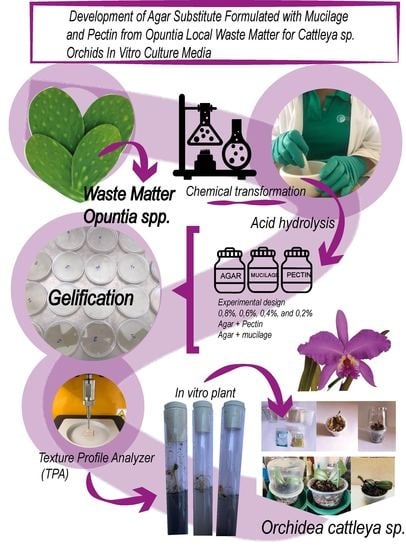
Development of Agar Substitute Formulated with Mucilage and Pectin from Opuntia Local Waste Matter for Cattleya sp. Orchids In Vitro Culture Media
Abstract
:1. Introduction
2. Materials and Methods
2.1. Extraction and Separation of Hydrocolloids
2.2. Agar Gel Preparation
2.3. Gel Characterization
2.3.1. Chemical Composition of Opuntia Hydrocolloids
2.3.2. Agar Substitute Stability
2.3.3. Agar Substitute Structure and Color
2.3.4. Mechanical Properties of Agar Substitute
2.3.5. Gelling Agent Validation in Cattleya sp. In Vitro
2.4. Statistics
3. Results
3.1. Chemical Composition of Hydrocolloids
3.2. Agar Substitute Stability
3.3. Mechanical Properties and Gel Characterization
3.4. The Functionality of Culture Mediums in Cattleya sp.
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TPA | Texture Profile Analysis |
| araf | Arabinofuranosyl |
| GalA | Galacturonic Acid |
| Ofip | Opuntia ficus indica pectin |
| Ofim | Opuntia ficus indica mucilage |
| Orp | Opuntia robusta pectin |
| Orm | Opuntia robusta mucilag |
| FTIR | Fourier Transform Infrared |
References
- Salazar, M.S.A.; Osorio, J.Y.M. Inclusion of organic components in culture medium to improve the in vitro propagation of Cattleya warscewiczii and Cattleya gaskelliana. South Afr. J. Bot. 2022, 148, 352–359. [Google Scholar] [CrossRef]
- Mayo-Mosqueda, A.; García-Hernández, E.; Noguera-Savelli, E.; Cetzal-Ix, W.; Alatorre-Cobos, F. Advances in Breeding, Bioprospecting, and In Vitro Culture of Laelia Orchid Species. Horticulturae 2022, 8, 103. [Google Scholar] [CrossRef]
- Mpatani, F.M.; Vuai, S.A.H. Performance of Low-Cost agar from Gracilaria salicornia on Tissue Culture of Pleurotus HK-37. Sci. World J. 2019, 2019, 2565692. [Google Scholar] [CrossRef]
- Magarelli, R.A.; Trupo, M.; Ambrico, A.; Larocca, V.; Martino, M.; Palazzo, S.; Balducchi, R.; Joutsjoki, V.; Pihlanto, A.; Bevivino, A. Designing a Waste-Based Culture Medium for the Production of Plant Growth Promoting Microorganisms Based on Cladodes Juice from Opuntia ficus-indica Pruning. Fermentation 2022, 8, 225. [Google Scholar] [CrossRef]
- Sun, J.; Ren, F.; Chang, Y.; Wang, P.; Li, Y.; Zhang, H.; Luo, J. Formation and structural properties of acid-induced casein–Agar double networks: Role of gelation sequence. Food Hydrocoll. 2018, 85, 291–298. [Google Scholar] [CrossRef]
- Öztürk-Kerimoğlu, B. A promising strategy for designing reduced-fat model meat emulsions by utilization of pea protein-Agar Agar gel complex. Food Struct. 2021, 29, 100205. [Google Scholar] [CrossRef]
- López-Escamilla, A.L.; López-Herrera, M.; Loaiza-Alanís, C. Efecto de diferentes agentes gelificantes en la germinación y desarrollo in vitro de plántulas de Echinocactus platyacanthus link et otto (cactaceae). Polibotánica 2016, 42, 153–166. [Google Scholar] [CrossRef] [Green Version]
- Pandya, Y.H.; Bakshi, M.; Sharma, A. Agar-Agar extraction, structural properties and applications: A review. Pharma Innov. 2022, 11, 1151–1157. Available online: https://www.thepharmajournal.com/archives/2022/vol11issue6S/PartO/S-11-6-106-220.pdf (accessed on 6 January 2023).
- Madera-Santana, T.J.; Herrera-Méndez, C.H.; Rodríguez-Núñez, J.R. An overview of the chemical modifications of chitosan and their advantages. Green Mater. 2018, 6, 131–142. [Google Scholar] [CrossRef] [Green Version]
- Gallegos-Villela, R.R.; Larrea-Zambrano, F.D.; Goyes-Lopez, C.E.; Perez-Sanchez, J.F.; Suarez-Dominguez, E.J.; Palacio-Perez, A. Effect of natural additives on concrete mechanical properties. Cogent Eng. 2021, 8, 1870790. [Google Scholar] [CrossRef]
- López-Díaz, A.S.; Méndez-Lagunas, L.L. Mucilage-Based film for applications. Food Rev. Int. 2022, 58, 6. [Google Scholar] [CrossRef]
- Taheri, A.; Jafari, S.M. Gum-based nanocarriers for the protection and delivery of food bioactive compounds. Adv. Colloid Interface Sci. 2019, 269, 277–295. [Google Scholar] [CrossRef] [PubMed]
- Otálora, M.C.; Wilches-Torres, A.; Gómez Castaño, J.A. Spray-Drying Microencapsulation of Pink Guava (Psidium guajava) Carotenoids Using Mucilage from Opuntia ficus-indica Cladodes and Aloe Vera Leaves as Encapsulating Materials. Polymers 2022, 14, 310. [Google Scholar] [CrossRef]
- Otálora, M.C.; Wilches-Torres, A.; Lara, C.R.; Cifuentes, G.R.; Gómez Castaño, J.A. Use of Opuntia ficus-indica Fruit Peel as a Novel Source of Mucilage with Coagulant Physicochemical/Molecular Characteristics. Polymers 2022, 14, 3832. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, C.D.; Pongener, A. Use of low cost agar alternative for in vitro propagation of commercially viable orchids is an attractive way for commercialization. South Afr. J. Bot. 2022, 150, 789–796. [Google Scholar] [CrossRef]
- Djekrif-Dakhmouche, S.; Gheribi-Aoulmi, Z.; Meraihi, Z.; Bennamoun, L. Application of a statistical design to the optimization of culture medium for α-amylase production by Aspergillus niger ATCC 16404 grown on orange waste powder. J. Food Eng. 2006, 73, 190–197. [Google Scholar] [CrossRef]
- Navarro, Q.R.; de Oliveira Corrêa, D.; Behling, A.; Noseda, M.D.; Ribas, L.L.F. Effect of microalga Desmodesmus subspicatus and plant growth regulators on the in vitro propagation of Cattleya warneri. Plant Cell Tissue Organ Cult. (PCTOC) 2023, 1–13. [Google Scholar] [CrossRef]
- Guadarrama-Lezama, A.Y.; Castaño, J.; Velázquez, G.; Carrillo-Navas, H.; Alvarez-Ramírez, J. Effect of nopal mucilage addition on physical, barrier and mechanical properties of citric pectin-based films. J. Food Sci. Technol. 2018, 55, 3739–3748. [Google Scholar] [CrossRef] [PubMed]
- Islam, F.; Wong, S.Y.; Li, X.; Arafat, M.T. Pectin and mucin modified cellulose-based superabsorbent hydrogel for controlled curcumin release. Cellulose 2022, 29, 5207–5222. [Google Scholar] [CrossRef]
- Stubley, S.J.; Cayre, O.J.; Murray, B.S.; Torres, I.C. Pectin-based microgels for rheological modification in the dilute to concentrated regimes. J. Colloid Interface Sci. 2022, 628, 684–695. [Google Scholar] [CrossRef]
- Stavi, I. Ecosystem services related with Opuntia ficus-indica (prickly pear cactus): A review of challenges and opportunities. Agroecol. Sustain. Food Syst. 2022, 46, 815–841. [Google Scholar] [CrossRef]
- Maceda, A.; Soto-Hernández, M.; Peña-Valdivia, C.B.; Trejo, C.; Terrazas, T. Characterization of lignocellulose of Opuntia (Cactaceae) species using FTIR spectroscopy: Possible candidates for renewable raw material. Biomass Convers. Biorefinery 2022, 12, 5165–5174. [Google Scholar] [CrossRef]
- Sánchez, M.; Corella, R. extracción, identificación y prueba microbiológica del agar extraído de gracilaria fortissima dawson (rhodophyta, gigartinales, gracilariaceae). Uniciencia 2008, 22, 99–106. [Google Scholar]
- Sánchez-Gutiérrez, A.E.; Soto-Zarazúa, G.M.; Rodríguez-González, S. Revalorización de Pencas Mermadas de Opuntia Mediante la Obtención de Hidrocoloides: Una Alternativa en Pymes Nopaleras. Revista Internacional de Investigación e Innovación Tecnológica. 2022. Available online: https://riiit.com.mx/apps/site/idem.php?module=Catalog&action=ViewItem&id=5039&item_id=85483&id=50394 (accessed on 6 January 2023).
- Goycoolea, F.M.; Cárdenas, A. Pectins from Opuntia spp.: A Short Review. J. Prof. Assoc. Cactus Dev. 2003, 5, 17–29. [Google Scholar]
- Fernandes, F.M.; Pais, S.S.; Neves, M.C. The preservation of Goodyera macrophylla Lowe by in vitro germination. Bol. Mus. Mun. Funchal 1999, 51, 43–52. Available online: https://publications.cm-funchal.pt/jspui/bitstream/100/878/1/Bolmmf-1999-art295.pdf (accessed on 6 January 2023).
- Foschi, M.L.; Juan, M.; Pascual, B.; Pascual-Seva, N. Influence of Lighting and Laser Irradiation on the Germination of Caper Seeds. Agriculture 2022, 12, 1612. [Google Scholar] [CrossRef]
- Zettler, L.W.; Dvorak, C.J. Tulasnella calospora Retains its effectiveness at facilitating orchid symbiotic germination in vitro after two decades of subculturing. Bot. Stud. 2021, 62, 14. [Google Scholar] [CrossRef]
- Jain, R.; Babbar, S.B. Xanthan gum: An economical substitute for Agar in plant tissue culture media. Plant Cell Rep. 2006, 25, 81–84. [Google Scholar] [CrossRef]
- Bayar, N.; Bouallegue, T.; Achour, M.; Kriaa, M.; Bougatef, A.; Kammoun, R. Ultrasonic extraction of pectin from Opuntia ficus indica cladodes after mucilage removal: Optimization of experimental conditions and evaluation of chemical and functional properties. Food Chem. 2017, 235, 275–282. [Google Scholar] [CrossRef]
- Hickson, T.G.L.; Polson, A. Some physical characteristics of the Agarose molecule. Biochim. Biophys. Acta 1968, 165, 43–58. [Google Scholar] [CrossRef]
- Armisén, R.; Gaiatas, F. Agar. In Handbook of Hydrocolloids, 2nd ed.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Sawston, UK, 2009; pp. 82–107. [Google Scholar] [CrossRef]
- Zale, P.J.; Clayton, A.; Nix, J.; Taylor, M. Asymbiotic in vitro seed germination, in vitro seedling development, and ex vitro acclimatization of Spiranthes. Appl. Plant Sci. 2020, 10, e11494. [Google Scholar] [CrossRef] [PubMed]
- Sidana, A.; Farooq, U. Sugarcane bagasse: A potential medium for fungal cultures. Chin. J. Biol. 2014, 2014, 840505. [Google Scholar] [CrossRef] [Green Version]
- Zdunek, A.; Pieczywek, P.M.; Cybulska, J. The primary, secondary, and structures of higher levels of pectin polysaccharides. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1101–1117. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Padilla, M.; Rodríguez-García, M.E.; Gutiérrez-Cortez, E.; del Carmen Valderrama-Bravo, M.; Rojas-Molina, J.I.; Rivera-Muñoz, E.M. Physicochemical and rheological characterization of Opuntia ficus mucilage at three different maturity stages of cladode. Eur. Polym. J. 2016, 78, 226–234. [Google Scholar] [CrossRef]
- Hernández-Becerra, E.; de los Angeles Aguilera-Barreiro, M.; Contreras-Padilla, M.; Pérez-Torrero, E.; Rodriguez-Garcia, M.E. Nopal cladodes (Opuntia ficus-indica): Nutritional properties and functional potential. J. Funct. Foods 2022, 95, 105183. [Google Scholar] [CrossRef]
- De Andrade Vieira, É.; Alcântara, M.A.; Dos Santos, N.A.; Gondim, A.D.; Iacomini, M.; Mellinger, C.; de Magalhães Cordeiro, A.M.T. Mucilages of cacti from Brazilian biodiversity: Extraction, physicochemical and technological properties. Food Chem. 2021, 346, 128892. [Google Scholar] [CrossRef]
- Asharuddin, S.M.; Othman, N.; Altowayti, W.A.H.; Bakar, N.A.; Hassan, A. Recent advancement in starch modification and its application as water treatment agent. Environ. Technol. Innov. 2021, 23, 101637. [Google Scholar] [CrossRef]
- Wang, T.; Park, Y.B.; Cosgrove, D.J.; Hong, M. Cellulose-pectin spatial contacts are inherent to never-dried Arabidopsis primary cell walls: Evidence from solid-state nuclear magnetic resonance. Plant Physiol. 2015, 168, 871–884. [Google Scholar] [CrossRef] [Green Version]
- Shekhar, C.N. An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol. Lett. 1999, 170, 265–270. [Google Scholar] [CrossRef]
- Khin, M.N.; Goff, H.D.; Nsor-Atindana, J.; Ahammed, S.; Liu, F.; Zhong, F. Effect of texture and structure of polysaccharide hydrogels containing maltose on release and hydrolysis of maltose during digestion: In vitro study. Food Hydrocoll. 2021, 112, 106326. [Google Scholar] [CrossRef]
- Wang, W.; Ma, X.; Jiang, P.; Hu, L.; Zhi, Z.; Chen, J.; Ding, T.; Liu, D. Characterization of pectin from grapefruit peel: A comparison of ultrasound-assisted and conventional heating extractions. Food Hydrocoll. 2016, 61, 730–739. [Google Scholar] [CrossRef]
- Nishinari, K.; Turcanu, M.; Nakauma, M.; Fang, Y. Role of fluid cohesiveness in safe swallowing. Sci. Food 2019, 3, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiao, D.; Shi, W.; Luo, M.; Hu, W.; Huang, Y.; Jiang, F.; Xie, F.; Zhang, B. Increasing xanthan gum content could enhance the performance of Agar/konjac glucomannan-based system. Food Hydrocoll. 2022, 132, 107845. [Google Scholar] [CrossRef]
- Al-Mayahi, A.M.W. The effect of humic acid (HA) and zinc oxide nanoparticles (ZnO-NPS) on in vitro regeneration of date palm (Phoenix dactylifera L.) cv. Quntar. Plant Cell Tissue Organ Cult. 2021, 145, 445–456. [Google Scholar] [CrossRef]
- Cardozo, J.A.S.; Pardo, R.Y.R.; Carvajal, M.X.Q.; Gonzalez, L.A.A. Evaluación de mezclas de agentes gelificantes como potenciales sustitutos del agar bacteriológico: Una aproximación mediante diseño de mezclas/Evaluating gelling-agent mixtures as potential substitutes for bacteriological agar: An approach by mixture design. Dyna 2019, 86, 171. [Google Scholar] [CrossRef]
- Quintero-Ramirez, M.; Martínez-Osorio, J.; Mujica-Niño, A.; Moreno-Quintero, M. Evaluación del efecto gelificante del agar de Gracilaria debilis en la elaboración de una salsa a base de tomate. Tecnol. Química 2020, 40, 690–704. Available online: http://scielo.sld.cu/scielo.php?pid=S2224-61852020000300690&script=sci_arttext&tlng=pt (accessed on 16 January 2023).
- Montecino, V.; Bahamonde, N.; Vásquez, J.A. Reseña de Alfredo Hipólito Llaña Garín (1913–1971): Primer ficólogo marino chileno. Rev. Biol. Mar. Y Oceanogr. 2021, 56, i–v. [Google Scholar] [CrossRef]
- Zheng, Z.; Bian, S.; Li, Z.; Zhang, Z.; Liu, Y.; Zhai, X.; Pan, H.; Zhao, X. Catechol modified quaternized chitosan enhanced wet adhesive and antibacterial properties of injectable thermo-sensitive hydrogel for wound healing. Carbohydr. Polym. 2020, 249, 116826. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, F.; He, J.; Xu, L.; Wang, T.; Feng, Z.-Q.; Zheng, J. Multiple Physical Bonds to Realize Highly Tough and Self-Adhesive Double-Network Hydrogels. ACS Appl. Polym. Mater. 2020, 2, 1031–1042. [Google Scholar] [CrossRef]
- López-Ramírez, A.M.; Duarte-Sierra, A. Avocado jelly: Formulation and optimization of an avocado gel using hydrocolloids. Int. J. Gastron. Food Sci. 2020, 21, 100234. [Google Scholar] [CrossRef]
- Paredes, J.E.M.; Castillo-González, A.M.; Valdéz-Aguilar, L.A.; Avitia-García, E.; del Rosario García-Mateos, M. Efecto de diferentes relaciones de luz azul: Roja en el crecimiento de plántulas de chile habanero (Capsicum chinense Jacq.). Biotecnia 2022, 24, 87–96. [Google Scholar] [CrossRef]
- Chirug, L.; Nagar, E.E.; Okun, Z.; Shpigelman, A. Effect of flavonoid structure and pH on iron-mediated pectin interaction. Food Hydrocoll. 2021, 116, 106654. [Google Scholar] [CrossRef]



| Treatments | Hardness | Hardness 2 | Cohesiveness | Adhesive Strength | Adhesive | Gumminess | Elasticity |
|---|---|---|---|---|---|---|---|
| Units | G | G | N | g/cm2 | MJ | G | mm |
| TC | 96.33 ± 0.07 e | 30.16 ± 1.41 e | 0.35 ± 0.16 a | 5.0 ± 0.70 c | 0.05 ± 0.03 a | 64.40 ± 0.88 b | 1.87 ± 0.04 a |
| T3 | 72.0 ± 0.86 f | 23.0 ± 3.53 f | 0.11 ± 0.03 d | 5.33 ± 1.06 c | 0.09 ± 0.04 a | 32.36 ± 1.13 d | 1.48 ± 0.21 c |
| T4 | 163.33 ± 0.70 c | 41.50 ± 1.06 d | 0.29 ± 0.16 a | 6.33 ± 3.01 c | 0.05 ± 0.03 a | 52.2 ± 9.26 b | 1.52 ± 0.12 c |
| T7 | 44.33 ± 1.44 h | 15.0 ± 0 i | 0.30 ± 0.27 a | 3.0 ± 1 d | 0.05 ± 0.04 a | 19.76 ± 4.45 e | 1.03 ± 0.27 f |
| T8 | 225.33 ± 2.82 b | 99.66 ± 0.70 a | 0.33 ± 0.13 a | 6.83 ± 0.70 b | 0.10 ± 0.06 a | 145.53 ± 14.56 a | 1.53 ± 0.01 d |
| T11 | 54.16 ± 0.35 g | 17.83 ± 0.28 h | 0.27 ± 0.05 a | 4.0 ± 0.5 cd | 0.06 ± 0.01 a | 37.5 ± 0.92 c | 1.59 ± 0.13 c |
| T12 | 133.66 ± 3.21 d | 71.50 ± 0.5 c | 0.28 ± 0.14 ad | 5.5 ± 1.32 c | 0.06 ± 0.01 a | 110.4 ± 35.63 a | 1.47 ± 0.14 c |
| T15 | 33.83 ± 3.88 i | 20.33 ± 1.44 g | −0.20 ± 0.55 c | 4.5 ± 1.32 c | 0.11 ± 0.05 a | −18.7 ± 0.49 b | 1.20 ± 0.11 e |
| T16 | 333.16 ± 2.47 a | 60.83 ± 0.70 b | 0.18 ± 0.005 b | 10.6 ± 1.41 a | 0.06 ± 0.01 a | 150.03 ± 6.29 a | 1.73 ± 0.08 b |
| Variable | Units | TC (Mean) | Variance | T3 (Mean) | Variance | F Value |
|---|---|---|---|---|---|---|
| NL | Portion | 1.33 | 0.33 | 3.66 | 0.066 | 24.5 |
| NR | Portion | 1 | 0.001 | 4.25 | 0.025 | 14.25 |
| RL | cm | 1.53 | 0.003 | 2.53 | 0.023 | 112.5 |
| SH | cm | 3.73 | 0.41 | 5.97 | 0.13 | 27.48 |
| LW | cm | 0.46 | 0.003 | 0.66 | 0.003 | 18 |
| LL | cm | 1.09 | 0.009 | 1.53 | 0.09 | 5.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Gutiérrez, A.E.; Soto-Zarazúa, G.M.; España-Sánchez, B.L.; Rodríguez-González, S.; Zamora-Castro, S. Development of Agar Substitute Formulated with Mucilage and Pectin from Opuntia Local Waste Matter for Cattleya sp. Orchids In Vitro Culture Media. Processes 2023, 11, 717. https://doi.org/10.3390/pr11030717
Sánchez-Gutiérrez AE, Soto-Zarazúa GM, España-Sánchez BL, Rodríguez-González S, Zamora-Castro S. Development of Agar Substitute Formulated with Mucilage and Pectin from Opuntia Local Waste Matter for Cattleya sp. Orchids In Vitro Culture Media. Processes. 2023; 11(3):717. https://doi.org/10.3390/pr11030717
Chicago/Turabian StyleSánchez-Gutiérrez, Arantza Elena, Genaro Martín Soto-Zarazúa, Beatriz Liliana España-Sánchez, Sarahí Rodríguez-González, and Sergio Zamora-Castro. 2023. "Development of Agar Substitute Formulated with Mucilage and Pectin from Opuntia Local Waste Matter for Cattleya sp. Orchids In Vitro Culture Media" Processes 11, no. 3: 717. https://doi.org/10.3390/pr11030717