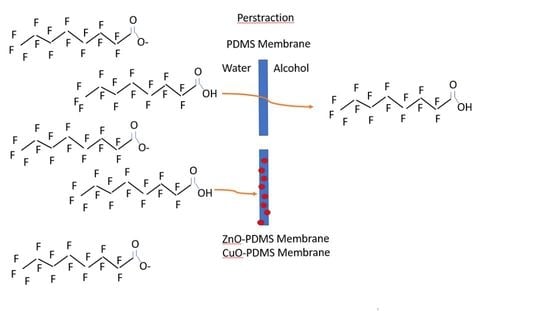
Perstraction: A Membrane-Assisted Liquid–Liquid Extraction of PFOA from Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Analytical Methods
2.3. Experimental Approach
2.3.1. Perstraction Test System
2.3.2. Partition Coefficients for PFOA in Selected Solvents
2.3.3. Determine the Solubility and Diffusivity of the Solvents in PDMS
2.3.4. Determine the Uptake of PFOA in PDMS
2.3.5. Determine the Effects of Particles Imbedded in the PDMS Membrane on PFOA Uptake and Solvent Absorption
2.3.6. Demonstrate the Perstraction Process for Removing PFOA from Water
3. Results
3.1. Partition Coefficients of PFOA in Selected Solvents
3.2. Determine the Solubility and Diffusivity of the Solvents in PDMS with and without Imbedded Particles
3.3. Absorption of PFOA in PDMS Membranes with and without Imbedded Particles
3.4. Perstraction of PFOA from Water to Solvent
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- EPA. Actions to Address PFAS|US EPA. Available online: https://www.epa.gov/pfas/epa-actions-address-pfas#:~:text=In%20May%202022%2C%20EPA%20took,or%20remediation%20activities%20are%20needed (accessed on 30 November 2022).
- Buck, R.C.; Franklin, J.; Berger, U.; Conder, J.M.; Cousins, I.T.; de Voogt, P.; Jensen, A.A.; Kannan, K.; Mabury, S.A.; van Leeuwenkk, S.P.J. Perfluoroalkyl and polyfluoroalkyl substances in the environment: Terminology, classification, and origins. Integr. Environ. Assess. Manag. 2008, 7, 513–541. [Google Scholar] [CrossRef]
- Lange, F.T.; Schmidt, C.; Brauch, H.-J. Perfluoroalkylcarboxylates and Sulfonates: Emerging Contaminants for Drinking Water Supplies? Association of River Waterworks—RIWA: Cranston, RI, USA, 2006; ISBN-10: 90-6683-116-2; ISBN-13: 978-90-6683-116-2; Available online: https://www.riwa-rijn.org/wp-content/uploads/2015/05/137_ptfe_report.pdf (accessed on 21 December 2022).
- Bartel, C.M. Occurrence and Distribution of Perfluorinated Surfactants in Groundwater Contaminated by Fire-Fighting Activity. Ph.D. Thesis, Oregon State University, Corvallis, OR, USA, 23 November 1999. Available online: https://www.proquest.com/docview/275713653?pq-origsite=gscholar&fromopenview=true (accessed on 21 December 2022).
- Post, G.B.; Cohn, P.D.; Cooper, K.R. Perfluorooctanoic acid (PFOA), an emerging drinking water contaminant: A critical review of recent literature. Environ. Res. 2012, 116, 93–117. [Google Scholar] [CrossRef] [PubMed]
- Houde, M.; Martin, J.W.; Letcher, R.J.; Solomon, K.R.; Muir, D.G. Biological monitoring of polyfluoroalkyl substances: A review. Environ. Sci. Technol. 2006, 40, 3463–3473. [Google Scholar] [CrossRef] [PubMed]
- Armitage, J.M.; Macleod, M.; Cousins, I.T. Comparative assessment of the global fate and transport of long-chain perfluorocarboxylic acids (PFCAs) and perfluorocarboxylates (PFCs) emitted from direct sources. Environ. Sci. Technol. 2009, 43, 5830–5836. [Google Scholar] [CrossRef] [PubMed]
- Muller, C.E.; Gerecke, A.C.; Bogdal, C.; Wang, Z.; Scheringer, M.; Hungerbuhler, K. Atmospheric fate of poly- and perfluorinated alkyl substances (PFCSs): I. Day-night patterns of air concentrations in summer in Zurich, Switzerland. Environ. Pollut. 2012, 169, 196–203. [Google Scholar] [CrossRef]
- Rayne, S.; Forest, K. Modeling the hydrolysis of perfluorinated compounds containing carboxylic and phosphoric acid ester functions and sulfonamide groups. J. Environ. Sci. Health Part A 2010, 45, 432–446. [Google Scholar] [CrossRef]
- Vecitis, C.D.; Park, H.; Cheng, J.; Mader, B.T.; Hoffmann, M.R. Treatment technologies for aqueous perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA). Environ. Sci. Eng. China 2009, 3, 129–151. [Google Scholar] [CrossRef]
- Available online: http://www.fluoridealert.org/pesticides/2005/effect.pfos.class.news.147.html (accessed on 30 November 2022).
- Merino, N.; Qu, Y.; Deeb, R.A.; Hawley, E.L.; Hoffmann, M.R.; Mahendra, S. Degradation and removal methods for perfluoroalkyl and polyfluoroalkyl substances in water. Environ. Eng. Sci. 2016, 33, 615–649. [Google Scholar] [CrossRef] [Green Version]
- McNamara, J.D.; Franco, R.; Mimna, R.; Zappa, L. Comparison of activated carbons for removal of perfluorinated compounds from drinking water. J.-Am. Water Work. Assoc. 2018, 110, E2–E14. [Google Scholar] [CrossRef]
- Subramanian, N.H.; Manigandan, P.; Wille, A.; Radhakrishnan, G. Determination of perfluorooctanoate and perfluorooctanesulfonate in water matrices by inline matrix elimination liquid chromatography with reversed phase separation and suppressed conductivity detection. J. Chromatogr. Sci. 2011, 49, 603–609. [Google Scholar] [CrossRef]
- Meng, P.; Deng, S.; Du, Z.; Wang, B.; Huang, J.; Wang, Y.; Yu, G.; Xing, B. Effect of hydro-oleophobic perfluorocarbon chain on interfacial behavior and mechanism of perfluorooctane sulfonate in oil-water mixture. Sci. Rep. Mar 2017, 16, 44694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EPA. Announces New Drinking Water Health Advisories for PFAS Chemicals, $1 Billion in Bipartisan Infrastructure Law Funding to Strengthen Health Protections|US EPA. Available online: https://www.epa.gov/newsreleases/epa-announces-new-drinking-water-health-advisories-pfas-chemicals-1-billion-bipartisan (accessed on 21 December 2022).
- Jimenez-Bonilla, P.; Wang, Y. In-situ biobutanol recovery from clostridial fermentations: A critical review. Crit. Rev. Biotechnol. 2018, 38, 469–482. [Google Scholar] [CrossRef] [PubMed]
- Outram, V.; Lalander, C.A.; Lee, J.G.M.; Davies, E.T.; Harvey, A.P. Applied in Situ Product Recovery in ABE Fermentation. Biotechnol. Prog. 2017, 33, 563–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, H.J.; Ramaswamy, S.; Liu, Y.Y. Separation and purification of biobutanol during bioconversion of biomass. Sep. Purif. Technol. 2014, 132, 513–540. [Google Scholar] [CrossRef]
- Abdehagh, N.; Tezel, F.H.; Thibault, J. Separation techniques in butanol production: Challenges and developments. Biomass Bioenergy 2014, 60, 222–246. [Google Scholar] [CrossRef]
- Qureshi, N.; Ezeji, T.C. Butanol, ‘a superior biofuel’ production from agricultural residues (renewable biomass): Recent progress in technology. Biofuels Bioprod. Biorefining 2008, 2, 319–330. [Google Scholar] [CrossRef]
- Longo, R.; Blackman, J.W.; Torley, P.J.; Rogiers, S.Y.; Schmidtke, L.M. Changes in volatile composition and sensory attributes of wines during alcohol content reduction. J. Sci. Food Agric. 2017, 97, 8–16. [Google Scholar] [CrossRef]
- Park, C.H.; Geng, Q.H. Simultaneous fermentation and separation in the ethanol and ABE fermentation. Sep. Purif. Methods 1992, 21, 127–174. [Google Scholar] [CrossRef]
- Sirkar, K.K. Membrane separation technologies: Current developments. Chem. Eng. Commun. 1997, 157, 145–184. [Google Scholar] [CrossRef]
- Merlet, G.; Uribe, F.; Aravena, C.; Rodriguez, M.; Cabezas, R.; Quijada-Maldonado, E.; Romero, J. Separation of fermentation products from ABE mixtures by perstraction using hydrophobic ionic liquids as extractants. J. Membr. Sci. 2017, 537, 337–343. [Google Scholar] [CrossRef]
- Qureshi, N.; Maddox, I.S. Reduction in butanol inhibition by perstraction: Utilization of concentrated lactose/whey permeate by Clostridium acetobutylicum to enhance butanol fermentation economics. Food Bioprod. Process. 2005, 83, 43–52. [Google Scholar] [CrossRef] [Green Version]
- Sam, F.E.; Ma, T.Z.; Salifu, R.; Wang, J.; Jiang, Y.M.; Zhang, B.; Han, S.Y. Techniques for dealcoholization of wines: Their impact on wine phenolic composition, volatile composition, and sensory characteristics. Foods 2021, 10, 2498. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.K.; Sawant, S.B.; Joshi, J.B.; Pangarkar, V.G. Perstraction of phenolic compounds from aqueous solution using a nonporous membrane. Sep. Sci. Technol. 1997, 32, 2669–2683. [Google Scholar] [CrossRef]
- Whelehan, M.; Marison, I.W. Capsular perstraction as a novel methodology for the recovery and purification of geldanamycin. Biotechnol. Prog. 2011, 27, 1068–1077. [Google Scholar] [CrossRef]
- Whelehan, M.; von Stockar, U.; Marison, I.W. Removal of pharmaceuticals from water: Using liquid-core microcapsules as a novel approach. Water Res. 2010, 44, 2314–2324. [Google Scholar] [CrossRef]
- Sylgard 184 Technical Data Sheet, Form No. 11-3184-01 C. (Dow Chemical Company, Midland, MI, USA). Available online: https://www.dow.com/content/dam/dcc/documents/en-us/productdatasheet/11/11-31/11-3184-sylgard-184-elastomer.pdf (accessed on 21 December 2022).
- Burns, D.C.; Ellis, D.A.; Li, H.; McMurdo, C.J.; Webster, E. Experimental pKa Determination for perfluorooctanoic acid (PFOA) and the potential impact of pKa concentration dependence on laboratory-measured partitioning phenomena and environmental modeling. Environ. Sci. Technol. 2008, 42, 9283–9288. [Google Scholar] [CrossRef]
- Available online: https://oxfordobserver.org/6065/community/city-council-hears-report-on-water-softening-solutions/ (accessed on 30 November 2022).
- Liu, J.; Zhen, X.J.; Tang, K.Y. Study on the gravimetric measurement of the swelling behaviors of polymer films. Rev. Adv. Mater. Sci. 2013, 33, 452–458. Available online: https://www.ipme.ru/e-journals/RAMS/no_53313/11_533_liu.pdf (accessed on 21 December 2022).
- Crank, J. The Mathematics of Diffusion; Oxford University Press: London, UK, 1956. [Google Scholar]
- Wang, J.; Guo, X. Adsorption kinetic models: Physical meanings, applications, and solving methods. J. Hazard. Mater. 2020, 390, 122156. [Google Scholar] [CrossRef]
- Wolf, M.P.; Salieb-Beugelaar, G.B.; Hunziker, P. PDMS with designer functionalities—Properties, modifications strategies, and applications. Prog. Polym. Sci. 2018, 83, 97–134. [Google Scholar] [CrossRef]
- Cocchi, G.; Grazia De Angelis, M.; Doghieri, F. Solubility and diffusivity of liquids for food and pharmaceutical applications in crosslinked polydimethylsiloxane (PDMS) films: I. Experimental data on pure organic components and vegetable oil. J. Membr. Sci. 2015, 492, 600–611. [Google Scholar] [CrossRef]
- Lubani, J.; De Angelis, F.; Meggiolaro, D.; Cartechini, L.; Fantacci, S. Modelling the interaction between carboxylic acids and zinc oxide: Insight into degradation of ZnO pigments. Molecules 2022, 27, 3362. [Google Scholar] [CrossRef] [PubMed]
- Segovia, M.; Lemus, K.; Moreno, M.; Santa Ana, M.A.; González, G.; Ballesteros, B.; Sotomayor, C.; Benavente, E. Zinc oxide/carboxylic acid lamellar structures. Mater. Res. Bull. 2011, 46, 2191–2195. [Google Scholar] [CrossRef]
- Shih, K.; Wang, F. Adsorption behavior of perfluorochemicals (PFCs) on boehmite: Influence of solution chemistry. Procedia Environ. Sci. 2013, 18, 106–113. [Google Scholar] [CrossRef] [Green Version]
- Available online: https://pfas-1.itrcweb.org/5-environmental-fate-and-transport-processes/ (accessed on 22 December 2022).
- Heerah, K.; Waclawek, S.; Konzuk, J.; Longstaffe, J.G. Benchtop19F NMR spectroscopy as a practical tool for testing of remedial technologies for the degradation of perfluorooctanoic acid, a persistent organic pollutant. Magn. Reson. Chem. 2020, 58, 1160–1167. [Google Scholar] [CrossRef] [PubMed]












| Experiment | Trial #1 | Trial #2 | Trial #3 |
|---|---|---|---|
| Test System 1 | |||
| Initial [PFOA] | 1000 mg/L PFOA | 350 mg/L PFOA | 500 mg/L PFOA |
| LHS | DI water | DI water | DI water |
| RHS | DI water | DI water | 1-hexanol |
| Membrane | PDMS | PDMS | PDMS |
| Test System 2 | |||
| Initial [PFOA] | 1000 mg/L PFOA | 350 mg/L PFOA | 500 mg/L PFOA |
| LHS | DI water | DI water | Tap water |
| RHS | DI water | 1-butanol | 1-hexanol |
| Membrane | 0.05 CuO-PDMS | PDMS | PDMS |
| Test System 3 | |||
| Initial [PFOA] | 1000 mg/L PFOA | 350 mg/L PFOA | 500 mg/L PFOA |
| LHS | DI water | DI water | DI water |
| RHS | DI water | 1-hexanol | 1-hexanol |
| Membrane | 0.05 ZnO-PDMS | PDMS | 0.1 ZnO-PDMS |
| Test System 4 | |||
| Initial [PFOA] | 1000 mg/L PFOA | 350 mg/L PFOA | 500 mg/L PFOA |
| LHS | DI water | DI water | Tap water |
| RHS | DI water | 1-octanol | 1-hexanol |
| Membrane | 0.15 ZnO-PDMS | PDMS | 0.1 ZnO-PDMS |
| Solvent | Aqueous Phase Deionized Water | Aqueous Phase Tap Water 1 |
|---|---|---|
| Hexane | N/A | 0.118 |
| Cyclohexane | N/A | 0.003 |
| Benzene | N/A | 0.025 |
| 1-Butanol | 16.4 ± 14.5 | 71.6 ± 29.2 |
| 1-Hexanol | 27.0 ± 16.7 | 31.8 ± 14.9 |
| 1-Octanol | 12.4 ± 5.6 | 5.4 ± 6.5 |
| Solvent | Membrane Material | Solubility (kg/m3) | Diffusivity 1 (1011 m2/s) |
|---|---|---|---|
| 1-Butanol | PDMS | 108 ± 9.7 | 2.6 ± 1.6 |
| 5 wt% ZnO in PDMS | 100 ± 4.6 | 1.6 ± 0.3 | |
| 10 wt% ZnO in PDMS | 111 ± 6.9 | 1.3 ± 0.003 | |
| 15 wt% ZnO in PDMS | 84.1 ± 6.5 | 1.5 ± 0.03 | |
| 5 wt% CuO in PDMS | 116 ± 11.4 | 1.8 ± 0.02 | |
| 10 wt% CuO in PDMS | 104 ± 20.6 | 1.6 ± 0.10 | |
| 15 wt% CuO in PDMS | 108 ± 9.6 | 1.4 ± 0.37 | |
| 1-Hexanol | PDMS | 92.1 ± 12.2 | 1.5 ± 0.12 |
| 5 wt% ZnO in PDMS | 79.4 ± 4.4 | 1.5 ± 0.26 | |
| 10 wt% ZnO in PDMS | 83.1 ± 6.7 | 1.2 ± 0.01 | |
| 15 wt% ZnO in PDMS | 76.1 ± 0.8 | 1.4 ± 0.29 | |
| 5 wt% CuO in PDMS | 116 ± 1.0 | 1.4 ± 0.07 | |
| 10 wt% CuO in PDMS | 104 ± 5.1 | 1.6 ± 0.16 | |
| 15 wt% CuO in PDMS | 101 ± 5.1 | 1.4 ± 0.07 | |
| 1-Octanol | PDMS | 52.1 ± 0.58 | 3.3 ± 0.45 |
| 5 wt% ZnO in PDMS | 41.3 ± 4.5 | 3.9 ± 0.62 | |
| 10 wt% ZnO in PDMS | 40.4 ± 3.9 | 4.1 ± 0.22 | |
| 15 wt% ZnO in PDMS | 41.7 ± 6.7 | 3.4 ± 1.1 | |
| 5 wt% CuO in PDMS | 44.4 ± 15 | 4.8 ± 3.8 | |
| 10 wt% CuO in PDMS | 48.6 ± 7.4 | 2.4 ± 0.54 | |
| 15 wt% CuO in PDMS | 44.7 ± 3.1 | 2.5 ± 0.27 |
| Initial PFOA Concentration (mg/L) | 0.10 ZnO-PDMS | 0.10 CuO-PDMS | ||
|---|---|---|---|---|
| k2 (mg Membrane/mg PFOA/day) | qe (mg PFOA/mg Membrane) | k2 (mg Membrane/mg PFOA/day) | qe (mg PFOA/mg Membrane) | |
| 200 | 0.0142 | 36 | 0.0083 | 18 |
| 400 | 0.0054 | 95 | 0.00055 | 104 |
| 600 | 0.0029 | 145 | 0.00071 | 143 |
| 800 | 0.0021 | 193 | 0.00074 | 182 |
| 1000 | 0.0016 | 234 | 0.00059 | 239 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almquist, C.B.; Garza, L.; Flood, M.; Carroll, A.; Armstrong, R.; Chen, S.; Marcellino, C. Perstraction: A Membrane-Assisted Liquid–Liquid Extraction of PFOA from Water. Processes 2023, 11, 217. https://doi.org/10.3390/pr11010217
Almquist CB, Garza L, Flood M, Carroll A, Armstrong R, Chen S, Marcellino C. Perstraction: A Membrane-Assisted Liquid–Liquid Extraction of PFOA from Water. Processes. 2023; 11(1):217. https://doi.org/10.3390/pr11010217
Chicago/Turabian StyleAlmquist, Catherine B., Linda Garza, Megan Flood, Anne Carroll, Ryan Armstrong, Sean Chen, and Christopher Marcellino. 2023. "Perstraction: A Membrane-Assisted Liquid–Liquid Extraction of PFOA from Water" Processes 11, no. 1: 217. https://doi.org/10.3390/pr11010217