Fatal Hypernatremic Dehydration in a Term Exclusively Breastfed Newborn
Abstract
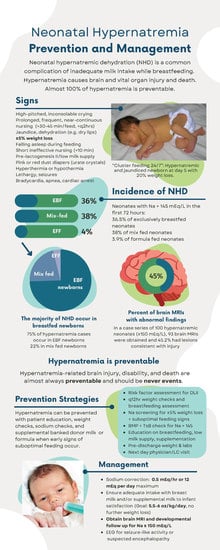
:1. Introduction
2. Case Report
3. Discussion
3.1. Mechanism of Disease
3.2. Risk Factors
3.3. Epidemiology
3.4. Signs and Symptoms
3.5. Long Term Sequelae
3.6. Prevention
3.7. Management
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferrández-González, M.; Bosch-Giménez, V.; López-Lozano, J.; Moreno-López, N.; Palazón-Bru, A.; Cortés-Castell, E. Weight loss thresholds to detect early hypernatremia in newborns. J. Pediatr. 2018, 95, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Uras, N.; Karadag, A.; Dogan, G.; Tonbul, A.; Tatli, M.M. Moderate hypernatremic dehydration in newborn infants: Retrospective evaluation of 64 cases. J. Matern. Neonatal Med. 2007, 20, 449–452. [Google Scholar] [CrossRef] [PubMed]
- Musapasaoglu, H.; Agildere, A.M.; TekSam, M.; Tarcan, A.; Gürakan, B. Hypernatraemic dehydration in a neonate: Brain MRI findings. Br. J. Radiol. 2008, 81, e57–e60. [Google Scholar] [CrossRef] [PubMed]
- Unal, S.; Arhan, E.; Kara, N.; Uncu, N.; Aliefendioğlu, D. Breast-feeding-associated hypernatremia: Retrospective analysis of 169 term newborns. Pediatrics Int. 2008, 50, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Ergenekon, E.; Unal, S.; Gücüyener, K.; Soysal, S.; Koç, E.; Okumus, N.; Türkyilmaz, C.; Onal, E.; Atalay, Y. Hypernatremic dehydration in the newborn period and long-term follow up. Pediatrics Int. 2007, 49, 19–23. [Google Scholar] [CrossRef]
- Meek, J.Y.; Noble, L.; Breastfeeding, S.O. Policy Statement: Breastfeeding and the Use of Human Milk. Pediatrics 2022, 150, e2022057988. [Google Scholar] [CrossRef]
- Ten Steps to Successful Breastfeeding. Available online: https://www.who.int/teams/nutrition-and-food-safety/food-and-nutrition-actions-in-health-systems/ten-steps-to-successful-breastfeeding (accessed on 9 August 2021).
- Jensen, D.; Wallace, S.; Kelsay, P. LATCH: A Breastfeeding charting system and documentation Tool. J. Obstet. Gynecol. Neonatal Nurs. 1994, 23, 27–32. [Google Scholar] [CrossRef]
- Shah, M.H.; Roshan, R.; Parikh, T.; Sathe, S.; Vaidya, U.; Pandit, A. LATCH Score at Discharge: A Predictor of Weight Gain and Exclusive Breastfeeding at 6 Weeks in Term Healthy Babies. J. Pediatric Gastroenterol. Nutr. 2021, 72, e48–e52. [Google Scholar] [CrossRef]
- Thach, B.T. Deaths and near deaths of healthy newborn infants while bed sharing on maternity wards. J. Perinatol. 2014, 34, 275–279. [Google Scholar] [CrossRef]
- Meena, A.; Singh, A.; Goyal, V.K.; Gupta, N.; Payal, V.; Chaturvedi, K. Brain Injury Patterns in Neonates with Hypernatremic De-hydration: Single Center Experience. Indian Pediatrics 2021, 58, 947–950. [Google Scholar] [CrossRef]
- Bolat, F.; Oflaz, M.B.; Güven, A.S.; Özdemir, G.; Alaygut, D.; Doğan, M.T.; Içağasoğlu, F.D.; Cevit, Ö.; Gültekin, A. What Is the Safe Approach for Neonatal Hypernatremic Dehydration? Pediatric Emerg. Care 2013, 29, 808–813. [Google Scholar] [CrossRef] [PubMed]
- Tarcan, A.; Tiker, F.; Vatandas, N.S.; Haberal, A.; Gurakan, B. Weight loss and hypernatremia in breast-fed babies: Frequency in neonates with non-hemolytic jaundice. J. Paediatr. Child Health 2005, 41, 484–487. [Google Scholar] [CrossRef] [PubMed]
- Yaseen, H.; Salem, M.; Darwich, M. Clinical presentation of hypernatremic dehydration in exclusively breast-fed neonates. Indian J. Pediatrics 2004, 71, 1059–1062. [Google Scholar] [CrossRef] [PubMed]
- Dewey, K.G.; Nommsen-Rivers, L.A.; Heinig, M.J.; Cohen, R.J. Risk Factors for Suboptimal Infant Breastfeeding Behavior, Delayed Onset of Lactation, and Excess Neonatal Weight Loss. Pediatrics 2003, 112 Pt 1, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Livingstone, V.H.; Willis, C.E.; Abdel-Wareth, L.O.; Thiessen, P.; Lockitch, G. Neonatal hypernatremic dehydration associated with breast-feeding malnutrition: A retrospective survey. Can. Med. Assoc. J. 2000, 162, 647–652. [Google Scholar]
- Gidrewicz, D.A.; Fenton, T.R. A systematic review and meta-analysis of the nutrient content of preterm and term breast milk. BMC Pediatrics 2014, 14, 216. [Google Scholar] [CrossRef]
- Neville, M.C.; Keller, R.; Seacat, J.; Lutes, V.; Neifert, M.; Casey, C.; Allen, J.; Archer, P. Studies in human lactation: Milk volumes in lactating women during the onset of lactation and full lactation. Am. J. Clin. Nutr. 1988, 48, 1375–1386. [Google Scholar] [CrossRef]
- FAO/WHO/UNU Expert Committee. Human Energy Requirements Report of a Joint FAO/WHO/UNU Expert Consultation. 17 October 2001. Available online: http://www.fao.org/3/y5686e/y5686e.pdf (accessed on 7 June 2021).
- Wight, N.; Marinelli, K.A.; Academy of Breastfeeding Medicine. ABM clinical protocol #1: Guidelines for blood glucose monitoring and treatment of hypoglycemia in term and late-preterm neonates, revised 2014. Breastfeed Med. 2014, 9, 173–179. [Google Scholar] [CrossRef]
- Futatani, T.; Ina, S.; Shimao, A.; Higashiyama, H.; Fujita, S.; Igarashi, N.; Hatasaki, K. Exclusive breast-feeding and postnatal changes in blood sodium, ketone, and glucose levels. Pediatrics Int. 2019, 61, 471–474. [Google Scholar] [CrossRef]
- Futatani, T.; Shimao, A.; Ina, S.; Higashiyama, H.; Fujita, S.; Ueno, K.; Igarashi, N.; Hatasaki, K. Capillary Blood Ketone Levels as an Indicator of Inadequate Breast Milk Intake in the Early Neonatal Period. J. Pediatrics 2017, 191, 76–81. [Google Scholar] [CrossRef]
- Bhutani, V.K.; Johnson, L.H. Newborn jaundice and kernicterus—Health and societal perspectives. Indian J. Pediatrics 2003, 70, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Seske, L.M.; Merhar, S.L.; Haberman, B.E. Late-Onset Hypoglycemia in Term Newborns with Poor Breastfeeding. Hosp. Pediatrics 2015, 5, 501–504. [Google Scholar] [CrossRef] [PubMed]
- Lavagno, C.; Camozzi, P.; Renzi, S.; Lava, S.A.G.; Simonetti, G.D.; Bianchetti, M.G.; Milani, G.P. Breastfeeding-Associated Hypernatremia. J. Hum. Lact. 2015, 32, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Matias, S.L.; Dewey, K.G.; Quesenberry, C.P.; Gunderson, E.P. Maternal prepregnancy obesity and insulin treatment during pregnancy are independently associated with delayed lactogenesis in women with recent gestational diabetes mellitus. Am. J. Clin. Nutr. 2014, 99, 115–121. [Google Scholar] [CrossRef]
- Salahudeen, M.S.; Koshy, A.M.; Sen, S. A study of the factors affecting time to onset of lactogenesis-II after parturition. J. Pharm. Res. 2013, 6, 68–72. [Google Scholar] [CrossRef]
- Chen, D.C.; Nommsen-Rivers, L.; Dewey, K.G.; Lönnerdal, B. Stress during labor and delivery and early lactation performance. Am. J. Clin. Nutr. 1998, 68, 335–344. [Google Scholar] [CrossRef]
- Chantry, C.J.; Nommsen-Rivers, L.A.; Peerson, J.M.; Cohen, R.J.; Dewey, K.G. Excess Weight Loss in First-Born Breastfed Newborns Relates to Maternal Intrapartum Fluid Balance. Pediatrics 2011, 127, e171–e179. [Google Scholar] [CrossRef]
- Nommsen-Rivers, L.A.; Chantry, C.J.; Peerson, J.M.; Cohen, R.J.; Dewey, K.G. Delayed onset of lactogenesis among first-time mothers is related to maternal obesity and factors associated with ineffective breastfeeding. Am. J. Clin. Nutr. 2010, 92, 574–584. [Google Scholar] [CrossRef]
- Preusting, I.; Brumley, J.; Odibo, L.; Spatz, D.L.; Louis, J. Obesity as a Predictor of Delayed Lactogenesis II. J. Hum. Lact. 2017, 33, 684–691. [Google Scholar] [CrossRef]
- Suliman, O.S.M. Dying for milk: A neonate with severe hypernatremia associated with inadequate breast feeding. Sudan J. Paediatr. 2015, 15, 55–62. [Google Scholar]
- Van Amerongen, R.H.; Moretta, A.C.; Gaeta, T.J. Severe hypernatremic dehydration and death in a breast-fed infant. Pediatric Emerg. Care 2001, 17, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Zaki, S.A.; Mondkar, J.; Shanbag, P.; Verma, R. Hypernatremic dehydration due to lactation failure in an exclusively breastfed neonate. Saudi J. Kidney Dis. Transplant. 2012, 23, 125–128. [Google Scholar]
- Neifert, M.; DeMarzo, S.; Seacat, J.; Young, D.; Leff, M.; Orleans, M. The influence of breast surgery, breast appearance, and pregnancy-induced breast changes on lactation sufficiency as measured by infant weight gain. Birth 1990, 17, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Kent, J.C.; Gardner, H.; Geddes, D.T. Breastmilk Production in the First 4 Weeks after Birth of Term Infants. Nutrients 2016, 8, 756. [Google Scholar] [CrossRef]
- Laing, I.A. Hypernatraemia in the first few days: Is the incidence rising? Arch. Dis. Child.-Fetal Neonatal Ed. 2002, 87, F158–F162. [Google Scholar] [CrossRef]
- PC-05. Available online: https://manual.jointcommission.org/releases/TJC2015B/MIF0170.html (accessed on 6 August 2022).
- Trotman, H.; Lord, C.; Barton, M.; Antoine, M. Hypernatraemic dehydration in Jamaican breastfed neonates: A 12-year review in a baby-friendly hospital. Ann. Trop. Paediatr. 2004, 24, 295–300. [Google Scholar] [CrossRef]
- Oddie, S.J.; Craven, V.; Deakin, K.; Westman, J.; Scally, A. Severe neonatal hypernatraemia: A population based study. Arch. Dis. Child.-Fetal Neonatal Ed. 2013, 98, F384–F387. [Google Scholar] [CrossRef]
- Moritz, M.L.; Manole, M.D.; Bogen, D.L.; Ayus, J.C. Breastfeeding-Associated Hypernatremia: Are We Missing the Diagnosis? Pediatrics 2005, 116, e343–e347. [Google Scholar] [CrossRef]
- Flaherman, V.J.; Schaefer, E.W.; Kuzniewicz, M.W.; Li, S.X.; Walsh, E.M.; Paul, I.M. Early Weight Loss Nomograms for Exclusively Breastfed Newborns. Pediatrics 2015, 135, e16–e23. [Google Scholar] [CrossRef]
- Flaherman, V.; Schaefer, E.W.; Kuzniewicz, M.W.; Li, S.X.; Walsh, E.M.; Paul, I.M. Health Care Utilization in the First Month After Birth and Its Relationship to Newborn Weight Loss and Method of Feeding. Acad. Pediatrics 2018, 18, 677–684. [Google Scholar] [CrossRef]
- Shroff, R.; Hignett, R.; Pierce, C.; Marks, S.; Hoff, W.V. Life-threatening hypernatraemic dehydration in breastfed babies. Arch. Dis. Child. 2006, 91, 1025–1026. [Google Scholar] [CrossRef] [PubMed]
- Yusupov, R.; Finegold, D.; Naylor, E.W.; Sahai, I.; Waisbren, S.; Levy, H.L. Sudden death in medium chain acyl-coenzyme a dehydrogenase deficiency (MCADD) despite newborn screening. Mol. Genet. Metab. 2010, 101, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Çaglar, M.; Özer, I.; Altugan, F. Risk factors for excess weight loss and hypernatremia in exclusively breast-fed infants. Braz. J. Med. Biol. Res. 2006, 39, 539–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korğalı, E.; Cihan, M.K.; Oğuzalp, T.; Şahinbaş, A.; Ekici, M. Hypernatremic Dehydration in Breastfed Term Infants: Retrospective Evaluation of 159 Cases. Breastfeed. Med. 2017, 12, 5–11. [Google Scholar] [CrossRef]
- Boensch, M.; Oberthuer, A.; Eifinger, F.; Roth, B. Life-Threatening Hypernatremic Dehydration in a 7-Week-Old Exclusively Breastfed Infant as a Cause of a Decline in Breastmilk Volume and Parental Language Barriers in a North African Family. Klin. Pädiatrie 2011, 223, 40–42. [Google Scholar] [CrossRef] [PubMed]
- Nommsen-Rivers, L.A.; Heinig, M.J.; Cohen, R.J.; Dewey, K.G. Newborn Wet and Soiled Diaper Counts and Timing of Onset of Lactation as Indicators of Breastfeeding Inadequacy. J. Hum. Lact. 2008, 24, 27–33. [Google Scholar] [CrossRef] [PubMed]
- The Academy of Breastfeeding Medicine Protocol Committee. ABM Clinical Protocol #3: Hospital Guidelines for the Use of Supplementary Feedings in the Healthy Term Breastfed Neonate, Revised 2009. Breastfeed. Med. 2009, 4, 175–182. [Google Scholar] [CrossRef]
- Kellams, A.; Harrel, C.; Omage, S.; Gregory, C.; Rosen-Carole, C.; the Academy of Breastfeeding Medicine. ABM Clinical Protocol #3: Supplementary Feedings in the Healthy Term Breastfed Neonate, Revised 2017. Breastfeed. Med. 2017, 12, 188–198. [Google Scholar] [CrossRef]
- Castillo, A.; Grogan, T.R.; Wegrzyn, G.H.; Ly, K.V.; Walker, V.P.; Calkins, K.L. Umbilical cord blood bilirubins, gestational age, and maternal race predict neonatal hyperbilirubinemia. PLoS ONE 2018, 13, e0197888. [Google Scholar] [CrossRef]
- Watchko, J.F. Identification of Neonates at Risk for Hazardous Hyperbilirubinemia: Emerging Clinical Insights. Pediatric Clin. N. Am. 2009, 56, 671–687. [Google Scholar] [CrossRef]
- Bentz, M.G.; Carmona, N.; Bhagwat, M.M.; Thimmig, L.M.; Saleh, J.; Eke, U.; Kokroko, J.; Dadasovich, R.; Rice, B.; Cabana, M.D. Beyond “Asian”: Specific East and Southeast Asian Races or Ethnicities Associated with Jaundice Readmission. Hosp. Pediatrics 2018, 8, 269–273. [Google Scholar] [CrossRef]
- Korkmaz, A.; Yiğit, Ş.; Fırat, M.; Oran, O. Cranial MRI in neonatal hypernatraemic dehydration. Pediatric Radiol. 2000, 30, 323–325. [Google Scholar] [CrossRef] [PubMed]
- Boskabadi, H.; Zakerihamidi, M.; Moradi, A. Predictability of prognosis of infantile hypernatremic dehydration: A prospective cohort study. J. Matern. Neonatal Med. 2020, 35, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Soman, T.B.; Moharir, M.; DeVeber, G.; Weiss, S. Infantile Spasms as an Adverse Outcome of Neonatal Cortical Sinovenous Thrombosis. J. Child Neurol. 2006, 21, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Koklu, E.; Gunes, T.; Ozturk, M.A.; Kose, M.; Kurtoglu, S.; Yuksel, F. A Review of 116 Cases of Breastfeeding-Associated Hypernatremia in Rural Area of Central Turkey. J. Trop. Pediatrics 2007, 53, 347–350. [Google Scholar] [CrossRef]
- Boskabadi, H.; Akhondian, J.; Afarideh, M.; Maamouri, G.; Bagheri, S.; Parizadeh, S.M.; Mobarhan, M.G.; Mohammadi, S.; Frens, G.A. Long-Term Neurodevelopmental Outcome of Neonates with Hypernatremic Dehydration. Breastfeed. Med. 2017, 12, 163–168. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Breastfeeding Report Card. Available online: https://www.cdc.gov/breastfeeding/data/reportcard.htm (accessed on 15 March 2022).
- Speer, R.; Schaefer, E.; Aholoukpe, M.; Leslie, D.; Gandhi, C. Trends in Costs of Birth Hospitalization and Readmissions for Late Preterm Infants. Children 2021, 8, 127. [Google Scholar] [CrossRef]
- Blumovich, A.; Mangel, L.; Yochpaz, S.; Mandel, D.; Marom, R. Risk factors for readmission for phototherapy due to jaundice in healthy newborns: A retrospective, observational study. BMC Pediatrics 2020, 20, 248. [Google Scholar] [CrossRef]
- Zia, M.T.; Golombek, S.; Nitkowski-Keever, S.; Paudel, U. Weight loss monitoring reduces the occurrence of neonatal hypernatremic dehydration in breastfeeding neonates. Int. J. Pediatrics Adolesc. Med. 2021, 9, 22–26. [Google Scholar] [CrossRef]
- Farah, E.; Barger, M.K.; Klima, C.; Rossman, B.; Hershberger, P. Impaired Lactation: Review of Delayed Lactogenesis and Insufficient Lactation. J. Midwifery Women’s Health 2021, 66, 631–640. [Google Scholar] [CrossRef]
- McCoy, M.B.; Heggie, P. In-Hospital Formula Feeding and Breastfeeding Duration. Pediatrics 2020, 146, e20192946. [Google Scholar] [CrossRef] [PubMed]
- Whipps, M.D.; Yoshikawa, H.; Demirci, J.R.; Hill, J. Estimating the Impact of In-Hospital Infant Formula Supplementation on Breastfeeding Success. Breastfeed. Med. 2021, 16, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Chantry, C.J.; Dewey, K.G.; Peerson, J.M.; Wagner, E.A.; Nommsen-Rivers, L.A. In-Hospital Formula Use Increases Early Breastfeeding Cessation among First-Time Mothers Intending to Exclusively Breastfeed. J. Pediatrics 2014, 164, 1339–1345.e5. [Google Scholar] [CrossRef] [Green Version]
- Murase, M.; Wagner, E.A.; JChantry, C.; Dewey, K.G.; Nommsen-Rivers, L.A. The Relation between Breast Milk Sodium to Potassium Ratio and Maternal Report of a Milk Supply Concern. J. Pediatr. 2017, 181, 294–297.e3. [Google Scholar] [CrossRef]
- Flaherman, V.J.; Aby, J.; Burgos, A.E.; Lee, K.A.; Cabana, M.D.; Newman, T.B. Effect of Early Limited Formula on Duration and Exclusivity of Breastfeeding in At-Risk Infants: An RCT. Pediatrics 2013, 131, 1059–1065. [Google Scholar] [CrossRef]
- Stranak, Z.; Feyereislova, S.; Cerna, M.; Kollárová, J.; Feyereisl, J. Limited Amount of Formula May Facilitate Breastfeeding: Randomized, Controlled Trial to Compare Standard Clinical Practice versus Limited Supplemental Feeding. PLoS ONE 2016, 11, e0150053. [Google Scholar] [CrossRef]
- Flaherman, V.J.; Narayan, N.R.; Hartigan-O’Connor, D.; Cabana, M.D.; McCulloch, C.E.; Paul, I.M. The Effect of Early Limited Formula on Breastfeeding, Readmission, and Intestinal Microbiota: A Randomized Clinical Trial. J. Pediatrics 2018, 196, 84–90.e1. [Google Scholar] [CrossRef]
- Flaherman, V.J.; Cabana, M.D.; McCulloch, C.E.; Paul, I. Effect of Early Limited Formula on Breastfeeding Duration in the First Year of Life: A Randomized Clinical Trial. JAMA Pediatrics 2019, 173, 729–735. [Google Scholar] [CrossRef]
- Kair, L.R.; Flaherman, V.J.; Colaizy, T.T. Effect of Donor Milk Supplementation on Breastfeeding Outcomes in Term Newborns: A Randomized Controlled Trial. Clin. Pediatrics 2019, 58, 534–540. [Google Scholar] [CrossRef]
- Kemper, A.R.; Newman, T.B.; Slaughter, J.L.; Maisels, M.B.M.J.; Watchko, J.F.; Downs, S.M.; Grout, R.W.; Bundy, D.G.; Stark, A.R.; Bogen, D.L.; et al. Clinical Practice Guideline Revision: Management of Hyperbilirubinemia in the Newborn Infant 35 or More Weeks of Gestation. Pediatrics 2022, 150, e2022058859. [Google Scholar] [CrossRef]
- Thornton, P.S.; Stanley, C.A.; De Leon, D.D.; Harris, D.; Haymond, M.W.; Hussain, K.; Levitsky, L.L.; Murad, M.H.; Rozance, P.J.; Simmons, R.A.; et al. Recommendations from the Pediatric Endocrine Society for Evaluation and Management of Persistent Hypoglycemia in Neonates, Infants, and Children. J. Pediatrics 2015, 167, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Durrani, N.U.R.; Imam, A.A.; Soni, N. Hypernatremia in Newborns: A Practical Approach to Management. Biomed. Hub 2022, 7, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Saini, A.G.; Vyas, S.; Kaur, A.; Singh, P.; Jayashree, M.; Sundaram, V.; Mukhopadhyay, K.; Singh, P. Neuroimaging Spectrum of Severe Hypernatremia in Infants with Neurological Manifestations. Neuropediatrics 2021, 52, 316–325. [Google Scholar] [CrossRef] [PubMed]
| Sodium Concentration (mEq/L) | Mortality |
|---|---|
| 150–160 | 3.6% |
| 161–170 | 17.3% |
| 171–189 | 66.6% |
| Neonatal Hypernatremic Dehydration | Delayed Lactogenesis II |
|---|---|
| Primiparity | Primiparity |
| Cesarean delivery | Cesarean delivery |
| Breast anomalies (surgery, no growth) | Flat or inverted nipples |
| Excessive pre-pregnancy weight | Maternal BMI > 27 kg/m2 |
| Delayed first breastfeeding | Advanced maternal age ≥ 30 years |
| No prior breastfeeding experience | Hypertension |
| Ineffective breastfeeding latch/transfer | Endocrine problems: GDM, hypothyroid |
| Insufficient or absent colostrum Inadequate mature milk supply | Postpartum hemorrhage (Sheehan syndrome) |
| Delayed or failed lactogenesis II | Complicated, prolonged staged II labor |
| Early Signs | Late Signs |
|---|---|
| Unsatisfied, frequent, prolonged nursing (>30–45 min/feed, <q2hrs) | Sleeping > 4 h w/o feeding |
| High-pitched, inconsolable crying | Sunken fontanelles |
| Body weight loss >5–7% | Lethargy |
| No wet diapers for 6 h (unreliable) | Kernicterus—opisthotonos, retrocollis |
| Pink or red dust in diapers (urate crystals) | Blank staring—encephalopathic facies |
| Short, ineffective, infrequent nursing | Hyperthermia |
| (<10 min, >q3hrs) | Seizures |
| Difficulty waking during feed | Hypothermia |
| Jaundice | Apnea, cyanosis |
| Poor skin turgor, dry lips | Bradycardia, cardiac arrest |
| Prevention Strategies | Recommendations |
|---|---|
| Weight loss monitoring | Weight check every 12 h after birth weight. Identify infants with weight loss >75th percentile on Newborn Weight Loss Tool and those with ≥5% weight loss for closer clinical and laboratory evaluation. |
| Sodium level screening | ≥5% with risk factors and/or signs of suboptimal feeding. |
| Basic metabolic panel | Check BMP for sodium levels >145 mEq/L and for clinically significant hyperbilirubinemia (AAP) [74] or hypoglycemia (PES) [75]. |
| Rescue supplementation | For alert, clinically dehydrated, distressed infants, offer supplementation after nursing with BDM or formula to satisfaction, if lactogenesis II has not occurred, while awaiting confirmation. Sub-threshold supplementation in response to early signs of hypernatremia can prevent the need for intravenous correction of hypernatremia, as well as phototherapy and/or exchange transfusion. |
| Pre-discharge screening | Weight check, BMP, and bilirubin for breastfed neonates. |
| Parent education | Assessment and counseling on risk factors for delayed lactogenesis II, low milk supply, and other breastfeeding challenges. Effective breastfeeding technique, signs of dehydration, hypernatremia, and suboptimal feeding; instruction on supplementation when signs occur followed by urgent pediatrician/LC visit. |
| Next day, frequent clinic follow-up | Next day and frequent visits until lactogenesis II and weight begins to increase. Evaluate for onset of lactogenesis II, breastfeeding frequency, duration, efficacy, infant behavior, signs of suboptimal feeding jaundice, dehydration, hypoglycemia, percent weight loss. LC evaluation of breastfeeding efficacy with weighted feed. Check BMP and bilirubin (TcB or TsB) for any signs of suboptimal feeding with ≥5% weight loss. Plan for supplementation for clinical or laboratory signs of suboptimal feeding present if lactogenesis II has not occurred or breast milk supply is inadequate to maintain weight. Counseling on protecting milk supply with adequate time breastfeeding, pumping for additional milk removal as needed. Hospital referral for hypernatremic dehydration ≥ 146 mEq/L, hyperbilirubinemia, hypoglycemia. |
| Hypernatremia Management |
|---|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
del Castillo-Hegyi, C.; Achilles, J.; Segrave-Daly, B.J.; Hafken, L. Fatal Hypernatremic Dehydration in a Term Exclusively Breastfed Newborn. Children 2022, 9, 1379. https://doi.org/10.3390/children9091379
del Castillo-Hegyi C, Achilles J, Segrave-Daly BJ, Hafken L. Fatal Hypernatremic Dehydration in a Term Exclusively Breastfed Newborn. Children. 2022; 9(9):1379. https://doi.org/10.3390/children9091379
Chicago/Turabian Styledel Castillo-Hegyi, Christie, Jennifer Achilles, B. Jody Segrave-Daly, and Lynnette Hafken. 2022. "Fatal Hypernatremic Dehydration in a Term Exclusively Breastfed Newborn" Children 9, no. 9: 1379. https://doi.org/10.3390/children9091379