Effectiveness of Non-Pharmacological Methods, Such as Breastfeeding, to Mitigate Pain in NICU Infants
Abstract
:1. Introduction
Nutrition and Neurodevelopmental Issues
2. Pain and Fetal Life
2.1. Types of Pain
2.1.1. Neonatal Trauma of NICU (IMTN)
2.1.2. Skin Injuries Associated with MARSIs
3. Pain Scales
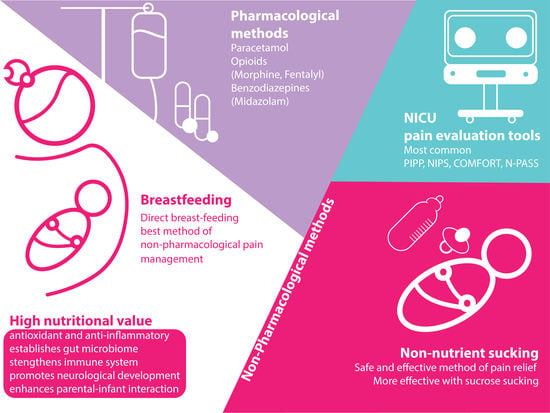
4. Non-Pharmacological Methods
4.1. Non-Nutrient Sucking
4.2. Breastfeeding
4.3. Non-Pharmacological Methods Used by Parents
5. Pharmacological Methods
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, J. History of pain theories. Neurosci. Bull. 2011, 27, 343–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rutter, N.; Doyal, L. Neonatal care and management of pain: Historical and ethical issues. Semin. Neonatol. 1998, 3, 297–302. [Google Scholar] [CrossRef]
- Moseley, G.L.; Butler, D.S. Fifteen years of explaining pain: The past, present, and future. J. Pain 2015, 16, 807–813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensen, T.S.; Finnerup, N.B. A brief history of pain. Lancet Neurol. 2014, 13, 872. [Google Scholar] [CrossRef]
- Hall, R.W.; Anand, K.J. Pain management in newborns. Clin. Perinatol. 2014, 41, 895–924. [Google Scholar] [CrossRef] [Green Version]
- Keels, E.; Sethna, N.; Watterberg, K.L.; Cummings, J.J.; Benitz, W.E.; Eichenwald, E.C.; Poindexter, B.B.; Stewart, D.L.; Aucott, S.W.; Goldsmith, J.P. Prevention and management of procedural pain in the neonate: An update. Pediatrics 2016, 137, 1–13. [Google Scholar]
- Yiğit, Ş.; Ecevit, A.; Köroğlu, Ö.A. Turkish Neonatal Society guideline on the neonatal pain and its management. Turk. Pediatri Arch. 2018, 53 (Suppl. 1), S161. [Google Scholar] [CrossRef]
- Harris, J.; Ramelet, A.-S.; van Dijk, M.; Pokorna, P.; Wielenga, J.; Tume, L.; Tibboel, D.; Ista, E. Clinical recommendations for pain, sedation, withdrawal and delirium assessment in critically ill infants and children: An ESPNIC position statement for healthcare professionals. Intensive Care Med. 2016, 42, 972–986. [Google Scholar] [CrossRef] [Green Version]
- Suresh, S.; Ecoffey, C.; Bosenberg, A.; Lonnqvist, P.-A.; De Oliveira, G.S.; de Leon Casasola, O.; De Andrés, J.; Ivani, G. The European society of regional anaesthesia and pain therapy/American society of regional anesthesia and pain medicine recommendations on local anesthetics and adjuvants dosage in pediatric regional anesthesia. Reg. Anesth. Pain Med. 2018, 43, 211–216. [Google Scholar] [CrossRef]
- Balice-Bourgois, C.; Zumstein-Shaha, M.; Vanoni, F.; Jaques, C.; Newman, C.J.; Simonetti, G.D. A systematic review of clinical practice guidelines for acute procedural pain on neonates. Clin. J. Pain 2020, 36, 390–398. [Google Scholar] [CrossRef]
- Perry, M.; Tan, Z.; Chen, J.; Weidig, T.; Xu, W.; Cong, X.S. Neonatal pain: Perceptions and current practice. Crit. Care Nurs. Clin. 2018, 30, 549–561. [Google Scholar] [CrossRef]
- Williams, M.D.; Lascelles, B.D.X. Early neonatal pain—A review of clinical and experimental implications on painful conditions later in life. Front. Pediatr. 2020, 8, 30. [Google Scholar] [CrossRef] [Green Version]
- Grunau, R.E.; Holsti, L.; Peters, J.W. Long-term consequences of pain in human neonates. Semin. Fetal Neonatal Med. 2006, 11, 268–275. [Google Scholar] [CrossRef]
- Walker, S.; Melbourne, A.; O’Reilly, H.; Beckmann, J.; Eaton-Rosen, Z.; Ourselin, S.; Marlow, N. Somatosensory function and pain in extremely preterm young adults from the UK EPICure cohort: Sex-dependent differences and impact of neonatal surgery. Br. J. Anaesth. 2018, 121, 623–635. [Google Scholar] [CrossRef] [Green Version]
- Agostoni, C.; Buonocore, G.; Carnielli, V.; De Curtis, M.; Darmaun, D.; Decsi, T.; Domellöf, M.; Embleton, N.D.; Fusch, C.; Genzel-Boroviczeny, O. Enteral nutrient supply for preterm infants: Commentary from the European Society of Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2010, 50, 85–91. [Google Scholar] [CrossRef]
- Horbar, J.D.; Ehrenkranz, R.A.; Badger, G.J.; Edwards, E.M.; Morrow, K.A.; Soll, R.F.; Buzas, J.S.; Bertino, E.; Gagliardi, L.; Bellù, R. Weight growth velocity and postnatal growth failure in infants 501 to 1500 grams: 2000–2013. Pediatrics 2015, 136, e84–e92. [Google Scholar] [CrossRef] [Green Version]
- Ramel, S.E.; Demerath, E.W.; Gray, H.L.; Younge, N.; Boys, C.; Georgieff, M.K. The relationship of poor linear growth velocity with neonatal illness and two-year neurodevelopment in preterm infants. Neonatology 2012, 102, 19–24. [Google Scholar] [CrossRef]
- Ehrenkranz, R.A.; Dusick, A.M.; Vohr, B.R.; Wright, L.L.; Wrage, L.A.; Poole, W.K.; Health, N.I.o.C.; Network, H.D.N.R. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. Pediatrics 2006, 117, 1253–1261. [Google Scholar] [CrossRef] [Green Version]
- Sammallahti, S.; Kajantie, E.; Matinolli, H.-M.; Pyhälä, R.; Lahti, J.; Heinonen, K.; Lahti, M.; Pesonen, A.-K.; Eriksson, J.G.; Hovi, P. Nutrition after preterm birth and adult neurocognitive outcomes. PLoS ONE 2017, 12, e0185632. [Google Scholar] [CrossRef] [Green Version]
- Grunau, R.E. Neonatal pain in very preterm infants: Long-term effects on brain, neurodevelopment and pain reactivity. Rambam Maimonides Med. J. 2013, 4, e0025. [Google Scholar]
- Briere, C.E.; McGrath, J.; Cong, X.; Cusson, R. An integrative review of factors that influence breastfeeding duration for premature infants after NICU hospitalization. J. Obstet. Gynecol. Neonatal Nurs. 2014, 43, 272–281. [Google Scholar] [CrossRef]
- Bujold, M.; Feeley, N.; Axelin, A.; Cinquino, C.; Dowling, D.; Thibeau, S. Expressing human milk in the NICU. Adv. Neonatal Care 2018, 18, 38–48. [Google Scholar] [CrossRef]
- Parker, M.G.; Stellwagen, L.M.; Noble, L.; Kim, J.H.; Poindexter, B.B.; Puopolo, K.M. Promoting human milk and breastfeeding for the very low birth weight infant. Pediatrics 2021, 148, e2021054272. [Google Scholar] [CrossRef] [PubMed]
- Lavallée, A.; De Clifford-Faugère, G.; Garcia, C.; Oviedo, A.N.F.; Héon, M.; Aita, M. PART 2: Practice and research recommendations for quality developmental care in the NICU. J. Neonatal Nurs. 2019, 25, 160–165. [Google Scholar] [CrossRef]
- Chetta, K.E.; Schulz, E.V.; Wagner, C.L. Outcomes improved with human milk intake in preterm and full-term infants. Semin. Perinatol. 2021, 45, 151384. [Google Scholar] [CrossRef]
- Ottolini, K.M.; Andescavage, N.; Keller, S.; Limperopoulos, C. Nutrition and the developing brain: The road to optimizing early neurodevelopment: A systematic review. Pediatr. Res. 2020, 87, 194–201. [Google Scholar] [CrossRef]
- Hortensius, L.M.; Janson, E.; van Beek, P.E.; Groenendaal, F.; Claessens, N.H.; Swanenburg de Veye, H.F.; Eijsermans, M.J.; Koopman-Esseboom, C.; Dudink, J.; van Elburg, R.M. Nutritional intake, white matter integrity, and neurodevelopment in extremely preterm born infants. Nutrients 2021, 13, 3409. [Google Scholar] [CrossRef]
- Belfort, M.B.; Inder, T.E. Human milk and preterm infant brain development: A narrative review. Clin. Ther. 2022, 44, 612–621. [Google Scholar] [CrossRef]
- Choudhury, V.; Amin, S.B.; Agarwal, A.; Srivastava, L.; Soni, A.; Saluja, S. Latent iron deficiency at birth influences auditory neural maturation in late preterm and term infants. Am. J. Clin. Nutr. 2015, 102, 1030–1034. [Google Scholar] [CrossRef] [Green Version]
- Andreas, N.J.; Kampmann, B.; Mehring Le-Doare, K. Human breast milk: A review on its composition and bioactivity. Early Hum. Dev. 2015, 91, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Paramasivam, K.; Michie, C.; Opara, E.; Jewell, A.P. Human breast milk immunology: A review. Int. J. Fertil. Women’s Med. 2006, 51, 208–217. [Google Scholar]
- Li, R.; Xia, W.; Zhang, Z.; Wu, K. S100B protein, brain-derived neurotrophic factor, and glial cell line-derived neurotrophic factor in human milk. PLoS ONE 2011, 6, e21663. [Google Scholar] [CrossRef] [Green Version]
- Dinakar, P.; Stillman, A.M. Pathogenesis of pain. Semin. Pediatr. Neurol. 2016, 23, 201–208. [Google Scholar] [CrossRef]
- Derbyshire, S.W. Fetal pain: Do we know enough to do the right thing? Reprod. Health Matters 2008, 16, 117–126. [Google Scholar] [CrossRef]
- Thill, B. Fetal Pain in the First Trimester. Linacre Q. 2022, 89, 73–100. [Google Scholar] [CrossRef]
- Goksan, S.; Hartley, C.; Emery, F.; Cockrill, N.; Poorun, R.; Moultrie, F.; Rogers, R.; Campbell, J.; Sanders, M.; Adams, E. fMRI reveals neural activity overlap between adult and infant pain. Elife 2015, 4, e06356. [Google Scholar] [CrossRef]
- Carbajal, R.; Rousset, A.; Danan, C.; Coquery, S.; Nolent, P.; Ducrocq, S.; Saizou, C.; Lapillonne, A.; Granier, M.; Durand, P. Epidemiology and treatment of painful procedures in neonates in intensive care units. Jama 2008, 300, 60–70. [Google Scholar] [CrossRef] [Green Version]
- Farmer, D.; Sitkin, N.; Lofberg, K.; Donkor, P.; Ozgediz, D. Surgical interventions for congenital anomalies. Dis. Control Priorities 2015, 1, 129–149. [Google Scholar]
- McPherson, C.; Grunau, R.E. Pharmacologic Analgesia and Sedation in Neonates. Clin. Perinatol. 2022, 49, 243–265. [Google Scholar] [CrossRef] [PubMed]
- Field, T. Preterm newborn pain research review. Infant Behav. Dev. 2017, 49, 141–150. [Google Scholar] [CrossRef]
- Thompson, D.K.; Lee, K.J.; Egan, G.F.; Warfield, S.K.; Doyle, L.W.; Anderson, P.J.; Inder, T.E. Regional white matter microstructure in very preterm infants: Predictors and 7 year outcomes. Cortex 2014, 52, 60–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Agata, A.; Sanders, M.; Grasso, D.; Young, E.; McGrath, J. UNPACKING THE BURDEN OF CARE FOR INFANTS IN THE NICU. Infant Ment. Health J. 2017, 38, 306–317. [Google Scholar] [CrossRef] [PubMed]
- Çekin, B.; Turan, T. The stress levels of parents of premature infants and related factors in Nenoatal Intensive Care Units. Turk. J. Pediatr. 2018, 60, 117–125. [Google Scholar] [CrossRef] [Green Version]
- D’Agata, A.L.; Young, E.E.; Cong, X.; Grasso, D.J.; McGrath, J.M. Infant Medical Trauma in the Neonatal Intensive Care Unit (IMTN): A Proposed Concept for Science and Practice. Adv. Neonatal Care Off. J. Natl. Assoc. Neonatal Nurses 2016, 16, 289–297. [Google Scholar] [CrossRef]
- Kuzniewicz, M.W.; Wi, S.; Qian, Y.; Walsh, E.M.; Armstrong, M.A.; Croen, L.A. Prevalence and neonatal factors associated with autism spectrum disorders in preterm infants. J. Pediatr. 2014, 164, 20–25. [Google Scholar] [CrossRef]
- O’Hara, M.W.; Wisner, K.L. Perinatal mental illness: Definition, description and aetiology. Best Pract. Res. Clin. Obstet. Gynaecol. 2014, 28, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.S.; van Rooij, S.J.H.; Jovanovic, T. Developmental Contributors to Trauma Response: The Importance of Sensitive Periods, Early Environment, and Sex Differences. Curr. Top. Behav. Neurosci. 2018, 38, 1–22. [Google Scholar] [CrossRef]
- Hutchinson, E.A.; De Luca, C.R.; Doyle, L.W.; Roberts, G.; Anderson, P.J.; Group, V.I.C.S. School-age outcomes of extremely preterm or extremely low birth weight children. Pediatrics 2013, 131, e1053–e1061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Xu, H.; Chen, S.; Lou, X.; Tan, J.; Xu, Y. Medical adhesive-related skin injuries and associated risk factors in a pediatric intensive care unit. Adv. Ski. Wound Care 2019, 32, 176–182. [Google Scholar] [CrossRef]
- Visscher, M.O.; Adam, R.; Brink, S.; Odio, M. Newborn infant skin: Physiology, development, and care. Clin. Dermatol. 2015, 33, 271–280. [Google Scholar] [CrossRef]
- Oranges, T.; Dini, V.; Romanelli, M. Skin Physiology of the Neonate and Infant: Clinical Implications. Adv. Wound Care 2015, 4, 587–595. [Google Scholar] [CrossRef] [Green Version]
- Johnson, E.; Hunt, R. Infant skin care: Updates and recommendations. Curr. Opin. Pediatr. 2019, 31, 476–481. [Google Scholar] [CrossRef]
- Talebi, F.R.; Asefnejad, A. The Effects of Gelatin Hydrogel on Neonatal Fibroblast Cells Viability in vitro. In Proceedings of the ICCBMS21, ICFNEAS21, Virtual, 19–20 August 2021. [Google Scholar]
- Cay, G.; Solanki, D.; Al Rumon, M.A.; Ravichandran, V.; Hoffman, L.; Laptook, A.; Padbury, J.; Salisbury, A.L.; Mankodiya, K. NeoWear: An IoT-connected e-textile wearable for neonatal medical monitoring. Pervasive Mob. Comput. 2022, 86, 101679. [Google Scholar] [CrossRef]
- McNichol, L.; Lund, C.; Rosen, T.; Gray, M. Medical adhesives and patient safety: State of the scienceconsensus statements for the assessment, prevention, and treatment of adhesive-related skin injuries. J. Wound Ostomy Cont. Nurs. 2013, 40, 365–380. [Google Scholar] [CrossRef] [PubMed]
- Mendes, L.A.; Costa, A.C.L.; Silva, D.C.Z.; Simões, D.A.d.S.; Côrrea, A.d.R.; Manzo, B.F. Adherence of the nursing team to patient safety actions in neonatal units. Rev. Bras. Enferm. 2021, 74, e20200765. [Google Scholar] [CrossRef]
- Thienhaus, O.; Cole, B.E. Classification of pain. In Pain Management: A Practical Guide for Clinicians; CRC Press: Boca Raton, FL, USA, 2001; pp. 27–36. ISBN 9781420093193. ISSN 9781420038927. [Google Scholar]
- Olsson, E.; Ahl, H.; Bengtsson, K.; Vejayaram, D.N.; Norman, E.; Bruschettini, M.; Eriksson, M. The use and reporting of neonatal pain scales: A systematic review of randomized trials. Pain 2021, 162, 353. [Google Scholar] [CrossRef]
- Slater, R.; Cantarella, A.; Franck, L.; Meek, J.; Fitzgerald, M. How well do clinical pain assessment tools reflect pain in infants? PLoS Med. 2008, 5, e129. [Google Scholar] [CrossRef] [Green Version]
- Gibbins, S.; Stevens, B.; McGrath, P.J.; Yamada, J.; Beyene, J.; Breau, L.; Camfield, C.; Finley, A.; Franck, L.; Johnston, C. Comparison of pain responses in infants of different gestational ages. Neonatology 2008, 93, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Campbell-Yeo, M.; Eriksson, M.; Benoit, B. Assessment and Management of Pain in Preterm Infants: A Practice Update. Children 2022, 9, 244. [Google Scholar] [CrossRef]
- Stevens, B.; Johnston, C.; Petryshen, P.; Taddio, A. Premature Infant Pain Profile: Development and initial validation. Clin. J. Pain 1996, 12, 13–22. [Google Scholar] [CrossRef]
- Stevens, B.J.; Gibbins, S.; Yamada, J.; Dionne, K.; Lee, G.; Johnston, C.; Taddio, A. The premature infant pain profile-revised (PIPP-R): Initial validation and feasibility. Clin. J. Pain 2014, 30, 238–243. [Google Scholar] [CrossRef]
- Lawrence, J.; Alcock, D.; McGrath, P.; Kay, J.; MacMurray, S.B.; Dulberg, C. The development of a tool to assess neonatal pain. Neonatal Netw. 1993, 12, 59–66. [Google Scholar] [CrossRef]
- Grunau, R.V.E.; Craig, K.D. Pain expression in neonates: Facial action and cry. Pain 1987, 28, 395–410. [Google Scholar] [CrossRef]
- Carbajal, R.; Paupe, A.; Hoenn, E.; Lenclen, R.; Olivier-Martin, M. APN: Evaluation behavioral scale of acute pain in newborn infants. Arch. Pediatr. 1997, 4, 623–628. [Google Scholar] [CrossRef]
- Ambuel, B.; Hamlett, K.W.; Marx, C.M.; Blumer, J.L. Assessing distress in pediatric intensive care environments: The COMFORT scale. J. Pediatr. Psychol. 1992, 17, 95–109. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, M.; Roofthooft, D.W.; Anand, K.J.; Guldemond, F.; de Graaf, J.; Simons, S.; de Jager, Y.; van Goudoever, J.B.; Tibboel, D. Taking up the challenge of measuring prolonged pain in (premature) neonates: The COMFORTneo scale seems promising. Clin. J. Pain 2009, 25, 607–616. [Google Scholar] [CrossRef]
- Hummel, P.; Puchalski, M.; Creech, S.D.; Weiss, M.G. Clinical reliability and validity of the N-PASS: Neonatal pain, agitation and sedation scale with prolonged pain. J. Perinatol. 2008, 28, 55–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giordano, V.; Edobor, J.; Deindl, P.; Wildner, B.; Goeral, K.; Steinbauer, P.; Werther, T.; Berger, A.; Olischar, M. Pain and Sedation Scales for Neonatal and Pediatric Patients in a Preverbal Stage of Development: A Systematic Review. JAMA Pediatr. 2019, 173, 1186–1197. [Google Scholar] [CrossRef]
- Vu-Ngoc, H.; Uyen, N.C.M.; Thinh, O.P.; Don, L.D.; Danh, N.V.T.; Truc, N.T.T.; Vi, V.T.; Vuong, N.L.; Huy, N.T.; Duong, P.D.T. Analgesic effect of non-nutritive sucking in term neonates: A randomized controlled trial. Pediatr. Neonatol. 2020, 61, 106–113. [Google Scholar] [CrossRef] [Green Version]
- Ludington-Hoe, S.M.; Johnson, M.W.; Morgan, K.; Lewis, T.; Gutman, J.; Wilson, P.D.; Scher, M.S. Neurophysiologic assessment of neonatal sleep organization: Preliminary results of a randomized, controlled trial of skin contact with preterm infants. Pediatrics 2006, 117, e909–e923. [Google Scholar] [CrossRef] [Green Version]
- Pillai Riddell, R.; Racine, N.; Turcotte, K.; Uman, L.; Horton, R.; Ahola Kohut, S.; Din Osmun, L.; Hillgrove-Stuart, J.; Stevens, B.; Lisi, D. Non-pharmacological management of infant and young child procedural pain: An abridged Cochrane review. Cochrane Database Syst. Rev. 2011, 10, CD006275. [Google Scholar] [CrossRef]
- Axelin, A.; Salanterä, S.; Lehtonen, L. ‘Facilitated tucking by parents’ in pain management of preterm infants—A randomized crossover trial. Early Hum. Dev. 2006, 82, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Chidambaram, A.G.; Manjula, S.; Adhisivam, B.; Vishnu Bhat, B. Effect of Kangaroo mother care in reducing pain due to heel prick among preterm neonates: A crossover trial. J. Matern. Fetal Neonatal Med. 2014, 27, 488–490. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.S.; Herbozo, C.; Aliwalas, L.L.; Shah, V.S. Breastfeeding or breast milk for procedural pain in neonates. Cochrane Database Syst. Rev. 2012. [Google Scholar] [CrossRef] [PubMed]
- Pacifici, G.M.; Allegaert, K. Clinical Pharmacology of Paracetamol in Neonates: A Review. Curr. Ther. Res. 2015, 77, 24–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anand, K.J. Pharmacological approaches to the management of pain in the neonatal intensive care unit. J. Perinatol. 2007, 27 (Suppl. S1), S4–S11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durrmeyer, X.; Vutskits, L.; Anand, K.J.; Rimensberger, P.C. Use of analgesic and sedative drugs in the NICU: Integrating clinical trials and laboratory data. Pediatr. Res. 2010, 67, 117–127. [Google Scholar] [CrossRef] [Green Version]
- Eccleston, C.; Cooper, T.E.; Fisher, E.; Anderson, B.; Wilkinson, N.M. Non-steroidal anti-inflammatory drugs (NSAIDs) for chronic non-cancer pain in children and adolescents. Cochrane Database Syst. Rev. 2017, 8, Cd012537. [Google Scholar] [CrossRef]
- Ng, E.; Taddio, A.; Ohlsson, A. Intravenous midazolam infusion for sedation of infants in the neonatal intensive care unit. Cochrane Database Syst. Rev. 2017, 1, CD002052. [Google Scholar] [CrossRef]
- Bergomi, P.; Chieppi, M.; Maini, A.; Mugnos, T.; Spotti, D.; Tzialla, C.; Scudeller, L. Nonpharmacological techniques to reduce pain in preterm infants who receive heel-lance procedure: A randomized controlled trial. Res. Theory Nurs. Pract. 2014, 28, 335–348. [Google Scholar] [CrossRef]
- Corrigan, M.J.; Keeler, J.R.; Miller, H.D.; Ben Khallouq, B.A.; Fowler, S.B. Music therapy and retinopathy of prematurity screening: Using recorded maternal singing and heartbeat for post exam recovery. J. Perinatol. 2020, 40, 1780–1788. [Google Scholar] [CrossRef]
- Dur, Ş.; Çevik, S.G.; Ustabaş Yıldız, N. The effect of white noise and classical music on pain and physiologic parameters in preterm infants during retinopathy of prematurity examinations: A randomized controlled trial. Early Child Dev. Care 2022, 1–12. [Google Scholar] [CrossRef]
- Campbell-Yeo, M.; Fernandes, A.; Johnston, C. Procedural pain management for neonates using nonpharmacological strategies: Part 2: Mother-driven interventions. Adv. Neonatal Care 2011, 11, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, D.; Inal, S. Effects of three different methods used during heel lance procedures on pain level in term neonates. Jpn. J. Nurs. Sci. 2020, 17, e12338. [Google Scholar] [CrossRef]
- Obeidat, H.M.; Shuriquie, M.A. Effect of Breast-Feeding and Maternal Holding in Relieving Painful Responses in Full-Term Neonates: A Randomized Clinical Trial. J. Perinat. Neonatal Nurs. 2015, 29, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Fallah, R.; Naserzadeh, N.; Ferdosian, F.; Binesh, F. Comparison of effect of kangaroo mother care, breastfeeding and swaddling on Bacillus Calmette-Guerin vaccination pain score in healthy term neonates by a clinical trial. J. Matern. Neonatal Med. 2017, 30, 1147–1150. [Google Scholar] [CrossRef]
- Marín Gabriel, M.; del Rey Hurtado de Mendoza, B.; Jiménez Figueroa, L.; Medina, V.; Iglesias Fernández, B.; Vázquez Rodríguez, M.; Escudero Huedo, V.; Medina Malagón, L. Analgesia with breastfeeding in addition to skin-to-skin contact during heel prick. Arch. Dis. Child. Fetal Neonatal Ed. 2013, 98, F499–F503. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.K.; Upadhyay, A.; Agarwal, A.; Goswami, G.; Kumar, J.; Sreenivas, V. Randomized controlled trial of topical EMLA and breastfeeding for reducing pain during wDPT vaccination. Eur. J. Pediatr. 2013, 172, 1527–1533. [Google Scholar] [CrossRef]
- Boroumandfar, K.; Khodaei, F.; Abdeyazdan, Z.; Maroufi, M. Comparison of vaccination-related pain in infants who receive vapocoolant spray and breastfeeding during injection. Iran. J. Nurs. Midwifery Res. 2013, 18, 33–37. [Google Scholar]
- Lima, A.H.; Hermont, A.P.; Friche, A.A. Analgesia in newborns: A case-control study of the efficacy of nutritive and non-nutritive sucking stimuli. Codas 2013, 25, 365–368. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Hong-Gu, H.; Zhou, X.; Wei, H.; Gao, Y.; Ye, B.; Liu, Z.; Chan, S.W. Pain relief effect of breast feeding and music therapy during heel lance for healthy-term neonates in China: A randomized controlled trial. Midwifery 2015, 31, 365–372. [Google Scholar] [CrossRef]
- Li, Q.; Tan, X.; Li, X.; Tang, W.; Mei, L.; Cheng, G.; Zou, Y. Efficacy and safety of combined oral sucrose and nonnutritive sucking in pain management for infants: A systematic review and meta-analysis. PLoS ONE 2022, 17, e0268033. [Google Scholar] [CrossRef]
- Stevens, B.; Yamada, J.; Ohlsson, A.; Haliburton, S.; Shorkey, A. Sucrose for analgesia in newborn infants undergoing painful procedures. Cochrane Database Syst. Rev. 2016, 7, Cd001069. [Google Scholar] [CrossRef] [PubMed]
- Gibbins, S.; Stevens, B. The influence of gestational age on the efficacy and short-term safety of sucrose for procedural pain relief. Adv. Neonatal Care Off. J. Natl. Assoc. Neonatal Nurses 2003, 3, 241–249. [Google Scholar]
- Ranjbar, A.; Bernstein, C.; Shariat, M.; Ranjbar, H. Comparison of facilitated tucking and oral dextrose in reducing the pain of heel stick in preterm infants: A randomized clinical trial. BMC Pediatr. 2020, 20, 162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandee, S.; Buachai, K.; Aroonpruksakul, N.; Tantemsapya, N.; Buasuk, T. Effects of Sucrose and Nonnutritive Sucking on Pain Behavior in Neonates and Infants undergoing Wound Dressing after Surgery: A Randomized Controlled Trial. Eur. J. Pediatr. Surg. 2021, 31, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Sawleshwarkar, K.; Singh, M.; Bajaj, R.; Loya, S.; Chikhlondhe, R.; Bhave, S. Implementing use of sucrose analgesia (non-pharmacological management of neonatal pain) in a standalone private facility level 3 neonatal care unit using point of care quality improvement methodology. BMJ Open Qual. 2022, 11, e001830. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.R. Oral feeding readiness in the neonatal intensive care unit. Neonatal Netw. 2012, 31, 148–155. [Google Scholar] [CrossRef]
- Briere, C.-E.; McGrath, J.M.; Cong, X.; Brownell, E.; Cusson, R. Direct-breastfeeding in the neonatal intensive care unit and breastfeeding duration for premature infants. Appl. Nurs. Res. 2016, 32, 47–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Academy of Breastfeeding Medicine Board of Directors. Position on breastfeeding. Breastfeed. Med. 2008, 3, 267–270. [Google Scholar] [CrossRef]
- Pediatrics, A.A.o. Breastfeeding and the use of human milk. Pediatrics 2012, 129, e827–e841. [Google Scholar] [CrossRef] [Green Version]
- Couto, G.R.; Dias, V.; de Jesus Oliveira, I. Benefits of exclusive breastfeeding: An integrative review. Nurs. Pract. Today 2020, 7, 245–254. [Google Scholar] [CrossRef]
- Komaroff, A.; Forest, S. Implementing a clinical protocol using breastfeeding to mitigate vaccination pain in infants. J. Pediatr. Nurs. 2020, 54, 50–57. [Google Scholar] [CrossRef]
- Callen, J.; Pinelli, J. A review of the literature examining the benefits and challenges, incidence and duration, and barriers to breastfeeding in preterm infants. Adv. Neonatal Care Off. J. Natl. Assoc. Neonatal Nurses 2005, 5, 72–88. [Google Scholar] [CrossRef]
- Terry, J. Teaching mothers to express and store breast milk. J. Fam. Health Care 2004, 14, 121–123. [Google Scholar] [PubMed]
- Benoit, B.; Martin-Misener, R.; Latimer, M.; Campbell-Yeo, M. Breast-Feeding Analgesia in Infants: An Update on the Current State of Evidence. J. Perinat. Neonatal Nurs. 2017, 31, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Napiórkowska-Orkisz, M.; Gutysz-Wojnicka, A.; Tanajewska, M.; Sadowska-Krawczenko, I. Evaluation of Methods to Minimize Pain in Newborns during Capillary Blood Sampling for Screening: A Randomized Clinical Trial. Int. J. Environ. Res. Public Health 2022, 19, 870. [Google Scholar] [CrossRef]
- Franck, L.S.; Oulton, K.; Bruce, E. Parental involvement in neonatal pain management: An empirical and conceptual update. J. Nurs. Scholarsh. 2012, 44, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Skene, C.; Franck, L.; Curtis, P.; Gerrish, K. Parental involvement in neonatal comfort care. J. Obstet. Gynecol. Neonatal Nurs. 2012, 41, 786–797. [Google Scholar] [CrossRef] [PubMed]
- Pölkki, T.; Korhonen, A.; Laukkala, H. Parents’ Use of Nonpharmacologic Methods to Manage Procedural Pain in Infants. J. Obstet. Gynecol. Neonatal Nurs. 2018, 47, 43–51. [Google Scholar] [CrossRef] [Green Version]
- Axelin, A.; Anderzén-Carlsson, A.; Eriksson, M.; Pölkki, T.; Korhonen, A.; Franck, L.S. Neonatal Intensive Care Nurses’ Perceptions of Parental Participation in Infant Pain Management: A Comparative Focus Group Study. J. Perinat. Neonatal Nurs. 2015, 29, 363–374. [Google Scholar] [CrossRef]
- Gates, A.; Shave, K.; Featherstone, R.; Buckreus, K.; Ali, S.; Scott, S.D.; Hartling, L. Procedural Pain: Systematic Review of Parent Experiences and Information Needs. Clin. Pediatr. 2018, 57, 672–688. [Google Scholar] [CrossRef] [PubMed]
- Maciel, H.I.A.; Costa, M.F.; Costa, A.C.L.; Marcatto, J.d.O.; Manzo, B.F.; Bueno, M. Pharmacological and nonpharmacological measures of pain management and treatment among neonates. Rev. Bras. Ter. Intensiv. 2019, 31, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Farnia, M.R.; Babaei, R.; Shirani, F.; Momeni, M.; Hajimaghsoudi, M.; Vahidi, E.; Saeedi, M. Analgesic effect of paracetamol combined with low-dose morphine versus morphine alone on patients with biliary colic: A double blind, randomized controlled trial. World J. Emerg. Med. 2016, 7, 25–29. [Google Scholar] [CrossRef] [Green Version]
- de Graaf, J.; van Lingen, R.A.; Valkenburg, A.J.; Weisglas-Kuperus, N.; Jebbink, L.G.; Wijnberg-Williams, B.; Anand, K.J.S.; Tibboel, D.; van Dijk, M. Does neonatal morphine use affect neuropsychological outcomes at 8 to 9 years of age? Pain 2013, 154, 449–458. [Google Scholar] [CrossRef]
- Rozé, J.C.; Denizot, S.; Carbajal, R.; Ancel, P.Y.; Kaminski, M.; Arnaud, C.; Truffert, P.; Marret, S.; Matis, J.; Thiriez, G.; et al. Prolonged sedation and/or analgesia and 5-year neurodevelopment outcome in very preterm infants: Results from the EPIPAGE cohort. Arch. Pediatr. Adolesc. Med. 2008, 162, 728–733. [Google Scholar] [CrossRef] [PubMed]
- Bellù, R.; Romantsik, O.; Nava, C.; de Waal, K.A.; Zanini, R.; Bruschettini, M. Opioids for newborn infants receiving mechanical ventilation. Cochrane Database Syst. Rev. 2021, 3, CD013732. [Google Scholar] [CrossRef]
- Abushanab, D.; Abounahia, F.F.; Alsoukhni, O.; Abdelaal, M.; Al-Badriyeh, D. Clinical and Economic Evaluation of the Impact of Midazolam on Morphine Therapy for Pain Relief in Critically III Ventilated Infants with Respiratory Distress Syndrome. Pediatr. Drugs 2021, 23, 143–157. [Google Scholar] [CrossRef]
- Witt, N.; Coynor, S.; Edwards, C.; Bradshaw, H. A guide to pain assessment and management in the neonate. Curr. Emerg. Hosp. Med. Rep. 2016, 4, 1–10. [Google Scholar] [CrossRef]

| Scale | Use | Indicators Used | Scoring |
|---|---|---|---|
| PIPP [62] | Premature infants in NICU | 3 behavioral (facial actions: brow bulge, eye squeeze, nasolabial furrow), 2 physiological (heart rate and oxygen saturation), and 2 contextual (GA and BS) items | Seven-item, four-point scale 21 points for preterm infants <28 weeks GA and 18 points for full-term infants |
| PIPP-R [63] | Extremely low gestational age (ELGA) infants | Same as PIPP | Seven-item, four-point scale |
| NIPS [64] | Infants < 1 year old | 5 behavioral, (facial expression, cry, arms, legs, and state of arousal) 1 physiological factor (breathing patterns), | Score 0–7, Score > 3 is indicative of pain |
| NFCS [65] | premature neonates, term-born neonates, and infants ≤18 months of age | 10 behavioral, (brow bulge, eye squeeze, nasolabial furrow, open lips, horizontal mouth stretch, vertical mouth stretch, taut tongue, lip purse, chin quiver, tongue protrusion.) Top 3 (brow bulge, eye squeeze, and nasolabial furrow) suffice for pain assessment. | Score 0–10 for premature infants: 10 Score 0–9 full term infants |
| COMFORTneo [67,68] | Premature infants in NICU | 6 behavioral, (alertness, calmness, muscle tone, physical movement, facial tension, and respiratory behavior/crying). Respiratory behavior in ventilated patients and Crying in nonventilated patients. | 6 items are scored on a 5-point scale, ranging from 1 to 5, with total score ranging from 6 to 30. |
| N-PASS [69] | NICU ventilated and/or postoperative infants 0–100 days of age, ≥ 23 weeks of gestation | 3 behavioral, (behavior/state, facial expression, extremities/tone) 4 physiological vital signs (heart rate, respiratory rate, blood pressure and/or oxygen saturation) | Score 0–10 |
| Pharmacological Methods | Type of Pain Management |
|---|---|
| Analgesics | |
| Paracetamol [77] | Mild to moderate pain |
| Opioids, mostly Morphine & Fentanyl [6] | Persistent pain |
| Methadone [78], ketamine, propofol, dexmedetomidine [79]. | Persistent pain, limited use |
| Non-Steroidal Anti-Inflammatory Drugs [80] | Not recommended for infants < 6 months of age, due to established adverse side effects |
| Sedatives | |
| Benzodiazepines- Midazolam, [81] | Sedation |
| Non-pharmacological methods | Type of Pain Management |
| Non-nutritive sucking [71]. Provision of a pacifier or the sucking of the fingers or the hand during painful event | Acute procedural Mild to moderate pain |
| Skin-to-skin care (kangaroo care) [72,73]. Newborns wearing only a diaper being held next to their mother’s bare chest | Acute procedural Mild to moderate pain |
| Swaddling/Facilitated tucking [74] Wraping the infant tightly/Holding the infant in the side-lying, flexed fetal-type position by hand | Acute procedural Mild pain |
| Rocking/holding [73,75]. Holding the neonate and swaying in an rocking motion | Acute procedural Mild pain |
| Music listening [82], Recorded maternal singing [83] White noise/classical music playing during painfull procedures [84] | Pasification, Recovery reinforcement of sucking, Acute procedural pain and stress relief |
| Breastfeeding for a duration of more than 2 min prior to a painful procedure [61,76,85,86,87,88,89,90,91,92,93] | Acute procedural pain mild to medium |
| Oral administration of Sucrose/glucose [94,95,96] | Acute procedural pain mild to medium, short lived duration |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koukou, Z.; Theodoridou, A.; Taousani, E.; Antonakou, A.; Panteris, E.; Papadopoulou, S.-S.; Skordou, A.; Sifakis, S. Effectiveness of Non-Pharmacological Methods, Such as Breastfeeding, to Mitigate Pain in NICU Infants. Children 2022, 9, 1568. https://doi.org/10.3390/children9101568
Koukou Z, Theodoridou A, Taousani E, Antonakou A, Panteris E, Papadopoulou S-S, Skordou A, Sifakis S. Effectiveness of Non-Pharmacological Methods, Such as Breastfeeding, to Mitigate Pain in NICU Infants. Children. 2022; 9(10):1568. https://doi.org/10.3390/children9101568
Chicago/Turabian StyleKoukou, Zoi, Anatoli Theodoridou, Eleftheria Taousani, Angeliki Antonakou, Eleftherios Panteris, Styliani-Stella Papadopoulou, Anna Skordou, and Stavros Sifakis. 2022. "Effectiveness of Non-Pharmacological Methods, Such as Breastfeeding, to Mitigate Pain in NICU Infants" Children 9, no. 10: 1568. https://doi.org/10.3390/children9101568