The Effect of Thyrotropin-Releasing Hormone and Antithyroid Drugs on Fetal Thyroid Function
Abstract
:1. Introduction
2. Materials and Methods
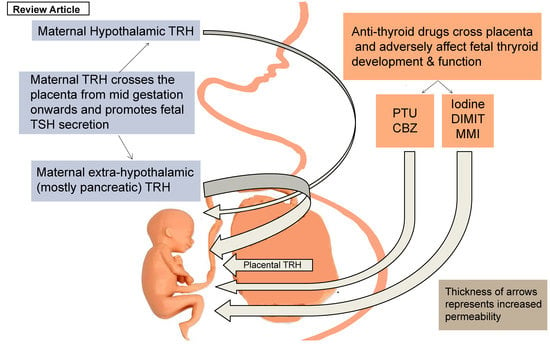
3. The Effect of Thyrotropin-Releasing Hormone (TRH) in the Development of Fetal Thyroid Function
4. The Effect of ATDs in the Development of Fetal Thyroid Function
5. TRH Analogues, Synthetic Drugs, and Their Action in the Development of Fetal Thyroid Function
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Epstein, F.H.; Burrow, G.N.; Fisher, D.A.; Larsen, P.R. Maternal and Fetal Thyroid Function. N. Engl. J. Med. 1994, 331, 1072–1078. [Google Scholar] [CrossRef] [Green Version]
- Andersen, S.L.; Olsen, J.; Laurberg, P. Foetal programming by maternal thyroid disease. Clin. Endocrinol. 2015, 83, 751–758. [Google Scholar] [CrossRef] [Green Version]
- Alemu, A.; Terefe, B.; Abebe, M.; Biadgo, B. Thyroid hormone dysfunction during pregnancy: A review. Int. J. Reprod. Biomed. 2016, 14, 677–686. [Google Scholar] [CrossRef] [Green Version]
- Vrachnis, N.; Kalampokas, E.; Sifakis, S.; Vitoratos, N.; Kalampokas, T.; Botsis, D.; Iliodromiti, Z. Placental growth factor (PlGF): A key to optimizing fetal growth. J. Matern. Neonatal Med. 2013, 26, 995–1002. [Google Scholar] [CrossRef]
- Forrest, D. The developing brain and maternal thyroid hormone: Finding the links. Endocrinology 2004, 145, 4034–4036. [Google Scholar] [CrossRef] [Green Version]
- Konstantakou, P.; Mastorakos, G.; Vrachnis, N.; Tomlinson, J.W.; Valsamakis, G. Dysregulation of 11beta-hydroxysteroid dehydrogenases: Implications during pregnancy and beyond. J. Matern. Neonatal Med. 2016, 30, 284–293. [Google Scholar] [CrossRef]
- James, S.R.; Franklyn, J.A.; Kilby, M.D. Placental transport of thyroid hormone. Best Pract. Res. Clin. Endocrinol. Metab. 2007, 21, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Landers, K.; Richard, K. Traversing barriers—How thyroid hormones pass placental, blood-brain and blood-cerebrospinal fluid barriers. Mol. Cell. Endocrinol. 2017, 458, 22–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deligeoroglou, E.; Athanasopoulos, N.; Tsimaris, P.; Dimopoulos, K.D.; Vrachnis, N.; Creatsas, G. Evaluation and management of adolescent amenorrhea. Ann. N. Y. Acad. Sci. 2010, 1205, 23–32. [Google Scholar] [CrossRef]
- Sakkas, E.G.; Paltoglou, G.; Linardi, A.; Gryparis, A.; Nteka, E.; Chalarakis, N.; Mantzou, A.; Vrachnis, N.; Iliodromiti, Z.; Koukkou, E.; et al. Associations of maternal oestradiol, cortisol, and TGF-β1 plasma concentrations with thyroid autoantibodies during pregnancy and postpartum. Clin. Endocrinol. 2018, 89, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Moleti, M.; Trimarchi, F.; Vermiglio, F. Thyroid Physiology in Pregnancy. Endocr. Pract. 2014, 20, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Springer, D.; Jiskra, J.; Limanova, Z.; Zima, T.; Potlukova, E. Thyroid in pregnancy: From physiology to screening. Crit. Rev. Clin. Lab. Sci. 2017, 54, 102–116. [Google Scholar] [CrossRef]
- Fliers, E.; Bauer, K.; Visser, T. Thyrotropin-Releasing Hormone (TRH). In Encyclopedia of Endocrine Diseases; Elsevier: Amsterdam, The Netherlands, 2004; pp. 577–580. [Google Scholar]
- Bílek, R.; Bičíková, M.; Šafařík, L. TRH-Like Peptides. Physiol. Res. 2011, 60, 207–215. [Google Scholar] [CrossRef]
- Roti, M.E.; Gnudi, A.; Braverman, L.E. The Placental Transport, Synthesis and Metabolism of Hormones and Drugs which Affect Thyroid Function*. Endocr. Rev. 1983, 4, 131–149. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, B.B.; Andersen, H.; Dige-Petersen, H.; Hummer, L. Pituitary-thyroid responsiveness to thyrotropin-releasing hormone in preterm and small-for-gestational age newborns. Acta Paediatr. Scand. 1977, 66, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Gregoriou, O.; Papadias, K.; Konidaris, S.; Bakalianou, K.; Salakos, N.; Vrachnis, N.; Creatsas, G. Treatment of hirsutism with combined pill containing drospirenone. Gynecol. Endocrinol. 2008, 24, 220–223. [Google Scholar] [CrossRef]
- Augoulea, A.; Vrachnis, N.; Lambrinoudaki, I.; Dafopoulos, K.; Iliodromiti, Z.; Daniilidis, A.; Varras, M.; Alexandrou, A.; Deligeoroglou, E.; Creatsas, G. Osteoprotegerin as a Marker of Atherosclerosis in Diabetic Patients. Int. J. Endocrinol. 2013, 2013, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Anselmo, J.; Cao, D.; Karrison, T.; Weiss, R.E.; Refetoff, S. Fetal Loss Associated With Excess Thyroid Hormone Exposure. JAMA 2004, 292, 691–695. [Google Scholar] [CrossRef] [Green Version]
- Ballabio, M.; Poshyachinda, M.; Ekins, R.P. Pregnancy-Induced Changes in Thyroid Function: Role of Human Chorionic Gonadotropin as Putative Regulator of Maternal Thyroid*. J. Clin. Endocrinol. Metab. 1991, 73, 824–831. [Google Scholar] [CrossRef]
- Gravidarum, H.; Tumors, T.; Chorionic, H. Human chorionic gonadotropin and the thyroid: Hyperemesis gravidarum and trophoblastic tumors. Thyroid 1999, 9, 653–657. [Google Scholar]
- Glinoer, D. What happens to the normal thyroid during pregnancy? Thyroid 1999, 9, 631–635. [Google Scholar] [CrossRef]
- Cao, X.-Y.; Jiang, X.-M.; Dou, Z.-H.; Rakeman, M.A.; Zhang, M.-L.; O’Donnell, K.; Ma, T.; Amette, K.; Delong, N.; Delong, G.R. Timing of Vulnerability of the Brain to Iodine Deficiency in Endemic Cretinism. N. Engl. J. Med. 1994, 331, 1739–1744. [Google Scholar] [CrossRef]
- Calina, D.; Docea, A.O.; Golokhvast, K.S.; Sifakis, S.; Tsatsakis, A.; Makrigiannakis, A. Management of Endocrinopathies in Pregnancy: A Review of Current Evidence. Int. J. Environ. Res. Public Health 2019, 16, 781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theodoropoulos, T.; Braverman, L.E.; Vagenakis, A.G. Thyrotropin-Releasing Hormone is not Required for Thyrotropin Secretion in the Perinatal Rat. J. Clin. Investig. 1979, 63, 588–594. [Google Scholar] [CrossRef] [Green Version]
- Polak, M. Human fetal thyroid function. Paediatr. Thyroidol. 2012, 26, 17–25. [Google Scholar]
- Fröhlich, E.; Wahl, R. The forgotten effects of thyrotropin-releasing hormone: Metabolic functions and medical applications. Front. Neuroendocr. 2019, 52, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Aratan-Spire, S.; Czernichow, P. Thyrotropin-Releasing Hormone-Degrading Activity of Neonatal Human Plasma. J. Clin. Endocrinol. Metab. 1980, 50, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Neary, J.T.; Nakamura, C.; Davies, I.J.; Soodak, M.; Maloof, F. Lower levels of thyrotropin-releasing hormone-degrading activity in human cord and in maternal sera than in the serum of euthyroid, nonpregnant adults. J. Clin. Investig. 1978, 62, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marchant, B.; Brownlie, B.E.W.; Hart, D.M.; Horton, P.W.; Alexander, W.D. The Placental Transfer of Propylthiouracil, Methimazole and Carbimazole. J. Clin. Endocrinol. Metab. 1977, 45, 1187–1193. [Google Scholar] [CrossRef] [PubMed]
- Capelo, L.; Beber, E.H.; Huang, S.A.; Zorn, T.M.; Bianco, A.C.; Gouveia, C.H. Deiodinase-mediated thyroid hormone inactivation minimizes thyroid hormone signaling in the early development of fetal skeleton. Bone 2008, 43, 921–930. [Google Scholar] [CrossRef] [Green Version]
- Napier, C.; Pearce, S.H.S. Rethinking antithyroid drugs in pregnancy. Clin. Endocrinol. 2015, 82, 475–477. [Google Scholar] [CrossRef] [PubMed]
- Yalamanchi, S.; Cooper, D.S. Thyroid disorders in pregnancy. Curr. Opin. Obstet. Gynecol. 2015, 27, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Koren, G. Motherisk Update Hyperthyroidism during pregnancy. Can. Fam. Physician 2009, 55, 701–703. [Google Scholar] [PubMed]
- Taylor, P.N.; Vaidya, B. Side Effects of Anti-Thyroid Drugs and Their Impact on the Choice of Treatment for Thyrotoxicosis in Pregnancy. Eur. Thyroid. J. 2012, 1, 176–185. [Google Scholar] [CrossRef] [Green Version]
- Khan, I.; Okosieme, O.; Lazarus, J. Antithyroid drug therapy in pregnancy: A review of guideline recommendations. Expert Rev. Endocrinol. Metab. 2017, 12, 269–278. [Google Scholar] [CrossRef]
- Alexander, E.K.; Pearce, E.N.; Brent, G.A.; Brown, R.S.; Chen, H.; Dosiou, C.; Grobman, W.A.; Laurberg, P.; Lazarus, J.; Mandel, S.J.; et al. 2017 Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum. Thyroid 2017, 27, 315–389. [Google Scholar] [CrossRef] [Green Version]
- Korevaar, T.I.M.; Medici, M.; Visser, T.J.; Peeters, R.P. Thyroid disease in pregnancy: New insights in diagnosis and clinical management. Nat. Rev. Endocrinol. 2017, 13, 610–622. [Google Scholar] [CrossRef]
- Moleti, M.; Di Mauro, M.; Sturniolo, G.; Russo, M.; Vermiglio, F. Hyperthyroidism in the pregnant woman: Maternal and fetal aspects. J. Clin. Transl. Endocrinol. 2019, 16, 100190. [Google Scholar] [CrossRef]
- Nazarpour, S.; Tehrani, F.R.; Simbar, M.; Azizi, F. Thyroid dysfunction and pregnancy outcomes. Iran. J. Reprod. Med. 2015, 13, 387–396. [Google Scholar]
- Moog, N.; Entringer, S.; Heim, C.; Wadhwa, P.; Kathmann, N.; Buss, C. Influence of maternal thyroid hormones during gestation on fetal brain development. Neuroscience 2017, 342, 68–100. [Google Scholar] [CrossRef] [Green Version]
- Roti, E.; Gnudi, A.; Braverman, L.E.; Robuschi, G.; Emanuele, R.; Bandini, P.; Benassi, L.; Pagliani, A.; Emerson, C.H. Human cord blood concentrations of thyrotropin, thyroglobulin, and iodothyronines after maternal administration of thyrotropin-releasing hormone. J. Clin. Endocrinol. Metab. 1981, 53, 813–817. [Google Scholar] [CrossRef] [PubMed]
- Shambaugh, G.; Kubek, M.; Wilber, J.F. Thyrotropin-Releasing Hormone Activity in the Human Placenta*. J. Clin. Endocrinol. Metab. 1979, 48, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Youngblood, W.W.; Lipton, M.A.; Humm, J.; Kizer, J.S. Thyrotropin-Releasing Hormone-Like Bioactivity in Placenta: Evidence for the Existence of Substances Other Than Pyroglu-His-Pro-NH 2 (TRH) Capable of Stimulating Pituitary Thyrotropin Release*. Endocrinology 1980, 106, 541–546. [Google Scholar] [CrossRef]
- Bagnoli, F.; Laura, F.; Sara, N.; Salvatore, G. Thyroid Function in Small for Gestational Age Newborns: A Review. J. Clin. Res. Pediatr. Endocrinol. 2012, 4, 2–7. [Google Scholar] [CrossRef]
- Engler, D.; Scanlon, M.F.; Jackson, I.M.D. Thyrotropin-releasing Hormone in the Systemic Circulation of the Neonatal Rat Is Derived from the Pancreas and Other Extraneural Tissues. J. Clin. Investig. 1981, 67, 800–808. [Google Scholar] [CrossRef] [Green Version]
- Moya, F.; Mena, P.; Heusser, F.; Foradori, A.; Paiva, E.; Yazigi, R.; Michaud, P.; Gross, I. Response of the Maternal, Fetal, and Neonatal Pituitary-Thyroid Axis to Thyrotropin-Releasing Hormone. Pediatr. Res. 1986, 20, 982–986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Napso, T.; Yong, H.E.J.; Lopez-Tello, J.; Sferruzzi-Perri, A. The Role of Placental Hormones in Mediating Maternal Adaptations to Support Pregnancy and Lactation. Front. Physiol. 2018, 9, 1091. [Google Scholar] [CrossRef] [PubMed]
- Azukizawa, M.; Murata, Y.; Ikenoue, T.; Martin, C.B.; Hershman, J.M. Effect of Thyrotropin-Releasing Hormone on Secretion of Thyrotropin, Prolactin, Thyroxine, and Triiodothyronine in Pregnant and Fetal Rhesus Monkeys. J. Clin. Endocrinol. Metab. 1976, 43, 1020–1028. [Google Scholar] [CrossRef]

| Hormone/Drug | Molecular Weight (g/mol) | Penetrability |
|---|---|---|
| Maternal hypothalamic TRH | 362.4 | + |
| Exogenous TRH | 362.4 | +++ |
| Placental TRH | * | +++ |
| Maternal pancreatic TRH | * | +++ |
| Maternal extrahypothalamic TRH | * | ++ |
| Iodine | 126.9 | +++ |
| DIMIT | 267.1 | +++ |
| PTU | 170.2 | ++ |
| CBZ | 186.2 | ++ |
| MMI | 114.1 | +++ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vrachnis, N.; Tsonis, O.; Vrachnis, D.; Antonakopoulos, N.; Paltoglou, G.; Barbounaki, S.; Mastorakos, G.; Paschopoulos, M.; Iliodromiti, Z. The Effect of Thyrotropin-Releasing Hormone and Antithyroid Drugs on Fetal Thyroid Function. Children 2021, 8, 454. https://doi.org/10.3390/children8060454
Vrachnis N, Tsonis O, Vrachnis D, Antonakopoulos N, Paltoglou G, Barbounaki S, Mastorakos G, Paschopoulos M, Iliodromiti Z. The Effect of Thyrotropin-Releasing Hormone and Antithyroid Drugs on Fetal Thyroid Function. Children. 2021; 8(6):454. https://doi.org/10.3390/children8060454
Chicago/Turabian StyleVrachnis, Nikolaos, Orestis Tsonis, Dionisios Vrachnis, Nikolaos Antonakopoulos, George Paltoglou, Stavroula Barbounaki, George Mastorakos, Minas Paschopoulos, and Zoi Iliodromiti. 2021. "The Effect of Thyrotropin-Releasing Hormone and Antithyroid Drugs on Fetal Thyroid Function" Children 8, no. 6: 454. https://doi.org/10.3390/children8060454