Insights into the Frequency and Distinguishing Features of Sleep Disorders in Pediatric Palliative Care Incorporating a Systematic Sleep Protocol
Abstract
:1. Introduction
2. Materials and Methods
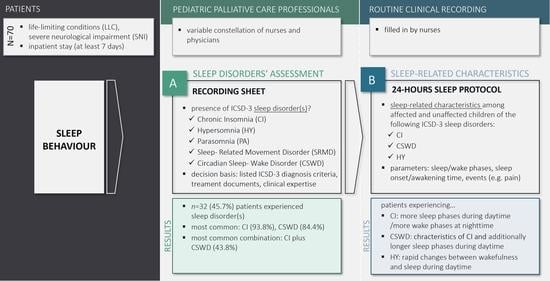
2.1. Participants
2.2. Assessment of Sleep Disorders
2.3. 24-Hours Sleep Protocol
2.4. Data Collection
2.5. Data Analysis
- Core phases: Sleep and wake phases during the daytime and nighttime (number and duration)
- Parameters of phase initiation: Sleep onset time (start of first sleep phase during the nighttime) and awakening time (start of first wake phase during the daytime)
- Events in the core phases during the daytime and nighttime (number, as duration could not be reliably interpreted due to the way of standard recording).
3. Results
3.1. Sample Characteristics
3.2. Sleep Disorders Ascribed by Professionals
3.3. Analyses of the 24-Hours Sleep Protocols
4. Discussion
Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Connor, S.R.; Downing, J.; Marston, J. Estimating the global need for palliative care for children: A cross-sectional analysis. J. Pain Symptom Manag. 2017, 53, 171–177. [Google Scholar] [CrossRef]
- Craig, F.; Abu-Saad Huijer, H.; Benini, F.; Kuttner, L.; Wood, C.; Feraris, P.C.; Zernikow, B. Impacct: Standards of paediatric palliative care. Schmerz 2008, 22, 401–408. [Google Scholar] [CrossRef]
- Hoell, J.I.; Weber, H.; Warfsmann, J.; Trocan, L.; Gagnon, G.; Danneberg, M.; Balzer, S.; Keller, T.; Janssen, G.; Kuhlen, M. Facing the large variety of life-limiting conditions in children. Eur. J. Pediatrics 2019, 178, 1893–1902. [Google Scholar] [CrossRef] [PubMed]
- Schwantes, S.; O’Brien, H.W. Pediatric palliative care for children with complex chronic medical conditions. Pediatric Clin. N. Am. 2014, 61, 797–821. [Google Scholar] [CrossRef] [PubMed]
- Hauer, J.M.; Wolfe, J. Supportive and palliative care of children with metabolic and neurological diseases. Curr. Opin. Support. Palliat. Care 2014, 8, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Borrington, C.; Akhtar, S.; Tirupatikumara, L.; McCathie, N. Doctor, my child won’t sleep. How can you help? Paediatr. Child Health 2017, 27, 427–431. [Google Scholar] [CrossRef]
- Siden, H.; Steele, R. Charting the territory: Children and families living with progressive life-threatening conditions. Paediatr. Child Health 2015, 20, 139–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tietze, A.-L.; Blankenburg, M.; Hechler, T.; Michel, E.; Koh, M.; Schlüter, B.; Zernikow, B. Sleep disturbances in children with multiple disabilities. Sleep Med. Rev. 2012, 16, 117–127. [Google Scholar] [CrossRef]
- Namisango, E.; Bristowe, K.; Allsop, M.J.; Murtagh, F.E.M.; Abas, M.; Higginson, I.J.; Downing, J.; Harding, R. Symptoms and concerns among children and young people with life-limiting and life-threatening conditions: A systematic review highlighting meaningful health outcomes. Patient 2019, 12, 15–55. [Google Scholar] [CrossRef]
- Malcolm, C.; Forbat, L.; Anderson, G.; Gibson, F.; Hain, R. Challenging symptom profiles of life-limiting conditions in children: A survey of care professionals and families. Palliat. Med. 2011, 25, 357–364. [Google Scholar] [CrossRef]
- Hemmingsson, H.; Stenhammar, A.M.; Paulsson, K. Sleep problems and the need for parental night-time attention in children with physical disabilities. Child Care Health Dev. 2009, 35, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Simard-Tremblay, E.; Constantin, E.; Gruber, R.; Brouillette, R.T.; Shevell, M. Sleep in children with cerebral palsy: A review. J. Child Neurol. 2011, 26, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Mol, E.M.; Monbaliu, E.; Ven, M.; Vergote, M.; Prinzie, P. The use of night orthoses in cerebral palsy treatment: Sleep disturbance in children and parental burden or not? Res. Dev. Disabil. 2012, 33, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Mörelius, E.; Hemmingsson, H. Parents of children with physical disabilities—Perceived health in parents related to the child’s sleep problems and need for attention at night. Child Care Health Dev. 2014, 40, 412–418. [Google Scholar] [CrossRef]
- American Academy of Sleep Medicine. International Classification of Sleep Disorders; American Academy of Sleep Medicine: Darien, IL, USA, 2014. [Google Scholar]
- Stores, G. Children’s sleep disorders: Modern approaches, developmental effects, and children at special risk. Dev. Med. Child Neurol. 1999, 41, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Scher, M.S. Topical review: Applying classifications of sleep disorders to children with neurologic conditions. J. Child Neurol. 1998, 13, 525–536. [Google Scholar] [CrossRef]
- Tsai, S.Y.; Lee, W.T.; Lee, C.C.; Jeng, S.F.; Weng, W.C. Agreement between actigraphy and diary-recorded measures of sleep in children with epilepsy. J. Nurs. Scholarsh. 2018, 50, 143–150. [Google Scholar] [CrossRef]
- Blankenburg, M.; Tietze, A.L.; Hechler, T.; Hirschfeld, G.; Michel, E.; Koh, M.; Zernikow, B. Snake: The development and validation of a questionnaire on sleep disturbances in children with severe psychomotor impairment. Sleep Med. 2013, 14, 339–351. [Google Scholar] [CrossRef]
- Tietze, A.L.; Zernikow, B.; Michel, E.; Blankenburg, M. Sleep disturbances in children, adolescents, and young adults with severe psychomotor impairment: Impact on parental quality of life and sleep. Dev. Med. Child Neurol. 2014, 56, 1187–1193. [Google Scholar] [CrossRef]
- Romeo, D.M.; Brogna, C.; Quintiliani, M.; Baranello, G.; Pagliano, E.; Casalino, T.; Sacco, A.; Ricci, D.; Mallardi, M.; Musto, E.; et al. Sleep disorders in children with cerebral palsy: Neurodevelopmental and behavioral correlates. Sleep Med. 2014, 15, 213–218. [Google Scholar] [CrossRef]
- Zambrelli, E.; Fossati, C.; Turner, K.; Taiana, M.; Vignoli, A.; Gervasini, C.; Russo, S.; Furia, F.; Masciadri, M.; Ajmone, P.; et al. Sleep disorders in cornelia de lange syndrome. Am. J. Med. Genet. Part C Semin. Med. Genet. 2016, 172, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Pera, M.C.; Romeo, D.M.; Graziano, A.; Palermo, C.; Messina, S.; Baranello, G.; Coratti, G.; Massaro, M.; Sivo, S.; Arnoldi, M.T.; et al. Sleep disorders in spinal muscular atrophy. Sleep Med. 2017, 30, 160–163. [Google Scholar] [CrossRef] [PubMed]
- Garske, D.; Schmidt, P.; Hasan, C.; Wager, J.; Zernikow, B. Palliativversorgung auf der pädiatrischen palliativstation “lichtblicke”—Eine retrospektive studie. Palliativmedizin 2016, 17, 302–307. [Google Scholar] [CrossRef]
- Alves, R.S.; Resende, M.B.; Skomro, R.P.; Souza, F.J.; Reed, U.C. Sleep and neuromuscular disorders in children. Sleep Med. Rev. 2009, 13, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Scholle, S.; Scholle, H.C.; Kemper, A.; Glaser, S.; Rieger, B.; Kemper, G.; Zwacka, G. First night effect in children and adolescents undergoing polysomnography for sleep-disordered breathing. Clin. Neurophysiol. 2003, 114, 2138–2145. [Google Scholar] [CrossRef]
- Agnew, H.W., Jr.; Webb, W.B.; Williams, R.L. The first night effect: An eeg study of sleep. Psychophysiology 1966, 2, 263–266. [Google Scholar] [CrossRef]
- European Commission. Peer review on “germany’s latest reforms ot the long-term care system”. In Social Affairs and Inclusion; DG Employment: Berlin, Germany, 2018. [Google Scholar]
- Kohrman, M.H.; Carney, P.R. Sleep-related disorders in neurologic disease during childhood. Pediatric Neurol. 2000, 23, 107–113. [Google Scholar] [CrossRef]
- Tolaymat, A.; Liu, Z. Sleep disorders in childhood neurological diseases. Children 2017, 4, 84. [Google Scholar] [CrossRef] [Green Version]
- Dorris, L.; Scott, N.; Zuberi, S.; Gibson, N.; Espie, C. Sleep problems in children with neurological disorders. Dev. Neurorehabilit. 2008, 11, 95–114. [Google Scholar] [CrossRef]
- Maski, K.; Owens, J.A. Insomnia, parasomnias, and narcolepsy in children: Clinical features, diagnosis, and management. Lancet Neurol. 2016, 15, 1170–1181. [Google Scholar] [CrossRef]
- Quine, L. Sleep problems in children with mental handicap. J. Intellect. Disabil. Res. 1991, 35, 269–290. [Google Scholar] [CrossRef] [PubMed]
- Atmawidjaja, R.W.; Wong, S.W.; Yang, W.W.; Ong, L.C. Sleep disturbances in malaysian children with cerebral palsy. Dev. Med. Child Neurol. 2014, 56, 681–685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horwood, L.; Mok, E.; Li, P.; Oskoui, M.; Shevell, M.; Constantin, E. Prevalence of sleep problems and sleep-related characteristics in preschool- and school-aged children with cerebral palsy. Sleep Med. 2018, 50, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Newman, C.J.; O’Regan, M.; Hensey, O. Sleep disorders in children with cerebral palsy. Dev. Med. Child Neurol. 2006, 48, 564–568. [Google Scholar] [CrossRef]
- Lim, M.M.; Szymusiak, R. Neurobiology of arousal and sleep: Updates and insights into neurological disorders. Curr. Sleep Med. Rep. 2015, 1, 91–100. [Google Scholar] [CrossRef] [Green Version]
- Thiriez, G.; Tournoud, M.; Wermenbol, V.; Vermeylen, D.; Ecochard, R.; Iwaz, J.; Lin, J.S.; Franco, P. Decreased spontaneous arousability in preterm newborns with impaired neurological outcome. J. Sleep Res. 2012, 21, 552–560. [Google Scholar] [CrossRef]

| ICD-10 Code—Group (n,% in Total Sample) Sleep Disorders According to ICSD-3 a (n,% within Underlying Disease) | ||||
|---|---|---|---|---|
| Chronic Insomnia | Hypersomnia | Parasomnia | Sleep-Related Movement Disorder | Circadian Sleep–Wake Disorder |
| G12—Spinal muscular atrophy and related syndromes (n = 3, 4.3) | ||||
| 1 (33.3) | - | - | - | 1 (33.3) |
| G31—Other degenerative diseases of nervous system, not elsewhere classified (n = 3, 4.3) | ||||
| 3 (100) | 1 (33.3) | - | 1 (33.3) | 2 (66.7) |
| G71—Primary disorders of muscles (n = 2, 2.9) | ||||
| - | - | - | - | 1 (50.0) |
| G93.1—Anoxic brain damage, not elsewhere classified (n = 7, 10.0) | ||||
| 2 (28.6) | 1 (14.3) | 1 (14.3) | 1 (14.3) | 1 (14.3) |
| Q04.8—Other specified congenital malformations of brain (n = 4, 5.7) | ||||
| 1 (25.0) | 1 (25.0) | - | - | 1 (25.0) |
| Q04.9—Congenital malformation of brain, unspecified (n = 5, 7.1) | ||||
| 3 (60.0) | 2 (40.0) | - | - | 2 (40.0) |
| Q93.8—Other specified disorders of brain (n = 3, 4.3) | ||||
| 2 (66.7) | 1 (33.3) | - | - | 2 (66.7) |
| E70–E90—Metabolic disorders (n = 11, 15.7) | ||||
| 6 (54.5) | 1 (9.1) | - | - | 6 (54.5) |
| Q87—Other specified congenital malformation syndromes affecting multiple systems (n = 10, 14.3) | ||||
| 6 (60.0) | 3 (30.0) | - | - | 5 (50.0) |
| Q89—Other congenital malformations, not elsewhere classified (n = 2, 2.9) | ||||
| 1 (50.0) | - | - | - | 1 (50.0) |
| Q93.9—Disorders of brain, unspecified (n = 2, 2.9) | ||||
| - | - | - | - | - |
| P91—Other disturbances of cerebral status of newborn (n = 18, 25.7) | ||||
| 5 (27.8) | - | 2 (11.1) | 2 (11.1) | 5 (27.8) |
| Type n (%) | ||||||
|---|---|---|---|---|---|---|
| Number of Assigned Sleep Disorders | Chronic Insomnia | Hypersomnia | Parasomnia | Sleep-Related Movement Disorder | Circadian Sleep–Wake Disorder | n (%) |
| 1 | 3 (9.4) | |||||
| 1 (3.1) | ||||||
| 2 | 14 (43.8) | |||||
| 1 (3.1) | ||||||
| 3 | 9 (28.1) | |||||
| 2 (6.3) | ||||||
| 1 (3.1) | ||||||
| 4 | 1 (3.1) | |||||
| 30 (93.8) | 10 (31.3) | 3 (9.4) | 4 (12.5) | 27 (84.4) | ||
| Nighttime | Daytime | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| core phases | sleep disorder | sleeping phase | waking phase | sleeping phase | waking phase | ||||
| nr | du | nr | du | nr | du | nr | du | ||
| Chronic Insomnia | 0.32 ** | −0.05 | 0.30 ** | 0.15 | 0.30 * | 0.19 | 0.32 ** | 0.03 | |
| Hypersomnia | 0.19 | −0.01 | 0.16 | 0.06 | 0.15 | 0.19 | 0.11 | −0.14 | |
| Circadian Sleep–Wake Disorder | 0.28 * | −0.19 | 0.25 * | 0.20 | 0.39 ** | 0.30 * | 0.35 ** | −0.14 | |
| phase initiation | sleep disorder | sleep onset time | awakening time | ||||||
| r | r | ||||||||
| Chronic Insomnia | −0.12 | −0.09 | |||||||
| Hypersomnia | −0.07 | −0.00 | |||||||
| Circadian Sleep–Wake Disorder | −0.09 | 0.03 | |||||||
| nighttime | daytime | ||||||||
| Events a | sleep disorder | sleeping phase | waking phase | sleeping phase | waking phase | ||||
| type | r | type | r | type | r | type | r | ||
| Chronic Insomnia | |||||||||
| Hypersomnia | QPC | 0.24 * | QPC | 0.24 * | |||||
| Circadian Sleep–Wake Disorder | QPC | 0.25 * | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dreier, L.A.; Zernikow, B.; Stening, K.; Wager, J. Insights into the Frequency and Distinguishing Features of Sleep Disorders in Pediatric Palliative Care Incorporating a Systematic Sleep Protocol. Children 2021, 8, 54. https://doi.org/10.3390/children8010054
Dreier LA, Zernikow B, Stening K, Wager J. Insights into the Frequency and Distinguishing Features of Sleep Disorders in Pediatric Palliative Care Incorporating a Systematic Sleep Protocol. Children. 2021; 8(1):54. https://doi.org/10.3390/children8010054
Chicago/Turabian StyleDreier, Larissa Alice, Boris Zernikow, Kathrin Stening, and Julia Wager. 2021. "Insights into the Frequency and Distinguishing Features of Sleep Disorders in Pediatric Palliative Care Incorporating a Systematic Sleep Protocol" Children 8, no. 1: 54. https://doi.org/10.3390/children8010054