Experiences of Caregivers and At-Risk Children Enrolled in a Prospective Pregnancy-Birth Cohort Study into the Causes of Type 1 Diabetes: The ENDIA Study
Abstract
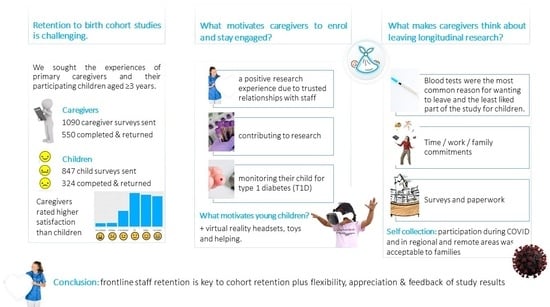
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Survey Design
2.2.1. Caregiver Survey
2.2.2. Child Survey
2.3. Survey Collection
2.4. Data Analysis
2.4.1. Quantitative Data
2.4.2. Qualitative Data
3. Results
3.1. Response Rate
3.2. Demographics
3.3. Overall Caregiver and Child Experience
3.4. Expectations of Study Participation
3.5. Motives for Participating in ENDIA
3.6. Thoughts of Leaving
3.7. Satisfaction with the Study Protocol
3.8. Regional Participation Program (RPP)
3.9. ENDIA during COVID-19
4. Discussion
4.1. Limitations
4.2. Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barned, C.; Dobson, J.; Stintzi, A.; Mack, D.; O’Doherty, K.C. Children’s perspectives on the benefits and burdens of research participation. AJOB Empir. Bioeth. 2018, 9, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Tromp, K.; Zwaan, C.M.; van de Vathorst, S. Motivations of children and their parents to participate in drug research: A systematic review. Eur. J. Pediatr. 2016, 175, 599–612. [Google Scholar] [CrossRef] [Green Version]
- Wendler, D.; Jenkins, T. Children’s and their parents’ views on facing research risks for the benefit of others. Arch. Pediatr. Adolesc. Med. 2008, 162, 9–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unguru, Y.; Sill, A.M.; Kamani, N. The experiences of children enrolled in pediatric oncology research: Implications for assent. Pediatrics 2010, 125, e876–e883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Pal, S.; Sozanska, B.; Madden, D.; Kosmeda, A.; Debinska, A.; Danielewicz, H.; Boznanski, A.; Detmar, S. Opinions of children about participation in medical genetic research. Public Health Genom. 2011, 14, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Swartling, U.; Hansson, M.G.; Ludvigsson, J.; Nordgren, A. “My parents decide if I can. I decide if I want to.” Children’s views on participation in medical research. J. Empir. Res. Hum. Res. Ethics 2011, 6, 68–75. [Google Scholar] [CrossRef] [PubMed]
- The National Health and Medical Research Council the Australian Research Council and Universities Australia. National Statement on Ethical Conduct in Human Research 2007 (Updated 2018); Commonwealth of Australia: Canberra, Australia, 2018. Available online: http://www.nhmrc.gov.au/guidelines/publications/e72 (accessed on 8 November 2022).
- Dennis, B.K. Understanding Participant Experiences: Reflections of a Novice Research Participant. Int. J. Qual. Methods 2014, 13, 395–410. [Google Scholar] [CrossRef]
- Ziegler, A.G.; Hoffmann, G.F.; Hasford, J.; Larsson, H.E.; Danne, T.; Berner, R.; Penno, M.; Koralova, A.; Dunne, J.; Bonifacio, E. Screening for asymptomatic beta-cell autoimmunity in young children. Lancet Child Adolesc. Health 2019, 3, 288–290. [Google Scholar] [CrossRef]
- Sims, E.K.; Besser, R.E.J.; Dayan, C.; Rasmussen, C.G.; Greenbaum, C.; Griffin, K.J.; Hagopian, W.; Knip, M.; Long, A.E.; Martin, F.; et al. Screening for Type 1 Diabetes in the General Population: A Status Report and Perspective. Diabetes 2022, 71, 610–623. [Google Scholar] [CrossRef]
- Penno, M.A.; Couper, J.J.; Craig, M.E.; Colman, P.G.; Rawlinson, W.D.; Cotterill, A.M.; Jones, T.W.; Harrison, L.C.; ENDIA Study Group. Environmental determinants of islet autoimmunity (ENDIA): A pregnancy to early life cohort study in children at-risk of type 1 diabetes. BMC Pediatr. 2013, 13, 124. [Google Scholar] [CrossRef] [Green Version]
- Penno, M.A.; Thomson, R.L.; Couper, J.J. Bunbury to Bundaberg, Darwin to Dover: Establishing a successful Regional Participation Program for the ENDIA type 1 diabetes cohort study. Med. J. Aust. 2016, 205, 486. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.W.; Allen, D.W.; Briese, T.; Couper, J.J.; Barry, S.C.; Colman, P.G.; Cotterill, A.M.; Davis, E.A.; Giles, L.C.; Harrison, L.C.; et al. Distinct gut virome profile of pregnant women with type 1 diabetes in the ENDIA study. Open Forum Infect. Dis. 2019, 6, ofz025. [Google Scholar] [CrossRef]
- Roth-Schulze, A.J.; Penno, M.A.S.; Ngui, K.M.; Oakey, H.; Bandala-Sanchez, E.; Smith, A.D.; Allnutt, T.R.; Thomson, R.L.; Vuillermin, P.J.; Craig, M.E.; et al. Type 1 Diabetes in Pregnancy Is Associated with Distinct Changes in the Composition and Function of the Gut Microbiome. Microbiome 2021, 9, 1–21. [Google Scholar] [CrossRef]
- Bandala-Sanchez, E.; Roth-Schulze, A.J.; Oakey, H.; Penno, M.A.S.; Bediaga, N.G.; Nasellia, G.; Ngui, K.M.; Smith, A.D.; Huang, D.; Zozaya-Valdesa, E.; et al. Women with type 1 diabetes exhibit a progressive increase in gut Saccharomyces cerevisiae in pregnancy associated with evidence of gut inflammation. Diabetes Res. Clin. Pract. 2022, 184, 109189. [Google Scholar] [CrossRef] [PubMed]
- Penno, M.A.S.; Anderson, A.J.; Thomson, R.L.; McGorm, K.; Barry, S.C.; Colman, P.G.; Craig, M.E.; Davis, E.A.; Harris, M.; Haynes, A.; et al. Evaluation of protocol amendments to the Environmental Determinants of Islet Autoimmunity (ENDIA) study during the COVID-19 pandemic. Diabet Med. 2021, 38, e14638. [Google Scholar] [CrossRef] [PubMed]
- McGorm, K.J.; Brown, J.D.; Thomson, R.L.; Oakey, H.; Moore, B.; Hendry, A.; Colman, P.G.; Craig, M.E.; Davis, E.A.; Harris, M.; et al. A Long-Term Evaluation of Facebook for Recruitment and Retention in the ENDIA Type 1 Diabetes Pregnancy-Birth Cohort Study. J. Diabetes Sci. Technol. 2022, 19322968221079867, epub ahead of print. [Google Scholar] [CrossRef]
- Lernmark, B.; Lynch, K.; Ballard, L.; Baxter, J.; Roth, R.; Simell, T.; Johnson, S.B. Reasons for Staying as a Participant in the Environmental Determinants of Diabetes in the Young (TEDDY) Longitudinal Study. J. Clin. Trials 2012, 2, 1000114. [Google Scholar] [CrossRef] [Green Version]
- Wong, D.; Baker, C. Pain in children: Comparison of assessment scales. Pediatr. Nurs. 1988, 14, 9–17. [Google Scholar]
- Harris, P.; Taylor, R.; Minor, B.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef] [Green Version]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 8 November 2022).
- Savicky, P. ‘Pspearman’: Spearman’s Rank Correlation Test. Version 0.3–1. Available online: https://cran.r-project.org/web/packages/pspearman/pspearman.pdf (accessed on 2 May 2022).
- Zhu, H.; Travison, T.; Tsai, T.; Beasley, W.; Xie, Y.; Yu, G.; Laurent, S.; Shepherd, R.; Sidi, Y.; Salzer, B.; et al. KableExtra: Construct Complex Table with Kable and Pipe Syntax. Version 1.3.4. Available online: https://cran.r-project.org/web/packages/kableExtra/kableExtra.pdf (accessed on 20 February 2021).
- Wickham, H. Package ‘ggplot2’: Elegant Graphics for Data Analysis, 2nd ed.; Springer: New York, NY, USA, 2016; ISBN 978-0-387-98141-3. Available online: https://ggplot2-book.org/ (accessed on 7 March 2023).
- Bryer, J.J.; Speerschneider, K. Analysis and Visualization Likert Items. Version 1.3.5. Available online: https://cran.r-project.org/web/packages/likert/likert.pdf (accessed on 29 December 2016).
- Chan, E.; Hovenden, M.; Ramage, E.; Ling, N.; Pham, J.H.; Rahim, A.; Lam, C.; Liu, L.; Foster, S.; Sambell, R.; et al. Virtual Reality for Pediatric Needle Procedural Pain: Two Randomized Clinical Trials. J. Pediatr. 2019, 209, 160–167.e164. [Google Scholar] [CrossRef] [PubMed]
- Rewers, M.; Hyöty, H.; Lernmark, Å.; Hagopian, W.; She, J.-X.; Schatz, D.; Ziegler, A.-G.; Toppari, J.; Akolkar, B.; the TEDDY Study Group; et al. The Environmental Determinants of Diabetes in the Young (TEDDY) Study: 2018 Update. Curr. Diab. Rep. 2018, 18, 136. [Google Scholar] [CrossRef] [PubMed]
- Lernmark, B.; Lynch, K.; Baxter, J.; Roth, R.; Simell, T.; Smith, L.; Swartling, U.; Johnson, S.B.; Teddy Study Group. Participant Experiences in the Environmental Determinants of Diabetes in the Young Study: Common Reasons for Withdrawing. J. Diabetes Res. 2016, 2016, 2720650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melin, J.; Lynch, K.F.; Lundgren, M.; Aronsson, C.A.; Larsson, H.E.; Johnson, S.B.; Rewers, M.; Barbour, A.; Bautista, K.; Baxter, J.; et al. Is staff consistency important to parents’ satisfaction in a longitudinal study of children at risk for type 1 diabetes: The TEDDY study. BMC Endocr. Disord. 2022, 22, 19. [Google Scholar] [CrossRef]
- Aslam, A.; Adams, T.L. “The workload is staggering”: Changing working conditions of stay-at-home mothers under COVID-19 lockdowns. Gend. Work Organ. 2022, 29, 1764–1778. [Google Scholar] [CrossRef]
- Johnson, S.B.; Baughcum, A.E.; Rafkin-Mervis, L.E.; Schatz, D.A.; DPT-1 Study Group. Participant and parent experiences in the oral insulin study of the Diabetes Prevention Trial for Type 1 Diabetes. Pediatr. Diabetes 2009, 10, 177–183. [Google Scholar] [CrossRef] [Green Version]




| 1.a. Parental Characteristics | Completers (n = 547) | Non-Completers (n = 543) | p-Value |
| Age of Mother (years) | |||
| Mean (SD) | 37.8 (4.87) | 37.1 (5.10) | 0.016 |
| Length of Study (years) | |||
| Median [Q1, Q3] | 5.13 [4.02, 6.41] | 5.37 [4.19, 6.42] | 0.073 a |
| Age of Father (years) | |||
| Mean (SD) | 39.6 (5.75) | 39.3 (5.81) | 0.45 |
| Missing | 21 (3.8%) | 32 (5.9%) | |
| Number of Children in ENDIA | |||
| One | 421 (76.97%) | 440 (81.03%) | 0.106 |
| Two | 114 (20.84%) | 98 (18.05%) | |
| Three | 12 (2.19%) | 5 (0.92%) | |
| 1.b. Child Characteristics | Completers (n = 685) | Non- Completers (n = 651) | p-Value |
| Age of Child (years) | |||
| Mean (SD) | 4.93 (1.73) | 5.17 (1.75) | 0.012 |
| Missing | 0 (0%) | 1 (0.2%) | |
| Days Since Last Visit | |||
| Median [Q1, Q3] | 127 [65.5, 194] | 229 [91.0, 805] | <0.001 b |
| Missing | 7 (1.0%) | 8 (1.2%) | |
| Current ENDIA Participation | |||
| Active | 648 (94.60%) | 466 (71.58%) | <0.001 |
| Inactive | 37 (5.40%) | 185 (28.42%) | |
| Proband Relationship(s) | |||
| Mother only | 395 (57.66%) | 413 (63.44%) | 0.02 |
| Father only | 209 (30.51%) | 154 (23.66%) | |
| Sibling only | 61 (8.91%) | 70 (10.75%) | |
| Multiple FDR probands c | 19 (2.77%) | 11 (1.69%) | |
| Other d | 1 (0.15%) | 3 (0.46%) | |
| Location | |||
| Site A | 202 (29.49%) | 159 (24.42%) | <0.001 |
| Site B | 158 (23.07%) | 123 (18.89%) | |
| Site C | 78 (11.39%) | 116 (17.82%) | |
| Site D | 93 (13.58%) | 103 (15.82%) | |
| Site E | 66 (9.64%) | 93 (14.29%) | |
| Regional | 88 (12.85%) | 57 (8.76%) | |
| Recruitment Time | |||
| Prenatal | 561 (81.90%) | 522 (80.18%) | 0.466 |
| Postnatal | 124 (18.10%) | 129 (19.82%) | |
| Islet autoantibodies Positive | |||
| Yes | 40 (5.84%) | 38 (5.84%) | 1 |
| No | 645 (94.16%) | 613 (94.16%) | |
| Coeliac autoantibodies Positive | |||
| Yes | 8 (1.17%) | 14 (2.15%) | 0.232 |
| No | 677 (98.83%) | 637 (97.85%) |
| Thoughts of Leaving | No (n = 402) | Yes (n = 122) | p-Value |
|---|---|---|---|
| Length of Study (years) | |||
| Median [Q1, Q3] | 4.64 [3.83, 6.04] | 5.41 [4.19, 6.57] | 0.003 a |
| Age of Child (years) | |||
| Mean (SD) | 4.82 (1.67) | 5.39 (1.87) | 0.003 |
| Days Since Last Visit | |||
| Median [Q1, Q3] | 125 [62.0, 183] | 152 [76.5, 320] | 0.001 b |
| Missing | 3 (0.7%) | 3 (2.5%) | |
| Current ENDIA Participation | |||
| Active | 387 (78.18%) | 108 (21.82%) | 0.002 |
| Inactive | 15 (51.72%) | 14 (48.28%) | |
| Proband Relationship(s) | |||
| Mother only | 235 (76.30%) | 73 (23.70%) | 0.78 |
| Father only | 120 (78.95%) | 32 (21.05%) | |
| Sibling only | 37 (75.51%) | 12 (24.49%) | |
| Multiple FDR probands c | 9 (64.29%) | 5 (35.71%) | |
| Other d | 1 (100.00%) | 0 (0.00%) | |
| Location | |||
| Site A | 129 (82.69%) | 27 17.31%) | <0.001 |
| Site B | 90 (78.26%) | 25 (21.74%) | |
| Site C | 32 (49.23%) | 33 (50.77%) | |
| Site D | 66 (94.29%) | 4 (5.71%) | |
| Site E | 51 (73.91%) | 18 (26.09%) | |
| Regional | 34 (69.39%) | 15 (30.61%) | |
| Recruitment Type | |||
| Prenatal | 333 (77.44%) | 97 (22.56%) | 0.481 |
| Postnatal | 69 (73.40%) | 25 (26.60%) | |
| IA Positive | |||
| Yes | 21 (77.78%) | 6 (22.22%) | 1 |
| No | 381 (76.66%) | 116 (23.34%) | |
| Coeliac Positive | |||
| Yes | 5 (83.33%) | 1 (16.67%) | 1 |
| No | 397 (76.64%) | 121 (23.36%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McGorm, K.J.; Brown, J.D.; Roberts, A.G.; Greenbank, S.; Brasacchio, D.; Sawyer, A.C.P.; Oakey, H.; Colman, P.G.; Craig, M.E.; Davis, E.A.; et al. Experiences of Caregivers and At-Risk Children Enrolled in a Prospective Pregnancy-Birth Cohort Study into the Causes of Type 1 Diabetes: The ENDIA Study. Children 2023, 10, 637. https://doi.org/10.3390/children10040637
McGorm KJ, Brown JD, Roberts AG, Greenbank S, Brasacchio D, Sawyer ACP, Oakey H, Colman PG, Craig ME, Davis EA, et al. Experiences of Caregivers and At-Risk Children Enrolled in a Prospective Pregnancy-Birth Cohort Study into the Causes of Type 1 Diabetes: The ENDIA Study. Children. 2023; 10(4):637. https://doi.org/10.3390/children10040637
Chicago/Turabian StyleMcGorm, Kelly J., James D. Brown, Alison G. Roberts, Susan Greenbank, Daniella Brasacchio, Alyssa C. P. Sawyer, Helena Oakey, Peter G. Colman, Maria E. Craig, Elizabeth A. Davis, and et al. 2023. "Experiences of Caregivers and At-Risk Children Enrolled in a Prospective Pregnancy-Birth Cohort Study into the Causes of Type 1 Diabetes: The ENDIA Study" Children 10, no. 4: 637. https://doi.org/10.3390/children10040637