From Metabolic Syndrome to Type 2 Diabetes in Youth
Abstract
:1. Introduction
2. Risk Factors for T2D in Young Individuals with MetS
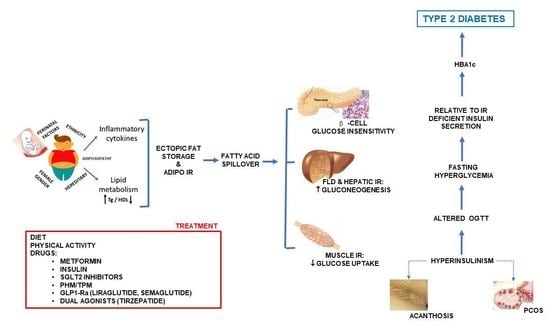
2.1. Role of Visceral Adiposity, Inflammation, and Oxidative Stress
2.2. FFAs and Tissue Insulin Resistance
2.3. Muscle–Adipose Tissue Cross-Talk
2.4. Role of Puberty
2.5. Role of Ethnicity
2.6. Role of Genetic Susceptibility
2.7. Role of Perinatal Factors
2.8. Role of Gender
2.9. Role of PCOS
2.10. Role of FLD
3. Screening for Prediabetes and T2D
4. Diagnosis and Clinical Features at the T2D Onset
5. Treatment
5.1. Nutritional Recommendations
5.2. Physical Activity
5.3. Drugs
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al-Hamad, D.; Raman, V. Metabolic syndrome in children and adolescents. Transl. Pediatr. 2017, 6, 397–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boney, C.M.; Verma, A.; Tucker, R.; Vohr, B.R. Metabolic syndrome in childhood: Association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics 2005, 115, e290–e296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiss, R.; Dziura, J.; Burgert, T.S.; Tamborlane, W.V.; Taksali, S.E.; Yeckel, C.W.; Allen, K.; Lopes, M.; Savoye, M.; Morrison, J.; et al. Obesity and the metabolic syndrome in children and adolescents. N. Engl. J. Med. 2004, 350, 2362–2374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Ferranti, S.D.; Gauvreau, K.; Ludwig, D.S.; Neufeld, E.J.; Newburger, J.W.; Rifai, N. Prevalence of the metabolic syndrome in American adolescents: Findings from the third national health and nutrition examination survey. Circulation 2004, 110, 2494–2497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruz, M.L.; Goran, M.I. The metabolic syndrome in children and adolescents. Curr. Diabetes Rep. 2004, 4, 53–62. [Google Scholar] [CrossRef]
- Alberti, K.G.M.; Zimmet, P.; Shaw, J. The metabolic syndrome—A new worldwide definition. Lancet 2005, 366, 1059–1062. [Google Scholar] [CrossRef]
- Agudelo, G.M.; Bedoya, G.; Estrada, A.; Patiño, F.A.; Muñoz, A.M.; Velásquez, C.M. Variations in the prevalence of metabolic syndrome in adolescents according to different criteria used for diagnosis: Which definition should be chosen for this age group? Metab. Syndr. Relat. Disord. 2014, 12, 202–209. [Google Scholar] [CrossRef]
- Friend, A.; Craig, L.; Turner, S. The prevalence of metabolic syndrome in children: A systematic review of the literature. Metab. Syndr. Relat. Disord. 2013, 11, 71–80. [Google Scholar] [CrossRef]
- Di Bonito, P.; Forziato, C.; Sanguigno, E.; Di Fraia, T.; Saitta, F.; Lardino, M.R.; Capaldo, B. Prevalence of the metabolic syndrome using ATP-derived definitions and its relation to insulin-resistance in a cohort of italian outpatient children. J. Endocrinol. Investig. 2010, 33, 806–809. [Google Scholar] [CrossRef]
- American Diabetes Association. 2. classification and diagnosis of diabetes: Standards of medical care in diabetes—2020. Diabetes Care 2020, 43 (Suppl. S1), S14–S31. [Google Scholar] [CrossRef] [Green Version]
- Mayer-Davis, E.J.; Kahkoska, A.R.; Jefferies, C.; Dabelea, D.; Balde, N.; Gong, C.X.; Aschner, P.; Craig, M.E. ISPAD clinical practice consensus guidelines 2018: Definition, epidemiology, and classification of diabetes in children and adolescents. Pediatr. Diabetes 2018, 19, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Giuliana, V. Diabete Tipo 2 e Obesità Pediatrica: Rassegna a Cura Dei Gruppi Di Studio Obesità Infantile e Diabete Della Società Italiana Di Endocrinologia e Diabetologia Pediatrica. G. Ital. Diabetol. Metab. 2017, 37, 213–230. [Google Scholar]
- Pedicelli, S.; Fintini, D.; Ravà, L.; Inzaghi, E.; Deodati, A.; Spreghini, M.R.; Bizzarri, C.; Mariani, M.; Cianfarani, S.; Cappa, M.; et al. Prevalence of prediabetes in children and adolescents by class of obesity. Pediatr. Obes. 2022, 17, e12900. [Google Scholar] [CrossRef] [PubMed]
- Iafusco, D. Dieci Domande Su Sindrome Metabolica e Diabete Mellito Tipo 2 Dell’adolescente. G. Ital. Diabetol. Metab. 2014, 34, 117–123. [Google Scholar]
- Shah, A.S.; Zeitler, P.S.; Wong, J.; Pena, A.S.; Wicklow, B.; Arslanian, S.; Chang, N.; Fu, J.; Dabadghao, P.; Pinhas-Hamiel, O.; et al. ISPAD clinical practice consensus guidelines 2022: Type 2 diabetes in children and adolescents. Pediatr. Diabetes 2022, 23, 872–902. [Google Scholar] [CrossRef]
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.-H.; Park, S.-Y.; Choi, C.S. Insulin resistance: From mechanisms to therapeutic strategies. Diabetes Metab. J. 2022, 46, 15–37. [Google Scholar] [CrossRef] [PubMed]
- Manco, M.; Calvani, M.; Mingrone, G. Effects of dietary fatty acids on insulin sensitivity and secretion. Diabetes Obes. Metab. 2004, 6, 402–413. [Google Scholar] [CrossRef]
- Deckelbaum, R.J.; Williams, C.L. Childhood obesity: The health issue. Obes. Res. 2001, 9, 239S–243S. [Google Scholar] [CrossRef]
- Goran, M.I.; Ball, G.D.C.; Cruz, M.L. Obesity and risk of type 2 diabetes and cardiovascular disease in children and adolescents. J. Clin. Endocrinol. Metab. 2003, 88, 1417–1427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martos-Moreno, G.Á.; Barrios, V.; Argente, J. Normative data for adiponectin, resistin, interleukin 6, and leptin/receptor ratio in a healthy spanish pediatric population: Relationship with sex steroids. Eur. J. Endocrinol. 2006, 155, 429–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paltoglou, G.; Fatouros, I.G.; Valsamakis, G.; Schoina, M.; Avloniti, A.; Chatzinikolaou, A.; Kambas, A.; Draganidis, D.; Mantzou, A.; Papagianni, M.; et al. Antioxidation improves in puberty in normal weight and obese boys, in positive association with exercise-stimulated growth hormone secretion. Pediatr. Res. 2015, 78, 158–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paltoglou, G.; Schoina, M.; Valsamakis, G.; Salakos, N.; Avloniti, A.; Chatzinikolaou, A.; Margeli, A.; Skevaki, C.; Papagianni, M.; Kanaka-Gantenbein, C.; et al. Interrelations among the adipocytokines leptin and adiponectin, oxidative stress and aseptic inflammation markers in pre- and early-pubertal normal-weight and obese boys. Endocrine 2017, 55, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Marseglia, L.; Manti, S.; D’Angelo, G.; Nicotera, A.; Parisi, E.; Di Rosa, G.; Gitto, E.; Arrigo, T. Oxidative stress in obesity: A critical component in human diseases. Int. J. Mol. Sci. 2014, 16, 378–400. [Google Scholar] [CrossRef] [Green Version]
- Greco, A.V.; Mingrone, G.; Giancaterini, A.; Manco, M.; Morroni, M.; Cinti, S.; Granzotto, M.; Vettor, R.; Camastra, S.; Ferrannini, E. Insulin resistance in morbid obesity. Diabetes 2002, 51, 144–151. [Google Scholar] [CrossRef] [Green Version]
- Raschke, S.; Eckel, J. Adipo-myokines: Two sides of the same coin—Mediators of inflammation and mediators of exercise. Mediat. Inflamm. 2013, 2013, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Graf, C.; Ferrari, N. Metabolic health—The role of adipo-myokines. Int. J. Mol. Sci. 2019, 20, 6159. [Google Scholar] [CrossRef] [Green Version]
- Peruzzi, B.; Urciuoli, E.; Mariani, M.; Chioma, L.; Tomao, L.; Montano, I.; Algeri, M.; Luciano, R.; Fintini, D.; Manco, M. Circulating extracellular vesicles impair mesenchymal stromal cell differentiation favoring adipogenic rather than osteogenic differentiation in adolescents with obesity. Int. J. Mol. Sci. 2022, 24, 447. [Google Scholar] [CrossRef]
- Magge, S.N.; Goodman, E.; Armstrong, S.C.; Daniels, S.; Corkins, M.; de Ferranti, S.; Golden, N.H.; Kim, J.H.; Schwarzenberg, S.J.; Sills, I.N.; et al. The metabolic syndrome in children and adolescents: Shifting the focus to cardiometabolic risk factor clustering. Pediatrics 2017, 140, e20171603. [Google Scholar] [CrossRef] [Green Version]
- Wittcopp, C.; Conroy, R. Metabolic syndrome in children and adolescents. Pediatr. Rev. 2016, 37, 193–202. [Google Scholar] [CrossRef]
- Divers, J.; Mayer-Davis, E.J.; Lawrence, J.M.; Isom, S.; Dabelea, D.; Dolan, L.; Imperatore, G.; Marcovina, S.; Pettitt, D.J.; Pihoker, C.; et al. Trends in incidence of type 1 and type 2 diabetes among youths—Selected counties and Indian reservations, United States, 2002–2015. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 161–165. [Google Scholar] [CrossRef]
- Frankenberg, A.D.; von Reis, A.F.; Gerchman, F. Relationships between adiponectin levels, the metabolic syndrome, and type 2 diabetes: A literature review. Arch. Endocrinol. Metab. 2017, 61, 614–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensen, E.T.; Dabelea, D. Type 2 diabetes in youth: New lessons from the search study. Curr. Diabetes Rep. 2018, 18, 36. [Google Scholar] [CrossRef] [PubMed]
- Silverman, B.L.; Metzger, B.E.; Cho, N.H.; Loeb, C.A. Impaired glucose tolerance in adolescent offspring of diabetic mothers: Relationship to fetal hyperinsulinism. Diabetes Care 1995, 18, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Todd, J.N.; Srinivasan, S.; Pollin, T.I. Advances in the genetics of youth-onset type 2 diabetes. Curr. Diabetes Rep. 2018, 18, 57. [Google Scholar] [CrossRef]
- Botnia Study Group; Almgren, P.; Lehtovirta, M.; Isomaa, B.; Sarelin, L.; Taskinen, M.R.; Lyssenko, V.; Tuomi, T.; Groop, L. Heritability and familiality of type 2 diabetes and related quantitative traits in the botnia study. Diabetologia 2011, 54, 2811–2819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarhangi, N.; Sharifi, F.; Hashemian, L.; Hassani Doabsari, M.; Heshmatzad, K.; Rahbaran, M.; Jamaldini, S.H.; Aghaei Meybodi, H.R.; Hasanzad, M. PPARG (Pro12Ala) genetic variant and risk of T2DM: A systematic review and meta-analysis. Sci. Rep. 2020, 10, 12764. [Google Scholar] [CrossRef]
- Hani, E.H.; Boutin, P.; Durand, E.; Inoue, H.; Permutt, M.A.; Velho, G.; Froguel, P. Missense mutations in the pancreatic islet beta cell inwardly rectifying K + channel gene (KIR6.2/BIR): A meta-analysis suggests a role in the polygenic basis of type II diabetes mellitus in caucasians. Diabetologia 1998, 41, 1511–1515. [Google Scholar] [CrossRef] [Green Version]
- Barroso, I.; Luan, J.; Middelberg, R.P.S.; Harding, A.-H.; Franks, P.W.; Jakes, R.W.; Clayton, D.; Schafer, A.J.; O’Rahilly, S.; Wareham, N.J. Candidate gene association study in type 2 diabetes indicates a role for genes involved in β-cell function as well as insulin action. PLoS Biol. 2003, 1, e20. [Google Scholar] [CrossRef]
- Ding, W.; Xu, L.; Zhang, L.; Han, Z.; Jiang, Q.; Wang, Z.; Jin, S. Meta-analysis of association between TCF7L2 polymorphism Rs7903146 and type 2 diabetes mellitus. BMC Med. Genet. 2018, 19, 38. [Google Scholar] [CrossRef] [Green Version]
- Verma, M.; Loh, N.Y.; Sabaratnam, R.; Vasan, S.K.; van Dam, A.D.; Todorčević, M.; Neville, M.J.; Toledo, E.; Karpe, F.; Christodoulides, C. TCF7L2 plays a complex role in human adipose progenitor biology, which might contribute to genetic susceptibility to type 2 diabetes. Metabolism 2022, 133, 155240. [Google Scholar] [CrossRef]
- Dabelea, D.; Dolan, L.M.; D’Agostino, R.; Hernandez, A.M.; McAteer, J.B.; Hamman, R.F.; Mayer-Davis, E.J.; Marcovina, S.; Lawrence, J.M.; Pihoker, C.; et al. Association testing of TCF7L2 polymorphisms with type 2 diabetes in multi-ethnic youth. Diabetologia 2011, 54, 535–539. [Google Scholar] [CrossRef] [Green Version]
- Giannini, C.; Polidori, N.; Chiarelli, F.; Mohn, A. The bad rainbow of COVID-19 time: Effects on glucose metabolism in children and adolescents with obesity and overweight. Int. J. Obes. 2022, 46, 1694–1702. [Google Scholar] [CrossRef]
- Vassy, J.L.; Durant, N.H.; Kabagambe, E.K.; Carnethon, M.R.; Rasmussen-Torvik, L.J.; Fornage, M.; Lewis, C.E.; Siscovick, D.S.; Meigs, J.B. A genotype risk score predicts type 2 diabetes from young adulthood: The cardia study. Diabetologia 2012, 55, 2604–2612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahajan, A.; Taliun, D.; Thurner, M.; Robertson, N.R.; Torres, J.M.; Rayner, N.W.; Payne, A.J.; Steinthorsdottir, V.; Scott, R.A.; Grarup, N.; et al. Fine-mapping type 2 diabetes loci to single-variant resolution using high-density imputation and islet-specific epigenome maps. Nat. Genet. 2018, 50, 1505–1513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morandi, A.; Bonnefond, A.; Lobbens, S.; Yengo, L.; Miraglia del Giudice, E.; Grandone, A.; Lévy-Marchal, C.; Weill, J.; Maffeis, C.; Froguel, P. Associations between type 2 diabetes-related genetic scores and metabolic traits, in obese and normal-weight youths. J. Clin. Endocrinol. Metab. 2016, 101, 4244–4250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balkhiyarova, Z.; Luciano, R.; Kaakinen, M.; Ulrich, A.; Shmeliov, A.; Bianchi, M.; Chioma, L.; Dallapiccola, B.; Prokopenko, I.; Manco, M. Relationship between glucose homeostasis and obesity in early life—A study of Italian children and adolescents. Hum. Mol. Genet. 2022, 31, 816–826. [Google Scholar] [CrossRef] [PubMed]
- Elliott, H.R.; Sharp, G.C.; Relton, C.L.; Lawlor, D.A. Epigenetics and gestational diabetes: A review of epigenetic epidemiology studies and their use to explore epigenetic mediation and improve prediction. Diabetologia 2019, 62, 2171–2178. [Google Scholar] [CrossRef] [Green Version]
- Candler, T.P.; Mahmoud, O.; Lynn, R.M.; Majbar, A.A.; Barrett, T.G.; Shield, J.P.H. Continuing rise of type 2 diabetes incidence in children and young people in the UK. Diabet. Med. 2018, 35, 737–744. [Google Scholar] [CrossRef]
- Bianchi, M.; Alisi, A.; Fabrizi, M.; Vallone, C.; Ravà, L.; Giannico, R.; Vernocchi, P.; Signore, F.; Manco, M. Maternal intake of N-3 polyunsaturated fatty acids during pregnancy is associated with differential methylation profiles in cord blood white cells. Front. Genet. 2019, 10, 1050. [Google Scholar] [CrossRef] [Green Version]
- Huebschmann, A.G.; Huxley, R.R.; Kohrt, W.M.; Zeitler, P.; Regensteiner, J.G.; Reusch, J.E.B. Sex differences in the burden of type 2 diabetes and cardiovascular risk across the life course. Diabetologia 2019, 62, 1761–1772. [Google Scholar] [CrossRef] [Green Version]
- Kufe, C.N.; Micklesfield, L.K.; Masemola, M.; Chikowore, T.; Kengne, A.P.; Karpe, F.; Norris, S.A.; Crowther, N.J.; Olsson, T.; Goedecke, J.H. Increased risk for type 2 diabetes in relation to adiposity in middle-aged black south African men compared to women. Eur. J. Endocrinol. 2022, 186, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Demirbag, R.; Yilmaz, R.; Erel, O. The association of total antioxidant capacity with sex hormones. Scand. Cardiovasc. J. 2005, 39, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Xiang, D.; Liu, Y.; Zhou, S.; Zhou, E.; Wang, Y. Protective effects of estrogen on cardiovascular disease mediated by oxidative stress. Oxidative Med. Cell. Longev. 2021, 2021, 1–15. [Google Scholar] [CrossRef]
- Kander, M.C.; Cui, Y.; Liu, Z. Gender difference in oxidative stress: A new look at the mechanisms for cardiovascular diseases. J. Cell. Mol. Med. 2017, 21, 1024–1032. [Google Scholar] [CrossRef]
- Bush, H.; Golabi, P.; Younossi, Z.M. Pediatric non-alcoholic fatty liver disease. Children 2017, 4, 48. [Google Scholar] [CrossRef] [Green Version]
- Manco, M.; Marcellini, M.; DeVito, R.; Comparcola, D.; Sartorelli, M.R.; Nobili, V. Metabolic syndrome and liver histology in paediatric non-alcoholic steatohepatitis. Int. J. Obes. 2008, 32, 381–387. [Google Scholar] [CrossRef] [Green Version]
- Newton, K.P.; Hou, J.; Crimmins, N.A.; Lavine, J.E.; Barlow, S.E.; Xanthakos, S.A.; Africa, J.; Behling, C.; Donithan, M.; Clark, J.M.; et al. Nonalcoholic steatohepatitis clinical research network. Prevalence of prediabetes and type 2 diabetes in children with nonalcoholic fatty liver disease. JAMA Pediatr. 2016, 170, e161971. [Google Scholar] [CrossRef] [PubMed]
- US Preventive Services Task Force; Davidson, K.W.; Barry, M.J.; Mangione, C.M.; Cabana, M.; Caughey, A.B.; Davis, E.M.; Donahue, K.E.; Doubeni, C.A.; Krist, A.H.; et al. Screening for prediabetes and type 2 diabetes: US preventive services task force recommendation statement. JAMA 2021, 326, 736. [Google Scholar] [CrossRef] [PubMed]
- US Preventive Services Task Force; Mangione, C.M.; Barry, M.J.; Nicholson, W.K.; Cabana, M.; Chelmow, D.; Coker, T.R.; Davidson, K.W.; Davis, E.M.; Donahue, K.E.; et al. Screening for prediabetes and type 2 diabetes in children and adolescents: US preventive services task force recommendation statement. JAMA 2022, 328, 963. [Google Scholar] [CrossRef]
- Zimmermann, E.; Bjerregaard, L.G.; Gamborg, M.; Vaag, A.A.; Sørensen, T.I.A.; Baker, J.L. Childhood body mass index and development of type 2 diabetes throughout adult life-a large-scale danish cohort study: Childhood body size and adult type 2 diabetes. Obesity 2017, 25, 965–971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- American Diabetes Association Professional Practice Committee. 14. children and adolescents: Standards of medical care in diabetes—2022. Diabetes Care 2022, 45 (Suppl. S1), S208–S231. [Google Scholar] [CrossRef]
- Savoye, M.; Caprio, S.; Dziura, J.; Camp, A.; Germain, G.; Summers, C.; Li, F.; Shaw, M.; Nowicka, P.; Kursawe, R.; et al. Reversal of early abnormalities in glucose metabolism in obese youth: Results of an intensive lifestyle randomized controlled trial. Diabetes Care 2014, 37, 317–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brar, P.C. Update on the current modalities used to screen high risk youth for prediabetes and/or type 2 diabetes mellitus. Ann. Pediatr. Endocrinol. Metab. 2019, 24, 71–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2010, 33 (Suppl. S1), S62–S69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- American Diabetes Association Professional Practice Committee. 2. classification and diagnosis of diabetes: Standards of medical care in diabetes—2022. Diabetes Care 2022, 45 (Suppl. S1), S17–S38. [Google Scholar] [CrossRef]
- Chen, M.E.; Aguirre, R.S.; Hannon, T.S. Methods for measuring risk for type 2 diabetes in youth: The oral glucose tolerance test (OGTT). Curr. Diabetes Rep. 2018, 18, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaw, J.E.; Zimmet, P.Z.; McCarty, D.; de Courten, M. Type 2 diabetes worldwide according to the new classification and criteria. Diabetes Care 2000, 23 (Suppl. S2), B5. [Google Scholar]
- Bergman, M.; Jagannathan, R.; Buysschaert, M.; Pareek, M.; Olsen, M.H.; Nilsson, P.M.; Medina, J.L.; Roth, J.; Chetrit, A.; Groop, L.; et al. Lessons learned from the 1-hour post-load glucose level during OGTT: Current screening recommendations for dysglycaemia should be revised. Diabetes Metab. Res. Rev. 2018, 34, e2992. [Google Scholar] [CrossRef]
- Andellini, M.; Manco, M.; Esposito, M.T.; Tozzi, A.E.; Bergman, M.; Ritrovato, M. A simulation model estimates lifetime health and economic outcomes of screening prediabetes using the 1-h plasma glucose. Acta Diabetol. 2022, 60, 9–17. [Google Scholar] [CrossRef]
- Kim, J.Y.; Goran, M.I.; Toledo-Corral, C.M.; Weigensberg, M.J.; Choi, M.; Shaibi, G.Q. One-hour glucose during an oral glucose challenge prospectively predicts β-cell deterioration and prediabetes in obese Hispanic youth. Diabetes Care 2013, 36, 1681–1686. [Google Scholar] [CrossRef] [Green Version]
- Garonzi, C.; Maguolo, A.; Maffeis, C. Pros and cons of current diagnostic tools for risk-based screening of prediabetes and type 2 diabetes in children and adolescents with overweight or obesity. Horm. Res. Paediatr. 2022; Ahead of print. [Google Scholar] [CrossRef]
- Vijayakumar, P.; Nelson, R.G.; Hanson, R.L.; Knowler, W.C.; Sinha, M. HbA1c and the prediction of type 2 diabetes in children and adults. Diabetes Care 2017, 40, 16–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blake, D.R.; Meigs, J.B.; Muller, D.C.; Najjar, S.S.; Andres, R.; Nathan, D.M. Impaired glucose tolerance, but not impaired fasting glucose, is associated with increased levels of coronary heart disease risk factors. Diabetes 2004, 53, 2095–2100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valerio, G.; Maffeis, C.; Saggese, G.; Ambruzzi, M.A.; Balsamo, A.; Bellone, S.; Bergamini, M.; Bernasconi, S.; Bona, G.; Calcaterra, V.; et al. Diagnosis, treatment and prevention of pediatric obesity: Consensus position statement of the Italian society for pediatric endocrinology and diabetology and the Italian society of pediatrics. Ital. J. Pediatr. 2018, 44, 88. [Google Scholar] [CrossRef] [Green Version]
- Delvecchio, M.; Mozzillo, E.; Salzano, G.; Iafusco, D.; Frontino, G.; Patera, P.I.; Rabbone, I.; Cherubini, V.; Grasso, V.; Tinto, N.; et al. Monogenic diabetes accounts for 6.3% of cases referred to 15 Italian pediatric diabetes centers during 2007 to 2012. J. Clin. Endocrinol. Metab. 2017, 102, 1826–1834. [Google Scholar] [CrossRef] [Green Version]
- Fajans, S.S.; Bell, G.I. MODY. Diabetes Care 2011, 34, 1878–1884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harsunen, M.; Kettunen, J.L.T.; Härkönen, T.; Dwivedi, O.; Lehtovirta, M.; Vähäsalo, P.; Veijola, R.; Ilonen, J.; Miettinen, P.J.; Knip, M.; et al. Identification of monogenic variants in more than ten per cent of children without type 1 diabetes-related autoantibodies at diagnosis in the Finnish pediatric diabetes register. Diabetologia 2022, 66, 438–449. [Google Scholar] [CrossRef]
- Copeland, K.C.; Silverstein, J.; Moore, K.R.; Prazar, G.E.; Raymer, T.; Shiffman, R.N.; Springer, S.C.; Thaker, V.V.; Anderson, M.; Spann, S.J.; et al. Management of newly diagnosed type 2 diabetes mellitus (T2DM) in children and adolescents. Pediatrics 2013, 131, 364–382. [Google Scholar] [CrossRef] [Green Version]
- Zeitler, P.; Arslanian, S.; Fu, J.; Pinhas-Hamiel, O.; Reinehr, T.; Tandon, N.; Urakami, T.; Wong, J.; Maahs, D.M. ISPAD clinical practice consensus guidelines 2018: Type 2 diabetes mellitus in youth. Pediatr. Diabetes 2018, 19, 28–46. [Google Scholar] [CrossRef]
- The TODAY Study Group. Treatment options for type 2 diabetes in adolescents and youth: A study of the comparative efficacy of metformin alone or in combination with rosiglitazone or lifestyle intervention in adolescents with type 2 diabetes. Pediatr. Diabetes 2007, 8, 74–87. [Google Scholar] [CrossRef] [Green Version]
- TODAY Study Group. A clinical trial to maintain glycemic control in youth with type 2 diabetes. N. Engl. J. Med. 2012, 366, 2247–2256. [Google Scholar] [CrossRef] [PubMed]
- Kaar, J.L.; Schmiege, S.J.; Drews, K.; Higgins, J.; Walders-Abramson, N.; Isganaitis, E.; Willi, S.M.; Marcus, M.D.; Zeitler, P.S.; Kelsey, M.M. Evaluation of the longitudinal change in health behavior profiles across treatment groups in the TODAY clinical trial. Pediatr. Diabetes 2020, 21, 224–232. [Google Scholar] [CrossRef]
- Arslanian, S.; Bacha, F.; Grey, M.; Marcus, M.D.; White, N.H.; Zeitler, P. Evaluation and management of youth-onset type 2 diabetes: A position statement by the American diabetes association. Diabetes Care 2018, 41, 2648–2668. [Google Scholar] [CrossRef] [Green Version]
- Chesser, H.; Srinivasan, S.; Puckett, C.; Gitelman, S.E.; Wong, J.C. Real-time continuous glucose monitoring in adolescents and young adults with type 2 diabetes can improve quality of life. J. Diabetes Sci. Technol. 2022; Ahead of print. [Google Scholar] [CrossRef]
- Smart, C.E.; Annan, F.; Bruno, L.P.; Higgins, L.A.; Acerini, C.L. Nutritional management in children and adolescents with diabetes. Pediatr. Diabetes 2014, 15, 135–153. [Google Scholar] [CrossRef]
- McGavock, J.; Dart, A.; Wicklow, B. Lifestyle therapy for the treatment of youth with type 2 diabetes. Curr. Diabetes Rep. 2015, 15, 568. [Google Scholar] [CrossRef] [Green Version]
- Bacha, F.; Cheng, P.; Gal, R.L.; Kollman, C.; Tamborlane, W.V.; Klingensmith, G.J.; Manseau, K.; Wood, J.; Beck, R.W.; Pediatric Diabetes Consortium. Initial presentation of type 2 diabetes in adolescents predicts durability of successful treatment with metformin monotherapy: Insights from the pediatric diabetes consortium T2D registry. Horm. Res. Paediatr. 2018, 89, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Andes, L.J.; Cheng, Y.J.; Rolka, D.B.; Gregg, E.W.; Imperatore, G. Prevalence of prediabetes among adolescents and young adults in the United States, 2005–2016. JAMA Pediatr. 2020, 174, e194498. [Google Scholar] [CrossRef]
- Barlow, S.E.; The Expert Committee. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: Summary report. Pediatrics 2007, 120 (Suppl. S4), S164–S192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Styne, D.M.; Arslanian, S.A.; Connor, E.L.; Farooqi, I.S.; Murad, M.H.; Silverstein, J.H.; Yanovski, J.A. Pediatric obesity—Assessment, treatment, and prevention: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2017, 102, 709–757. [Google Scholar] [CrossRef] [Green Version]
- Smart, C.E.; Annan, F.; Higgins, L.A.; Jelleryd, E.; Lopez, M.; Acerini, C.L. ISPAD clinical practice consensus guidelines 2018: Nutritional management in children and adolescents with diabetes. Pediatr. Diabetes 2018, 19, 136–154. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.A.; Kim, D.H.; Kim, S.M.; Park, Y.G.; Kim, N.H.; Baik, S.H.; Choi, K.M.; Han, K.; Yoo, H.J. Impact of the dynamic change of metabolic health status on the incident type 2 diabetes: A nationwide population-based cohort study. Endocrinol. Metab. 2019, 34, 406. [Google Scholar] [CrossRef] [PubMed]
- The TODAY Study Group. Design of a family-based lifestyle intervention for youth with type 2 diabetes: The TODAY study. Int. J. Obes. 2010, 34, 217–226. [Google Scholar] [CrossRef] [Green Version]
- Stoner, L.; Pontzer, H.; Barone Gibbs, B.; Moore, J.B.; Castro, N.; Skidmore, P.; Lark, S.; Williams, M.A.; Hamlin, M.J.; Faulkner, J. Fitness and fatness are both associated with cardiometabolic risk in preadolescents. J. Pediatr. 2020, 217, 39–45.e1. [Google Scholar] [CrossRef] [PubMed]
- Fedewa, M.V.; Gist, N.H.; Evans, E.M.; Dishman, R.K. Exercise and insulin resistance in youth: A meta-analysis. Pediatrics 2014, 133, e163–e174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colberg, S.R.; Sigal, R.J.; Yardley, J.E.; Riddell, M.C.; Dunstan, D.W.; Dempsey, P.C.; Horton, E.S.; Castorino, K.; Tate, D.F. Physical activity/exercise and diabetes: A position statement of the American diabetes association. Diabetes Care 2016, 39, 2065–2079. [Google Scholar] [CrossRef] [Green Version]
- Colberg, S.R.; Sigal, R.J.; Fernhall, B.; Regensteiner, J.G.; Blissmer, B.J.; Rubin, R.R.; Chasan-Taber, L.; Albright, A.L.; Braun, B. Exercise and type 2 diabetes. Diabetes Care 2010, 33, e147–e167. [Google Scholar] [CrossRef] [Green Version]
- Shaibi, G.Q.; Ball, G.D.C.; Cruz, M.L.; Weigensberg, M.J.; Salem, G.J.; Goran, M.I. Cardiovascular fitness and physical activity in children with and without impaired glucose tolerance. Int. J. Obes. 2006, 30, 45–49. [Google Scholar] [CrossRef] [Green Version]
- Dias, I.; Farinatti, P.; De Souza, M.D.G.C.; Manhanini, D.P.; Balthazar, E.; Dantas, D.L.S.; De Andrade Pinto, E.H.; Bouskela, E.; Kraemer-Aguiar, L.G. Effects of resistance training on obese adolescents. Med. Sci. Sport. Exerc. 2015, 47, 2636–2644. [Google Scholar] [CrossRef]
- Inge, T.H.; Laffel, L.M.; Jenkins, T.M.; Marcus, M.D.; Leibel, N.I.; Brandt, M.L.; Haymond, M.; Urbina, E.M.; Dolan, L.M.; Zeitler, P.S.; et al. Comparison of surgical and medical therapy for type 2 diabetes in severely obese adolescents. JAMA Pediatr. 2018, 172, 452. [Google Scholar] [CrossRef]
- United States Food and Drug Administration. FDA Approves Treatment for Chronic Weight Management in Pediatric Patients Aged 12 Years and Older. 2022. Available online: https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-treatment-chronic-weight-management-pediatric-patients-aged-12-years-and-older (accessed on 26 December 2022).
- Friedrichsen, M.; Breitschaft, A.; Tadayon, S.; Wizert, A.; Skovgaard, D. The Effect of Semaglutide 2.4 mg once weekly on energy intake, appetite, control of eating, and gastric emptying in adults with obesity. Diabetes Obes. Metab. 2021, 23, 754–762. [Google Scholar] [CrossRef]
- Weghuber, D.; Barrett, T.; Barrientos-Pérez, M.; Gies, I.; Hesse, D.; Jeppesen, O.K.; Kelly, A.S.; Mastrandrea, L.D.; Sørrig, R.; Arslanian, S. Once-weekly Semaglutide in adolescents with obesity. N. Engl. J. Med. 2022, 387, 2245–2257. [Google Scholar] [CrossRef]
- Bensignor, M.O.; Kelly, A.S.; Arslanian, S. Anti-obesity pharmacotherapy for treatment of pediatric type 2 diabetes: Review of the literature and lessons learned from adults. Front. Endocrinol. 2022, 13, 1043650. [Google Scholar] [CrossRef]
- Okuyaz, C.; Kursel, O.; Komur, M.; Tamer, L. Evaluation of appetite-stimulating hormones in prepubertal children with epilepsy during topiramate treatment. Pediatr. Neurol. 2012, 47, 423–426. [Google Scholar] [CrossRef]
- Inge, T.H.; Courcoulas, A.P.; Jenkins, T.M.; Michalsky, M.P.; Brandt, M.L.; Xanthakos, S.A.; Dixon, J.B.; Harmon, C.M.; Chen, M.K.; Xie, C.; et al. Five-year outcomes of gastric bypass in adolescents as compared with adults. N. Engl. J. Med. 2019, 380, 2136–2145. [Google Scholar] [CrossRef] [PubMed]
- Son, J.W.; Kim, S. Comprehensive review of current and upcoming anti-obesity drugs. Diabetes Metab. J. 2020, 44, 802–818. [Google Scholar] [CrossRef] [PubMed]
- Kelly, A.S.; Bensignor, M.O.; Hsia, D.S.; Shoemaker, A.H.; Shih, W.; Peterson, C.; Varghese, S.T. Phentermine/topiramate for the treatment of adolescent obesity. NEJM Evid. 2022, 1, EVIDoa2200014. [Google Scholar] [CrossRef]
- Tamborlane, W.V.; Laffel, L.M.; Weill, J.; Gordat, M.; Neubacher, D.; Retlich, S.; Hettema, W.; Hoesl, C.E.; Kaspers, S.; Marquard, J. Randomized, double-blind, placebo-controlled dose-finding study of the dipeptidyl peptidase-4 inhibitor linagliptin in pediatric patients with type 2 diabetes. Pediatr. Diabetes 2018, 19, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Laffel, L.M.B.; Tamborlane, W.V.; Yver, A.; Simons, G.; Wu, J.; Nock, V.; Hobson, D.; Hughan, K.S.; Kaspers, S.; Marquard, J. Pharmacokinetic and pharmacodynamic profile of the sodium-glucose co-transporter-2 inhibitor empagliflozin in young people with type 2 diabetes: A randomized trial. Diabet. Med. 2018, 35, 1096–1104. [Google Scholar] [CrossRef] [PubMed]
- Malik, I.O.; Petersen, M.C.; Klein, S. Glucagon-like peptide -1, Glucose-dependent insulinotropic polypeptide, and glucagon receptor poly-agonists: A new era in obesity pharmacotherapy. Obesity 2022, 30, 1718–1721. [Google Scholar] [CrossRef] [PubMed]
- Papamargaritis, D.; le Roux, C.W.; Holst, J.J.; Davies, M.J. New therapies for obesity. Cardiovasc. Res. 2022, cvac176. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iafusco, D.; Franceschi, R.; Maguolo, A.; Guercio Nuzio, S.; Crinò, A.; Delvecchio, M.; Iughetti, L.; Maffeis, C.; Calcaterra, V.; Manco, M. From Metabolic Syndrome to Type 2 Diabetes in Youth. Children 2023, 10, 516. https://doi.org/10.3390/children10030516
Iafusco D, Franceschi R, Maguolo A, Guercio Nuzio S, Crinò A, Delvecchio M, Iughetti L, Maffeis C, Calcaterra V, Manco M. From Metabolic Syndrome to Type 2 Diabetes in Youth. Children. 2023; 10(3):516. https://doi.org/10.3390/children10030516
Chicago/Turabian StyleIafusco, Dario, Roberto Franceschi, Alice Maguolo, Salvatore Guercio Nuzio, Antonino Crinò, Maurizio Delvecchio, Lorenzo Iughetti, Claudio Maffeis, Valeria Calcaterra, and Melania Manco. 2023. "From Metabolic Syndrome to Type 2 Diabetes in Youth" Children 10, no. 3: 516. https://doi.org/10.3390/children10030516