Organoid Technology and Its Role for Theratyping Applications in Cystic Fibrosis
Abstract
:1. Introduction
2. Alternatives to Conventional Clinical Trials
3. Cell Models for Studying CF Disease Pathogenesis and Therapy
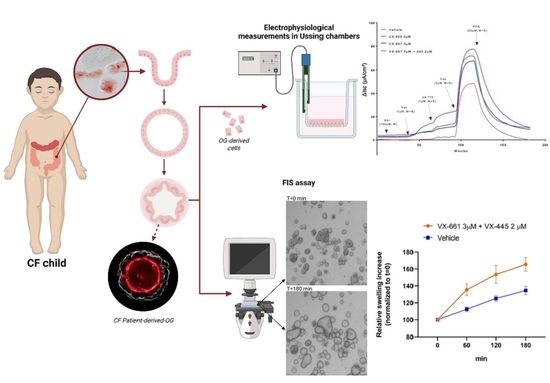
4. Disease Modeling of Intestinal OG in Infants and Children
5. CFTR Bioassay in Intestinal Organoids
6. Limitations of Organoid-Based Assays
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Castellani, C.; Duff, A.J.A.; Bell, S.C.; Heijerman, H.G.M.; Munck, A.; Ratjen, F.; Sermet-Gaudelus, I.; Southern, K.W.; Barben, J.; Flume, P.A.; et al. ECFS Best Practice Guidelines: The 2018 Revision. J. Cyst. Fibros. 2018, 17, 153–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Athanazio, R.A.; Filho, L.V.R.F.S.; Vergara, A.A.; Ribeiro, A.F.; Riedi, C.A.; Procianoy, E.; Adde, F.V.; Reis, F.J.C.; Ribeiro, J.D.; Torres, L.A.; et al. Brazilian Guidelines for the Diagnosis and Treatment of Cystic Fibrosis. J. Bras. Pneumol. 2017, 43, 219–245. [Google Scholar] [CrossRef] [Green Version]
- Cohen-Cymberknoh, M.; Shoseyov, D.; Kerem, E. Managing Cystic Fibrosis: Strategies That Increase Life Expectancy and Improve Quality of Life. Am. J. Respir. Crit. Care Med. 2011, 183, 1463–1471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narayanan, S.; Mainz, J.G.; Gala, S.; Tabori, H.; Grossoehme, D. Adherence to Therapies in Cystic Fibrosis: A Targeted Literature Review. Expert Rev. Respir. Med. 2017, 11, 129–145. [Google Scholar] [CrossRef] [PubMed]
- Campbell, P.W.; White, T.B. Newborn Screening for Cystic Fibrosis: An Opportunity to Improve Care and Outcomes. J. Pediatr. 2005, 147, S2–S5. [Google Scholar] [CrossRef] [PubMed]
- Coffey, M.J.; Whitaker, V.; Gentin, N.; Junek, R.; Shalhoub, C.; Nightingale, S.; Hilton, J.; Wiley, V.; Wilcken, B.; Gaskin, K.J.; et al. Differences in Outcomes between Early and Late Diagnosis of Cystic Fibrosis in the Newborn Screening Era. J. Pediatr. 2017, 181, 137–145. [Google Scholar] [CrossRef]
- Wagener, J.S.; Zemanick, E.T.; Sontag, M.K. Newborn Screening for Cystic Fibrosis. Curr. Opin. Pediatr. 2012, 24, 329–335. [Google Scholar] [CrossRef] [Green Version]
- Rosenthal, M. Newborn Screening for Cystic Fibrosis: The Motion against—Voices in the Wilderness. Paediatr. Respir. Rev. 2008, 9, 295–300. [Google Scholar] [CrossRef]
- McKay, K.; Wilcken, B. Newborn Screening for Cystic Fibrosis Offers an Advantage over Symptomatic Diagnosis for the Long Term Benefit of Patients: The Motion For. Paediatr. Respir. Rev. 2008, 9, 290–294. [Google Scholar] [CrossRef]
- Southern, K.W.; Mérelle, M.M.E.; Dankert-Roelse, J.E.; Nagelkerke, A. Newborn Screening for Cystic Fibrosis. Cochrane Database Syst. Rev. 2009. [Google Scholar] [CrossRef]
- Grosse, S.D.; Rosenfeld, M.; Devine, O.J.; Lai, H.J.; Farrell, P.M. Potential Impact of Newborn Screening for Cystic Fibrosis on Child Survival: A Systematic Review and Analysis. J. Pediatr. 2006, 149, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Szczesniak, R.D.; Li, D.; Su, W.; Brokamp, C.; Pestian, J.; Seid, M.; Clancy, J.P. Phenotypes of Rapid Cystic Fibrosis Lung Disease Progression during Adolescence and Young Adulthood. Am. J. Respir. Crit. Care Med. 2017, 196, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, M.; Cunningham, S.; Harris, W.T.; Lapey, A.; Regelmann, W.E.; Sawicki, G.S.; Southern, K.W.; Chilvers, M.; Higgins, M.; Tian, S.; et al. An Open-Label Extension Study of Ivacaftor in Children with CF and a CFTR Gating Mutation Initiating Treatment at Age 2–5 Years (KLIMB). J. Cyst. Fibros. 2019, 18, 838–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenfeld, M.; Wainwright, C.E.; Higgins, M.; Wang, L.T.; McKee, C.; Campbell, D.; Tian, S.; Schneider, J.; Cunningham, S.; Davies, J.C.; et al. Ivacaftor Treatment of Cystic Fibrosis in Children Aged 12 to <24 Months and with a CFTR Gating Mutation (ARRIVAL): A Phase 3 Single-Arm Study. Lancet Respir. Med. 2018, 6, 545–553. [Google Scholar] [CrossRef]
- Milla, C.E.; Ratjen, F.; Marigowda, G.; Liu, F.; Waltz, D.; Rosenfeld, M. Lumacaftor/Ivacaftor in Patients Aged 6–11 Years with Cystic Fibrosis and Homozygous for F508del-CFTR. Am. J. Respir. Crit. Care Med. 2017, 195, 912–920. [Google Scholar] [CrossRef] [Green Version]
- Taylor-Cousar, J.L.; Munck, A.; McKone, E.F.; van der Ent, C.K.; Moeller, A.; Simard, C.; Wang, L.T.; Ingenito, E.P.; McKee, C.; Lu, Y.; et al. Tezacaftor–Ivacaftor in Patients with Cystic Fibrosis Homozygous for Phe508del. N. Engl. J. Med. 2017, 377, 2013–2023. [Google Scholar] [CrossRef]
- Springsteel, M.F.; Galietta, L.J.V.; Ma, T.; By, K.; Berger, G.O.; Yang, H.; Dicus, C.W.; Choung, W.; Quan, C.; Shelat, A.A.; et al. Benzoflavone Activators of the Cystic Fibrosis Transmembrane Conductance Regulator: Towards a Pharmacophore Model for the Nucleotide-Binding Domain. Bioorg. Med. Chem. 2003, 11, 4113–4120. [Google Scholar] [CrossRef]
- Pranke, I.; Golec, A.; Hinzpeter, A.; Edelman, A.; Sermet-Gaudelus, I. Emerging Therapeutic Approaches for Cystic Fibrosis. From Gene Editing to Personalized Medicine. Front. Pharmacol. 2019, 10, 121. [Google Scholar] [CrossRef] [Green Version]
- Sosnay, P.R.; Siklosi, K.R.; Van Goor, F.; Kaniecki, K.; Yu, H.; Sharma, N.; Ramalho, A.S.; Amaral, M.D.; Dorfman, R.; Zielenski, J.; et al. Defining the Disease Liability of Variants in the Cystic Fibrosis Transmembrane Conductance Regulator Gene. Nat. Genet. 2013, 45, 1160–1167. [Google Scholar] [CrossRef] [Green Version]
- Sosnay, P.R.; Salinas, D.B.; White, T.B.; Ren, C.L.; Farrell, P.M.; Raraigh, K.S.; Girodon, E.; Castellani, C. Applying Cystic Fibrosis Transmembrane Conductance Regulator Genetics and CFTR2 Data to Facilitate Diagnoses. J. Pediatr. 2017, 181, S27–S32. [Google Scholar] [CrossRef]
- Kraiczy, J.; Nayak, K.M.; Howell, K.J.; Ross, A.; Forbester, J.; Salvestrini, C.; Mustata, R.; Perkins, S.; Andersson-Rolf, A.; Leenen, E.; et al. DNA Methylation Defines Regional Identity of Human Intestinal Epithelial Organoids and Undergoes Dynamic Changes during Development. Gut 2019, 68, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Kerschner, J.L.; Gosalia, N.; Neems, D.; Gorsic, L.K.; Safi, A.; Crawford, G.E.; Kosak, S.T.; Leir, S.-H.; Harris, A. Differential Contribution of Cis -Regulatory Elements to Higher Order Chromatin Structure and Expression of the CFTR Locus. Nucleic Acids Res. 2016, 44, 3082–3094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zielenski, J.; Tsui, L.-C. Cystic Fibrosis: Genotypic And Phenotypic Variations. Annu. Rev. Genet. 1995, 29, 777–807. [Google Scholar] [CrossRef] [PubMed]
- Welsh, M.J.; Smith, A.E. Molecular Mechanisms of CFTR Chloride Channel Dysfunction in Cystic Fibrosis. Cell 1993, 73, 1251–1254. [Google Scholar] [CrossRef] [PubMed]
- Marson, F.A.L. Personalized or Precision Medicine? The Example of Cystic Fibrosis. Front. Pharmacol. 2017, 8, 390. [Google Scholar] [CrossRef]
- Schork, N.J. Personalized Medicine: Time for One-Person Trials. Nature 2015, 520, 609–611. [Google Scholar] [CrossRef] [Green Version]
- Dobra, R.; Bentley, S.; Edmondson, C.; Ovens, M.; Saunders, C.; Short, C.; Wilson, G.; Davies, J.C.; Bush, A. Going the Extra Mile: Why Clinical Research in Cystic Fibrosis Must Include Children. Children 2022, 9, 1080. [Google Scholar] [CrossRef]
- Gruenert, D.C.; Willems, M.; Cassiman, J.J.; Frizzell, R.A. Established Cell Lines Used in Cystic Fibrosis Research. J. Cyst. Fibros. 2004, 3, 191–196. [Google Scholar] [CrossRef] [Green Version]
- Bednarski, C.; Tomczak, K.; vom Hövel, B.; Weber, W.-M.; Cathomen, T. Targeted Integration of a Super-Exon into the CFTR Locus Leads to Functional Correction of a Cystic Fibrosis Cell Line Model. PLoS ONE 2016, 11, e0161072. [Google Scholar] [CrossRef] [Green Version]
- Bellec, J.; Bacchetta, M.; Losa, D.; Anegon, I.; Chanson, M.; Nguyen, T. CFTR Inactivation by Lentiviral Vector-Mediated RNA Interference and CRISPR-Cas9 Genome Editing in Human Airway Epithelial Cells. Curr. Gene Ther. 2015, 15, 447–459. [Google Scholar] [CrossRef]
- Ehrhardt, C.; Collnot, E.-M.; Baldes, C.; Becker, U.; Laue, M.; Kim, K.-J.; Lehr, C.-M. Towards an in Vitro Model of Cystic Fibrosis Small Airway Epithelium: Characterisation of the Human Bronchial Epithelial Cell Line CFBE41o-. Cell Tissue Res. 2006, 323, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, A.S.; Randell, S.H.; Stewart, S.A.; Elenbaas, B.; Hartwell, K.A.; Brooks, M.W.; Fleming, M.D.; Olsen, J.C.; Miller, S.W.; Weinberg, R.A.; et al. Immortalization and Transformation of Primary Human Airway Epithelial Cells by Gene Transfer. Oncogene 2002, 21, 4577–4586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopes-Pacheco, M.; Bacalhau, M.; Ramalho, S.S.; Silva, I.A.L.; Ferreira, F.C.; Carlile, G.W.; Thomas, D.Y.; Farinha, C.M.; Hanrahan, J.W.; Amaral, M.D. Rescue of Mutant CFTR Trafficking Defect by the Investigational Compound MCG1516A. Cells 2022, 11, 136. [Google Scholar] [CrossRef] [PubMed]
- Pedemonte, N.; Tomati, V.; Sondo, E.; Galietta, L.J.V. Influence of Cell Background on Pharmacological Rescue of Mutant CFTR. Am. J. Physiol.-Cell Physiol. 2010, 298, C866–C874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ostedgaard, L.S.; Rogers, C.S.; Dong, Q.; Randak, C.O.; Vermeer, D.W.; Rokhlina, T.; Karp, P.H.; Welsh, M.J. Processing and Function of CFTR-ΔF508 Are Species-Dependent. Proc. Natl. Acad. Sci. USA 2007, 104, 15370–15375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durmowicz, A.G.; Lim, R.; Rogers, H.; Rosebraugh, C.J.; Chowdhury, B.A. The U.S. Food and Drug Administration’s Experience with Ivacaftor in Cystic Fibrosis. Establishing Efficacy Using In Vitro Data in Lieu of a Clinical Trial. Ann. Am. Thorac. Soc. 2018, 15, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Vertex Pharmaceuticals Inc. Highlights of Prescribing Information: Trikafta® (Elexacaftor/Tezacaftor/Ivacaftor). 2021. Available online: https://pi.vrtx.com/files/uspi_elexacaftor_tezacaftor_ivacaftor.pdf (accessed on 31 October 2022).
- Pranke, I.M.; Hatton, A.; Simonin, J.; Jais, J.P.; Le Pimpec-Barthes, F.; Carsin, A.; Bonnette, P.; Fayon, M.; Stremler-Le Bel, N.; Grenet, D.; et al. Correction of CFTR Function in Nasal Epithelial Cells from Cystic Fibrosis Patients Predicts Improvement of Respiratory Function by CFTR Modulators. Sci. Rep. 2017, 7, 7375. [Google Scholar] [CrossRef] [Green Version]
- Gianotti, A.; Delpiano, L.; Caci, E. In Vitro Methods for the Development and Analysis of Human Primary Airway Epithelia. Front. Pharmacol. 2018, 9, 1176. [Google Scholar] [CrossRef] [Green Version]
- Sachs, N.; Papaspyropoulos, A.; Zomer-van Ommen, D.D.; Heo, I.; Böttinger, L.; Klay, D.; Weeber, F.; Huelsz-Prince, G.; Iakobachvili, N.; Amatngalim, G.D.; et al. Long-term Expanding Human Airway Organoids for Disease Modeling. EMBO J. 2019, 38, e100300. [Google Scholar] [CrossRef]
- Vonk, A.M.; van Mourik, P.; Ramalho, A.S.; Silva, I.A.L.; Statia, M.; Kruisselbrink, E.; Suen, S.W.F.; Dekkers, J.F.; Vleggaar, F.P.; Houwen, R.H.J.; et al. Protocol for Application, Standardization and Validation of the Forskolin-Induced Swelling Assay in Cystic Fibrosis Human Colon Organoids. STAR Protoc. 2020, 1, 100019. [Google Scholar] [CrossRef]
- de Courcey, F.; Zholos, A.V.; Atherton-Watson, H.; Williams, M.T.S.; Canning, P.; Danahay, H.L.; Elborn, J.S.; Ennis, M. Development of Primary Human Nasal Epithelial Cell Cultures for the Study of Cystic Fibrosis Pathophysiology. Am. J. Physiol.-Cell Physiol. 2012, 303, C1173–C1179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dekkers, J.F.; Wiegerinck, C.L.; de Jonge, H.R.; Bronsveld, I.; Janssens, H.M.; de Winter-de Groot, K.M.; Brandsma, A.M.; de Jong, N.W.M.; Bijvelds, M.J.C.; Scholte, B.J.; et al. A Functional CFTR Assay Using Primary Cystic Fibrosis Intestinal Organoids. Nat. Med. 2013, 19, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Scudieri, P.; Musante, I.; Venturini, A.; Guidone, D.; Genovese, M.; Cresta, F.; Caci, E.; Palleschi, A.; Poeta, M.; Santamaria, F.; et al. Ionocytes and CFTR Chloride Channel Expression in Normal and Cystic Fibrosis Nasal and Bronchial Epithelial Cells. Cells 2020, 9, 2090. [Google Scholar] [CrossRef] [PubMed]
- Sette, G.; Lo Cicero, S.; Blaconà, G.; Pierandrei, S.; Bruno, S.M.; Salvati, V.; Castelli, G.; Falchi, M.; Fabrizzi, B.; Cimino, G.; et al. Theratyping Cystic Fibrosis in Vitro in ALI Culture and Organoid Models Generated from Patient-Derived Nasal Epithelial Conditionally Reprogrammed Stem Cells. Eur. Respir. J. 2021, 58, 2100908. [Google Scholar] [CrossRef]
- Barkauskas, C.E.; Chung, M.-I.; Fioret, B.; Gao, X.; Katsura, H.; Hogan, B.L.M. Lung Organoids: Current Uses and Future Promise. Development 2017, 144, 986–997. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Anderson, J.D.; Deng, L.; Mackay, S.; Bailey, J.; Kersh, L.; Rowe, S.M.; Guimbellot, J.S. Human Nasal Epithelial Organoids for Therapeutic Development in Cystic Fibrosis. Genes 2020, 11, 603. [Google Scholar] [CrossRef]
- Amatngalim, G.D.; Rodenburg, L.W.; Aalbers, B.L.; Raeven, H.H.; Aarts, E.M.; Sarhane, D.; Spelier, S.; Lefferts, J.W.; Silva, I.A.; Nijenhuis, W.; et al. Measuring Cystic Fibrosis Drug Responses in Organoids Derived from 2D Differentiated Nasal Epithelia. Life Sci. Alliance 2022, 5, e202101320. [Google Scholar] [CrossRef]
- Golec, A.; Pranke, I.; Scudieri, P.; Hayes, K.; Dreano, E.; Dunlevy, F.; Hatton, A.; Downey, D.G.; Galietta, L.; Sermet, I. Isolation, Cultivation, and Application of Primary Respiratory Epithelial Cells Obtained by Nasal Brushing, Polyp Samples, or Lung Explants. STAR Protoc. 2022, 3, 101419. [Google Scholar] [CrossRef]
- Wong, S.L.; Awatade, N.T.; Astore, M.A.; Allan, K.M.; Carnell, M.J.; Slapetova, I.; Chen, P.; Setiadi, J.; Pandzic, E.; Fawcett, L.K.; et al. Molecular Dynamics and Theratyping in Airway and Gut Organoids Reveal R352Q-CFTR Conductance Defect. Am. J. Respir Cell Mol. Biol. 2022, 67, 99–111. [Google Scholar] [CrossRef]
- Silva, I.A.L.; Railean, V.; Duarte, A.; Amaral, M.D. Personalized Medicine Based on Nasal Epithelial Cells: Comparative Studies with Rectal Biopsies and Intestinal Organoids. J. Pers. Med. 2021, 11, 421. [Google Scholar] [CrossRef]
- Clancy, J.P.; Szczesniak, R.D.; Ashlock, M.A.; Ernst, S.E.; Fan, L.; Hornick, D.B.; Karp, P.H.; Khan, U.; Lymp, J.; Ostmann, A.J.; et al. Multicenter Intestinal Current Measurements in Rectal Biopsies from CF and Non-CF Subjects to Monitor CFTR Function. PLoS ONE 2013, 8, e73905. [Google Scholar] [CrossRef] [PubMed]
- Bagheri-Hanson, A.; Nedwed, S.; Rueckes-Nilges, C.; Naehrlich, L. Intestinal Current Measurement versus Nasal Potential Difference Measurements for Diagnosis of Cystic Fibrosis: A Case–Control Study. BMC Pulm. Med. 2014, 14, 156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, T.; Vries, R.G.; Snippert, H.J.; van de Wetering, M.; Barker, N.; Stange, D.E.; van Es, J.H.; Abo, A.; Kujala, P.; Peters, P.J.; et al. Single Lgr5 Stem Cells Build Crypt-Villus Structures in Vitro without a Mesenchymal Niche. Nature 2009, 459, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Clevers, H. Growing Self-Organizing Mini-Guts from a Single Intestinal Stem Cell: Mechanism and Applications. Science 2013, 340, 1190–1194. [Google Scholar] [CrossRef] [Green Version]
- Sato, T.; Stange, D.E.; Ferrante, M.; Vries, R.G.J.; van Es, J.H.; van den Brink, S.; van Houdt, W.J.; Pronk, A.; van Gorp, J.; Siersema, P.D.; et al. Long-Term Expansion of Epithelial Organoids From Human Colon, Adenoma, Adenocarcinoma, and Barrett’s Epithelium. Gastroenterology 2011, 141, 1762–1772. [Google Scholar] [CrossRef]
- Servidoni, M.F.; Sousa, M.; Vinagre, A.M.; Cardoso, S.R.; Ribeiro, M.A.; Meirelles, L.R.; de Carvalho, R.B.; Kunzelmann, K.; Ribeiro, A.F.; Ribeiro, J.D.; et al. Rectal Forceps Biopsy Procedure in Cystic Fibrosis: Technical Aspects and Patients Perspective for Clinical Trials Feasibility. BMC Gastroenterol. 2013, 13, 91. [Google Scholar] [CrossRef] [Green Version]
- De Boeck, K. Cystic Fibrosis: Terminology and Diagnostic Algorithms. Thorax 2006, 61, 627–635. [Google Scholar] [CrossRef] [Green Version]
- Sermet-Gaudelus, I.; Girodon, E.; Vermeulen, F.; Solomon, G.M.; Melotti, P.; Graeber, S.Y.; Bronsveld, I.; Rowe, S.M.; Wilschanski, M.; Tümmler, B.; et al. ECFS Standards of Care on CFTR-Related Disorders: Diagnostic Criteria of CFTR Dysfunction. J. Cyst. Fibros. 2022, 21, 922–936. [Google Scholar] [CrossRef]
- Farrell, P.M.; White, T.B.; Ren, C.L.; Hempstead, S.E.; Accurso, F.; Derichs, N.; Howenstine, M.; McColley, S.A.; Rock, M.; Rosenfeld, M.; et al. Diagnosis of Cystic Fibrosis: Consensus Guidelines from the Cystic Fibrosis Foundation. J. Pediatr. 2017, 181, S4–S15. [Google Scholar] [CrossRef] [Green Version]
- Kunzelmann, K.; Mall, M. Electrolyte Transport in the Mammalian Colon: Mechanisms and Implications for Disease. Physiol. Rev. 2002, 82, 245–289. [Google Scholar] [CrossRef]
- Mall, M.; Wissner, A.; Seydewitz, H.H.; Kuehr, J.; Brandis, M.; Greger, R.; Kunzelmann, K. Defective Cholinergic Cl—Secretion and Detection of K + Secretion in Rectal Biopsies from Cystic Fibrosis Patients. Am. J. Physiol.-Gastrointest. Liver Physiol. 2000, 278, G617–G624. [Google Scholar] [CrossRef] [PubMed]
- Veeze, H.J.; Sinaasappel, M.; Bijman, J.; Bouquet, J.; De Jonge, H.R. Ion Transport Abnormalities in Rectal Suction Biopsies from Children with Cystic Fibrosis. Gastroenterology 1991, 101, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Graeber, S.Y.; Vitzthum, C.; Mall, M.A. Potential of Intestinal Current Measurement for Personalized Treatment of Patients with Cystic Fibrosis. J. Pers. Med. 2021, 11, 384. [Google Scholar] [CrossRef] [PubMed]
- Chusilp, S.; Li, B.; Lee, D.; Lee, C.; Vejchapipat, P.; Pierro, A. Intestinal Organoids in Infants and Children. Pediatr. Surg. Int. 2020, 36, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sachs, N.; de Ligt, J.; Kopper, O.; Gogola, E.; Bounova, G.; Weeber, F.; Balgobind, A.V.; Wind, K.; Gracanin, A.; Begthel, H.; et al. A Living Biobank of Breast Cancer Organoids Captures Disease Heterogeneity. Cell 2018, 172, 373–386.e10. [Google Scholar] [CrossRef] [Green Version]
- Broutier, L.; Mastrogiovanni, G.; Verstegen, M.M.; Francies, H.E.; Gavarró, L.M.; Bradshaw, C.R.; Allen, G.E.; Arnes-Benito, R.; Sidorova, O.; Gaspersz, M.P.; et al. Human Primary Liver Cancer–Derived Organoid Cultures for Disease Modeling and Drug Screening. Nat. Med. 2017, 23, 1424–1435. [Google Scholar] [CrossRef] [Green Version]
- Dekkers, J.F.; Berkers, G.; Kruisselbrink, E.; Vonk, A.; de Jonge, H.R.; Janssens, H.M.; Bronsveld, I.; van de Graaf, E.A.; Nieuwenhuis, E.E.S.; Houwen, R.H.J.; et al. Characterizing Responses to CFTR-Modulating Drugs Using Rectal Organoids Derived from Subjects with Cystic Fibrosis. Sci. Transl. Med. 2016, 8, 344ra84. [Google Scholar] [CrossRef]
- Beekman, J.M. Individualized Medicine Using Intestinal Responses to CFTR Potentiators and Correctors: Individualized Medicine Using Intestinal Responses. Pediatr. Pulmonol. 2016, 51, S23–S34. [Google Scholar] [CrossRef]
- de Poel, E.; Lefferts, J.W.; Beekman, J.M. Intestinal Organoids for Cystic Fibrosis Research. J. Cyst. Fibros. 2020, 19, S60–S64. [Google Scholar] [CrossRef] [Green Version]
- Ramalho, A.S.; Fürstová, E.; Vonk, A.M.; Ferrante, M.; Verfaillie, C.; Dupont, L.; Boon, M.; Proesmans, M.; Beekman, J.M.; Sarouk, I.; et al. Correction of CFTR Function in Intestinal Organoids to Guide Treatment of Cystic Fibrosis. Eur. Respir. J. 2021, 57, 1902426. [Google Scholar] [CrossRef]
- Dekkers, J.F.; Gogorza Gondra, R.A.; Kruisselbrink, E.; Vonk, A.M.; Janssens, H.M.; de Winter-de Groot, K.M.; van der Ent, C.K.; Beekman, J.M. Optimal Correction of Distinct CFTR Folding Mutants in Rectal Cystic Fibrosis Organoids. Eur. Respir. J. 2016, 48, 451–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Winter-de Groot, K.M.; Janssens, H.M.; van Uum, R.T.; Dekkers, J.F.; Berkers, G.; Vonk, A.; Kruisselbrink, E.; Oppelaar, H.; Vries, R.; Clevers, H.; et al. Stratifying Infants with Cystic Fibrosis for Disease Severity Using Intestinal Organoid Swelling as a Biomarker of CFTR Function. Eur. Respir. J. 2018, 52, 1702529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berkers, G.; van Mourik, P.; Vonk, A.M.; Kruisselbrink, E.; Dekkers, J.F.; de Winter-de Groot, K.M.; Arets, H.G.M.; Marck-van der Wilt, R.E.P.; Dijkema, J.S.; Vanderschuren, M.M.; et al. Rectal Organoids Enable Personalized Treatment of Cystic Fibrosis. Cell Rep. 2019, 26, 1701–1708.e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aalbers, B.L.; Brunsveld, J.E.; van der Ent, C.K.; van den Eijnden, J.C.; Beekman, J.M.; Heijerman, H.G.M. Forskolin Induced Swelling (FIS) Assay in Intestinal Organoids to Guide Eligibility for Compassionate Use Treatment in a CF Patient with a Rare Genotype. J. Cyst. Fibros. 2022, 21, 254–257. [Google Scholar] [CrossRef]
- Pranke, I.; Hatton, A.; Masson, A.; Flament, T.; Le Bourgeois, M.; Chedevergne, F.; Bailly, C.; Urbach, V.; Hinzpeter, A.; Edelman, A.; et al. Might Brushed Nasal Cells Be a Surrogate for CFTR Modulator Clinical Response? Am. J. Respir. Crit. Care Med. 2019, 199, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Amato, F.; Scudieri, P.; Musante, I.; Tomati, V.; Caci, E.; Comegna, M.; Maietta, S.; Manzoni, F.; Di Lullo, A.M.; Wachter, E.; et al. Two CFTR Mutations within Codon 970 Differently Impact on the Chloride Channel Functionality. Hum. Mutat. 2019, 40, 742–748. [Google Scholar] [CrossRef]
- Terlizzi, V.; Amato, F.; Castellani, C.; Ferrari, B.; Galietta, L.J.V.; Castaldo, G.; Taccetti, G. Ex Vivo Model Predicted in Vivo Efficacy of CFTR Modulator Therapy in a Child with Rare Genotype. Mol. Genet. Genom. Med. 2021, 9, e1656. [Google Scholar] [CrossRef]
- Mosler, K.; Coraux, C.; Fragaki, K.; Zahm, J.-M.; Bajolet, O.; Bessaci-Kabouya, K.; Puchelle, E.; Abély, M.; Mauran, P. Feasibility of Nasal Epithelial Brushing for the Study of Airway Epithelial Functions in CF Infants. J. Cyst. Fibros. 2008, 7, 44–53. [Google Scholar] [CrossRef]
- Lyman, G.H.; Moses, H.L. Biomarker Tests for Molecularly Targeted Therapies—The Key to Unlocking Precision Medicine. N. Engl. J. Med. 2016, 375, 4–6. [Google Scholar] [CrossRef] [Green Version]
- Van Goor, F.; Hadida, S.; Grootenhuis, P.D.J.; Burton, B.; Stack, J.H.; Straley, K.S.; Decker, C.J.; Miller, M.; McCartney, J.; Olson, E.R.; et al. Correction of the F508del-CFTR Protein Processing Defect in Vitro by the Investigational Drug VX-809. Proc. Natl. Acad. Sci. USA 2011, 108, 18843–18848. [Google Scholar] [CrossRef]
- Van Goor, F.; Hadida, S.; Grootenhuis, P.D.J.; Burton, B.; Cao, D.; Neuberger, T.; Turnbull, A.; Singh, A.; Joubran, J.; Hazlewood, A.; et al. Rescue of CF Airway Epithelial Cell Function in Vitro by a CFTR Potentiator, VX-770. Proc. Natl. Acad. Sci. USA 2009, 106, 18825–18830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moon, C.; VanDussen, K.L.; Miyoshi, H.; Stappenbeck, T.S. Development of a Primary Mouse Intestinal Epithelial Cell Monolayer Culture System to Evaluate Factors That Modulate IgA Transcytosis. Mucosal Immunol. 2014, 7, 818–828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozuka, K.; He, Y.; Koo-McCoy, S.; Kumaraswamy, P.; Nie, B.; Shaw, K.; Chan, P.; Leadbetter, M.; He, L.; Lewis, J.G.; et al. Development and Characterization of a Human and Mouse Intestinal Epithelial Cell Monolayer Platform. Stem Cell Rep. 2017, 9, 1976–1990. [Google Scholar] [CrossRef] [Green Version]
- Ciciriello, F.; Bijvelds, M.J.C.; Alghisi, F.; Meijsen, K.F.; Cristiani, L.; Sorio, C.; Melotti, P.; Fiocchi, A.G.; Lucidi, V.; De Jonge, H.R. Theratyping of the Rare CFTR Variants E193K and R334W in Rectal Organoid-Derived Epithelial Monolayers. J. Pers. Med. 2022, 12, 632. [Google Scholar] [CrossRef] [PubMed]
- Ferrera, L.; Baroni, D.; Moran, O. Lumacaftor-Rescued F508del-CFTR Has a Modified Bicarbonate Permeability. J. Cyst. Fibros. 2019, 18, 602–605. [Google Scholar] [CrossRef]
- Fiore, M.; Picco, C.; Moran, O. Correctors Modify the Bicarbonate Permeability of F508del-CFTR. Sci. Rep. 2020, 10, 8440. [Google Scholar] [CrossRef] [PubMed]
- Zomer-van Ommen, D.D.; de Poel, E.; Kruisselbrink, E.; Oppelaar, H.; Vonk, A.M.; Janssens, H.M.; van der Ent, C.K.; Hagemeijer, M.C.; Beekman, J.M. Comparison of Ex Vivo and in Vitro Intestinal Cystic Fibrosis Models to Measure CFTR-Dependent Ion Channel Activity. J. Cyst. Fibros. 2018, 17, 316–324. [Google Scholar] [CrossRef]
- Cuyx, S.; Ramalho, A.S.; Corthout, N.; Fieuws, S.; Fürstová, E.; Arnauts, K.; Ferrante, M.; Verfaillie, C.; Munck, S.; Boon, M.; et al. Rectal Organoid Morphology Analysis (ROMA) as a Promising Diagnostic Tool in Cystic Fibrosis. Thorax 2021, 76, 1146–1149. [Google Scholar] [CrossRef] [PubMed]
- Amaral, M.D.; de Boeck, K.; Amaral, M.; Davies, J.C.; de Boeck, K.; Drevinek, P.; Elborn, S.; Kerem, E.; Lee, T. Theranostics by Testing CFTR Modulators in Patient-Derived Materials: The Current Status and a Proposal for Subjects with Rare CFTR Mutations. J. Cyst. Fibros. 2019, 18, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.G.; Mallon, B.S.; Park, K.; Robey, P.G.; McKay, R.D.G.; Gottesman, M.M.; Zheng, W. Pluripotent Stem Cell Platforms for Drug Discovery. Trends Mol. Med. 2018, 24, 805–820. [Google Scholar] [CrossRef]
- Fatehullah, A.; Tan, S.H.; Barker, N. Organoids as an in Vitro Model of Human Development and Disease. Nat. Cell Biol. 2016, 18, 246–254. [Google Scholar] [CrossRef]


| Modulator | License Age and Characteristics | Mutations |
|---|---|---|
| Ivacaftor | Class III gating mutations (Table 2) | |
| Lumacaftor/Ivacaftor | Homozygous F508del | |
| Tezacaftor/Ivacaftor | Homozygous F508del or at least one copy of responsive mutations (Table 3) | |
| Ivacaftor/Tezacaftor/Elexacaftor | At least one F508del mutation or at least one copy of responsive mutations (Table 4) |
| 711 + 3A→G | D1152H | G194R | I807M | Q237H | R553Q | S1159F |
| 2789 + 5 G→A | D1270N | G314E | I1027T | Q359R | R668C | S1159P |
| 3272–26A→G | E56K | G551D | I1139V | Q1291R | R792G | S1251N |
| 3849 + 10kbC→T | E193K | G551S | K1060T | R74W | R933G | S1255P |
| A120T | E822K | G576A | L206W | R75Q | R1070Q | T338I |
| A234D | E831X | G970D | L320V | R117C | R1070W | T1053I |
| A349V | F311del | G1069R | L967S | R117G | R1162L | V232D |
| A455E | F311L | G1244E | L997F | R117H | R1283M | V562I |
| A1067T | F508C | G1249R | L1480P | R117L | S549N | V754M |
| D110E | F508C/S1251N | G1349D | M152V | R117P | S549R | V1293G |
| D110H | F1052V | H939R | M952I | R170H | S589N | W1282R |
| D192G | F1074L | H1375P | M952T | R347H | S737F | Y1014C |
| D579G | G178E | I148T | P67L | R347L | S945L | Y1032C |
| D924N | G178R | I175V | Q237E | R352Q | S977F |
| 546insCTA | D1152H | G126D | I601F | P5L | R334L | S912L |
| 711 + 3A→G | D1270N | G178E | I618T | P67L | R334Q | S945L |
| 2789 + 5 G→A | E56K | G178R | I807M | P205S | R347H | S977F |
| 3272–26A→G | E60K | G194R | I980K | Q98R | R347L | S1159F |
| 3849 + 10kbC→T | E92K | G194V | I1027T | Q237E | R347P | S1159P |
| A120T | E116K | G314E | I1139V | Q237H | R352Q | S1251N |
| A234D | E193K | G551D | I1269N | Q359R | R352W | S1255P |
| A349V | E403D | G551S | I1366N | Q1291R | R553Q | T338I |
| A455E | E588V | G576A | K1060T | R31L | R668C | T1036N |
| A554E | E822K | G576A/R668C | L15P | R74Q | R751L | T1053I |
| A1006E | E831X | G622D | L206W | R74W | R792G | V201M |
| A1067T | F191V | G970D | L320V | R74W/D1270N | R933G | V232D |
| D110E | F311del | G1069R | L346P | R74W/V201M | R1066H | V562I |
| D110H | F311L | G1244E | L967S | R74W/V201M/D1270N | R1070Q | V754M |
| D192G | F508C | G1249R | L997F | R75Q | R1070W | V1153E |
| D443Y | F508C/S1251N | G1349D | L1324P | R117C | R1162L | V1240G |
| D443Y/G576A/R668C | F508del | H939R | L1335P | R117G | R1283M | V1293G |
| D579G | F575Y | H1054D | L1480P | R117H | R1283S | W1282R |
| D614G | F1016S | H1375P | M152V | R117L | S549N | Y109N |
| D836Y | F1052V | I148T | M265R | R117P | S549R | Y161S |
| D924N | F1074L | I175V | M952I | R170H | S589N | Y1014C |
| D979V | F1099L | I336K | M952T | R258G | S737F | Y1032C |
| 3141del9 | E193K | G551D | I980K | P574H | R352W | S1255P |
| 546insCTA | E403D | G551S | I1027T | Q98R | R553Q | T338I |
| A46D | E474K | G576A | I1139V | Q237E | R668C | T1036N |
| A120T | E588V | G576A/R668C | I1269N | Q237H | R751L | T1053I |
| A234D | E822K | G622D | I1366N | Q359R | R792G | V201M |
| A349V | F191V | G628R | K1060T | Q1291R | R933G | V232D |
| A455E | F311del | G970D | L15P | R31L | R1066H | V456A |
| A554E | F311L | G1061R | L165S | R74Q | R1070Q | V456F |
| A1006E | F508C | G1069R | L206W | R74W | R1070W | V562I |
| A1067T | F508C/S1251N | G1244E | L320V | R74W/D1270N | R1162L | V754M |
| D110E | F508del | G1249R | L346P | R74W/V201M | R1283M | V1153E |
| D110H | F575Y | G1349D | L453S | R74W/V201M/D1270N | R1283S | V1240G |
| D192G | F1016S | H139R | L967S | R75Q | S13F | V1293G |
| D443Y | F1052V | H199Y | L997F | R117C | S341P | W361R |
| D443Y/G576A/R668C | F1074L | H939R | L1077P | R117G | S364P | W1098C |
| D579G | F1099L | H1054D | L1324P | R117H | S492F | W1282R |
| D614G | G27R | H1085P | L1335P | R117L | S549N | Y109N |
| D836Y | G85E | H1085R | L1480P | R117P | S549R | Y161D |
| D924N | G126D | H1375P | M152V | R170H | S589N | Y161S |
| D979V | G178E | I148T | M265R | R258G | S737F | Y563N |
| D1152H | G178R | I175V | M952I | R334L | S912L | Y1014C |
| D1270N | G194R | I336K | M952T | R334Q | S945L | Y1032C |
| E56K | G194V | I502T | M1101K | R347H | S977F | |
| E60K | G314E | I601F | P5L | R347L | S1159F | |
| E92K | G463V | I618T | P67L | R347P | S1159P | |
| E116K | G480C | I807M | P205S | R352Q | S1251N |
| Criticisms | Solutions | Refs |
|---|---|---|
|
| [40] |
|
| [68] |
|
| [83,84,85] |
| In progress | |
| In progress | |
| In progress |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conti, J.; Sorio, C.; Melotti, P. Organoid Technology and Its Role for Theratyping Applications in Cystic Fibrosis. Children 2023, 10, 4. https://doi.org/10.3390/children10010004
Conti J, Sorio C, Melotti P. Organoid Technology and Its Role for Theratyping Applications in Cystic Fibrosis. Children. 2023; 10(1):4. https://doi.org/10.3390/children10010004
Chicago/Turabian StyleConti, Jessica, Claudio Sorio, and Paola Melotti. 2023. "Organoid Technology and Its Role for Theratyping Applications in Cystic Fibrosis" Children 10, no. 1: 4. https://doi.org/10.3390/children10010004