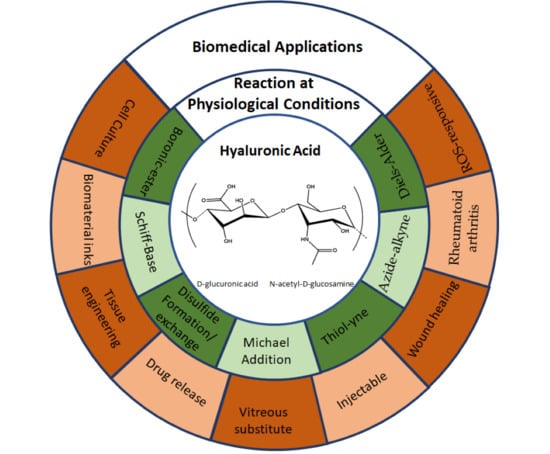
Hyaluronic Acid Hydrogels Crosslinked in Physiological Conditions: Synthesis and Biomedical Applications
Abstract
:1. Introduction
2. Overview of HA Crosslinking Reactions Carried out at Physiological Conditions
2.1. Boronic-Ester Formation
2.2. Schiff-Base Formation
2.3. Thiol Chemistry
2.4. Cycloaddition Reactions
2.4.1. Azide-Alkyne Cycloaddition Reaction
2.4.2. Diels–Alder Formation
3. Thermoreversible Gelation of HA Hydrogels at Physiological Temperature
Combination with Natural Polymers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Name |
| ADH | Adipic acid dihydrazide |
| ASCs | Adipose-derived stem cells |
| pKa | Acid dissociation constant |
| HA-AA | Azide-modified HA |
| BP | BMP-2 mimicking peptide |
| CEC | Carboxyethyl-chitosan |
| CPCs | Cartilage-derived progenitor cells |
| CS | Chitosan |
| Dex | Dextran |
| Dexa | Dexamethasone |
| DA | Diels-Alder |
| Dox | Doxorubicin |
| DHCA | Dihydrocaffeic acid |
| DVS | Divinyl sulfone |
| ECM | Extracellular matrix |
| EWG | Electron withdrawing group |
| Fru | Fructose |
| GP | Glycerophosphate |
| GC | Glycol Chitosan |
| GLU | Gluconamide |
| HA | Hyaluronic acid |
| HA-tet | HA-tetrazine |
| HA-TCO | HA- trans-cyclooctene |
| hMSC | Human mesenchymal stem cells |
| BMP-2 | Human bone morphogenetic protein 2 |
| HPCH | Hydroxypropyl chitin |
| IEDDA | Inverse electron demand Diels-Alder |
| G‘‘ | Loss Modulus |
| LCST | Low critical solution temperature |
| Mal | Maltose |
| HA-mCOH | Mono-aldehyde HA |
| NIPA | N-isopropylacrylamide |
| CS-OB | Oxanorbonadiene-modified CS |
| OHA | Oxidized Hyaluronic acid |
| PBA | Phenylboronic acid |
| PECs | Polyelectrolyte complexes |
| PEG | Polyethylene glycol |
| PEGDA | Polyethylene glycol di-acrylate |
| PEGDMA | Polyethylene glycol di-methacrylate |
| PEO | Poly (ethylene oxide) |
| PNIPAm | Poly (N-isopropylacrylamide) |
| PPO | Poly (propylene oxide) |
| QbD | Quality by design approach |
| ROS | Reactive Oxygen Species |
| NaOH | Sodium hydroxide |
| NaIO4 | sodium periodate |
| T | Temperature |
| SH | Thiol group |
| 3D | Three-dimensional |
| G’ | Storage Modulus |
| SPAAC | Strain-promoted azide–alkyne cycloaddition |
| UV | Ultraviolet |
References
- Ghosh, K. Biocompatibility of hyaluronic acid: From cell recognition to therapeutic applications. In Natural-Based Polymers for Biomedical Application; Woodhead Publishing: Sawston, UK, 2008; pp. 716–737. ISBN 9781845692643. [Google Scholar]
- Huynh, A.; Priefer, R. Hyaluronic acid applications in ophthalmology, rheumatology, and dermatology. Carbohydr. Res. 2020, 489, 107950. [Google Scholar] [CrossRef] [PubMed]
- Burdick, J.A.; Prestwich, G.D. Hyaluronic acid hydrogels for biomedical applications. Adv. Mater. 2011, 23, 41–56. [Google Scholar] [CrossRef] [PubMed]
- Khunmanee, S.; Jeong, Y.; Park, H. Crosslinking method of hyaluronic-based hydrogel for biomedical applications. J. Tissue Eng. 2017, 8, 2041731417726464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schanté, C.E.; Zuber, G.; Herlin, C.; Vandamme, T.F. Chemical modifications of hyaluronic acid for the synthesis of derivatives for a broad range of biomedical applications. Carbohydr. Polym. 2011, 85, 469–489. [Google Scholar] [CrossRef]
- Oliveira, A.V.; Marcelo, A.; Rosa da Costa, A.M.; Silva, G.A. Evaluation of cystamine-modified hyaluronic acid/chitosan polyplex as retinal gene vector. Mater. Sci. Eng. C 2016, 58, 264–272. [Google Scholar] [CrossRef] [Green Version]
- Chan, L.C.; Cox, B.G. Kinetics of amide formation through carbodiimide/N-hydroxybenzotriazole (HOBt) couplings. J. Org. Chem. 2007, 72, 8863–8869. [Google Scholar] [CrossRef]
- Gřundělová, L.; Gregorova, A.; Mráček, A.; Vícha, R.; Smolka, P.; Minařík, A. Viscoelastic and mechanical properties of hyaluronan films and hydrogels modified by carbodiimide. Carbohydr. Polym. 2015, 119, 142–148. [Google Scholar] [CrossRef]
- Li, J.; Huo, M.; Wang, J.; Zhou, J.; Mohammad, J.M.; Zhang, Y.; Zhu, Q.; Waddad, A.Y.; Zhang, Q. Redox-sensitive micelles self-assembled from amphiphilic hyaluronic acid-deoxycholic acid conjugates for targeted intracellular delivery of paclitaxel. Biomaterials 2012, 33, 2310–2320. [Google Scholar] [CrossRef]
- Pandit, A.H.; Mazumdar, N.; Ahmad, S. Periodate oxidized hyaluronic acid-based hydrogel scaffolds for tissue engineering applications. Int. J. Biol. Macromol. 2019, 137, 853–869. [Google Scholar] [CrossRef]
- Kenne, L.; Gohil, S.; Nilsson, E.M.; Karlsson, A.; Ericsson, D.; Helander Kenne, A.; Nord, L.I. Modification and cross-linking parameters in hyaluronic acid hydrogels—Definitions and analytical methods. Carbohydr. Polym. 2013, 91, 410–418. [Google Scholar] [CrossRef] [Green Version]
- Xue, Y.; Chen, H.; Xu, C.; Yu, D.; Xu, H.; Hu, Y. Synthesis of hyaluronic acid hydrogels by crosslinking the mixture of high-molecular-weight hyaluronic acid and low-molecular-weight hyaluronic acid with 1,4-butanediol diglycidyl ether. RSC Adv. 2020, 10, 7206–7213. [Google Scholar] [CrossRef]
- Andrade del Olmo, J.; Alonso, J.M.; Sáez Martínez, V.; Ruiz-Rubio, L.; Pérez González, R.; Vilas-Vilela, J.L.; Pérez-Álvarez, L. Biocompatible hyaluronic acid-divinyl sulfone injectable hydrogels for sustained drug release with enhanced antibacterial properties against Staphylococcus aureus. Mater. Sci. Eng. C 2021, 125, 112102. [Google Scholar] [CrossRef] [PubMed]
- Tsanaktsidou, E.; Kammona, O.; Kiparissides, C. On the synthesis and characterization of biofunctional hyaluronic acid based injectable hydrogels for the repair of cartilage lesions. Eur. Polym. J. 2019, 114, 47–56. [Google Scholar] [CrossRef]
- Borzacchiello, A.; Russo, L.; Malle, B.M.; Schwach-Abdellaoui, K.; Ambrosio, L. Hyaluronic Acid Based Hydrogels for Regenerative Medicine Applications. BioMed Res. Int. 2015, 2015, 871218. [Google Scholar] [CrossRef] [PubMed]
- Alonso, J.M.; Del Olmo, J.A.; Gonzalez, R.P.; Saez-martinez, V. Injectable hydrogels: From laboratory to industrialization. Polymers 2021, 13, 650. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.Y. Relationship between structure and cytocompatibility of divinyl sulfone cross-linked hyaluronic acid. Carbohydr. Polym. 2014, 101, 203–212. [Google Scholar] [CrossRef]
- Gajendiran, M.; Rhee, J.S.; Kim, K. Recent Developments in Thiolated Polymeric Hydrogels for Tissue Engineering Applications. Tissue Eng.-Part B Rev. 2018, 24, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Leichner, C.; Jelkmann, M.; Bernkop-Schnürch, A. Thiolated polymers: Bioinspired polymers utilizing one of the most important bridging structures in nature. Adv. Drug Deliv. Rev. 2019, 151–152, 191–221. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Fernandes-Cunha, G.M.; Myung, D. In situ-forming hyaluronic acid hydrogel through visible light-induced thiol-ene reaction. React. Funct. Polym. 2018, 131, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Choi, B.; Hu, J.; Lee, M. Injectable chitosan hyaluronic acid hydrogels for cartilage tissue engineering. Acta Biomater. 2013, 9, 4779–4786. [Google Scholar] [CrossRef]
- Bi, X.; Liang, A. In Situ-Forming Cross-linking Hydrogel Systems: Chemistry and Biomedical Applications. Emerg. Concepts Anal. Appl. Hydrogels 2016, 86, 541–547. [Google Scholar]
- Echalier, C.; Valot, L.; Martinez, J.; Mehdi, A.; Subra, G. Chemical cross-linking methods for cell encapsulation in hydrogels. Mater. Today Commun. 2019, 20, 100536. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Chen, J.; Deng, C.; Suuronen, E.J.; Zhong, Z. Click hydrogels, microgels and nanogels: Emerging platforms for drug delivery and tissue engineering. Biomaterials 2014, 35, 4969–4985. [Google Scholar] [CrossRef]
- Rodell, C.B.; Kaminski, A.L.; Burdick, J.A. Rational design of network properties in guest-host assembled and shear-thinning hyaluronic acid hydrogels. Biomacromolecules 2013, 14, 4125–4134. [Google Scholar] [CrossRef]
- Rodell, C.B.; Wade, R.J.; Purcell, B.P.; Dusaj, N.N.; Burdick, J.A. Selective Proteolytic Degradation of Guest–Host Assembled, Injectable Hyaluronic Acid Hydrogels. ACS Biomater. Sci. Eng. 2015, 1, 277–286. [Google Scholar] [CrossRef]
- Rosales, A.M.; Rodell, C.B.; Chen, M.H.; Morrow, M.G.; Anseth, K.S.; Burdick, J.A. Reversible Control of Network Properties in Azobenzene-Containing Hyaluronic Acid-Based Hydrogels. Bioconjug. Chem. 2018, 29, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, L.; Highley, C.B.; Rodell, C.B.; Sun, W.; Burdick, J.A. 3D Printing of Shear-Thinning Hyaluronic Acid Hydrogels with Secondary Cross-Linking. ACS Biomater. Sci. Eng. 2016, 2, 1743–1751. [Google Scholar] [CrossRef] [PubMed]
- Gaffey, A.C.; Chen, M.H.; Trubelja, A.; Venkataraman, C.M.; Chen, C.W.; Chung, J.J.; Schultz, S.; Sehgal, C.M.; Burdick, J.A.; Atluri, P. Delivery of progenitor cells with injectable shear-thinning hydrogel maintains geometry and normalizes strain to stabilize cardiac function after ischemia. J. Thorac. Cardiovasc. Surg. 2019, 157, 1479–1490. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Kong, X.; Xiong, Y.; Zhang, X.; Chen, H.; Jiang, W.; Niu, Y.; Xu, W.; Ren, C. An overview of dynamic covalent bonds in polymer material and their applications. Eur. Polym. J. 2020, 141, 110094. [Google Scholar] [CrossRef]
- Chakma, P.; Konkolewicz, D. Dynamic Covalent Bonds in Polymeric Materials. Angew. Chem.-Int. Ed. 2019, 58, 9682–9695. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.-H.; Yu, C.-H.; Yeh, Y.-C. Engineering nanocomposite hydrogels using dynamic bonds. Acta Biomater. 2021, 1. [Google Scholar] [CrossRef]
- Tang, S.; Richardson, B.M.; Anseth, K.S. Dynamic covalent hydrogels as biomaterials to mimic the viscoelasticity of soft tissues. Prog. Mater. Sci. 2021, 120, 100738. [Google Scholar] [CrossRef]
- Hernández, R.; Mijangos, C. Determining the Rheological Properties of Polymer Hydrogels for the Development of Advanced Applications. In Rheology: Theory, Properties and Practical Applications; Mitchell, G., Ed.; Centre for Rapid and Sustainable Product Development, Polytechnic Institute of Leiria: Marinha Grande, Portugal, 2014; p. 494. ISBN 978-1-62618-963-8. [Google Scholar]
- Aeridou, E.; Diáz Diáz, D.; Alemán, C.; Pérez-Madrigal, M.M. Advanced Functional Hydrogel Biomaterials Based on Dynamic B-O Bonds and Polysaccharide Building Blocks. Biomacromolecules 2020, 21, 3984–3996. [Google Scholar] [CrossRef]
- Shi, W.; Hass, B.; Kuss, M.A.; Zhang, H.; Ryu, S.; Zhang, D.; Li, T.; Li, Y.; Duan, B. Fabrication of versatile dynamic hyaluronic acid-based hydrogels. Carbohydr. Polym. 2020, 233, 115803. [Google Scholar] [CrossRef]
- Yan, J.; Springsteen, G.; Deeter, S.; Wang, B. The relationship among pK a, pH, and binding constants in the interactions between boronic acids and diols-It is not as simple as it appears. Tetrahedron 2004, 60, 11205–11209. [Google Scholar] [CrossRef]
- Brooks, W.L.A.; Sumerlin, B.S. Synthesis and Applications of Boronic Acid-Containing Polymers: From Materials to Medicine. Chem. Rev. 2016, 116, 1375–1397. [Google Scholar] [CrossRef]
- Figueiredo, T.; Ogawa, Y.; Jing, J.; Cosenza, V.; Jeacomine, I.; Olsson, J.D.M.; Gerfaud, T.; Boiteau, J.G.; Harris, C.; Auzély-Velty, R. Self-crosslinking smart hydrogels through direct complexation between benzoxaborole derivatives and diols from hyaluronic acid. Polym. Chem. 2020, 11, 3800–3811. [Google Scholar] [CrossRef]
- Dufort, B.M.; Tibbitt, M.W. Design of moldable hydrogels for biomedical applications using dynamic covalent boronic esters. Mater. Today Chem. 2019, 12, 16–33. [Google Scholar] [CrossRef]
- Van Duin, M.; Peters, J.A.; Kieboom, A.P.G.; Van Bekkum, H. Studies on borate esters 1. The ph dependence of the stability of esters of boric acid and borate in aqueous medium as studied by 11B NMR. Tetrahedron 1984, 40, 2901–2911. [Google Scholar] [CrossRef]
- Tarus, D.; Hachet, E.; Messager, L.; Catargi, B.; Ravaine, V.; Auzély-Velty, R. Readily prepared dynamic hydrogels by combining phenyl boronic acid-and maltose-modifi ed anionic polysaccharides at neutral pH. Macromol. Rapid Commun. 2014, 35, 2089–2095. [Google Scholar] [CrossRef]
- de Oliveira, M.M.; Nakamura, C.V.; Auzély-Velty, R. Boronate-ester crosslinked hyaluronic acid hydrogels for dihydrocaffeic acid delivery and fibroblasts protection against UVB irradiation. Carbohydr. Polym. 2020, 247, 116845. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Ma, R.; Liu, G.; Liu, X.; Gao, Y.; Shen, J.; An, Y.; Shi, L. Effect of coordination on the glucose-responsiveness of PEG-b-(PAA-co-PAAPBA) micelles. Macromol. Rapid Commun. 2010, 31, 1628–1634. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, T.; Jing, J.; Jeacomine, I.; Olsson, J.; Gerfaud, T.; Boiteau, J.G.; Rome, C.; Harris, C.; Auzély-Velty, R. Injectable Self-Healing Hydrogels Based on Boronate Ester Formation between Hyaluronic Acid Partners Modified with Benzoxaborin Derivatives and Saccharides. Biomacromolecules 2020, 21, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, T.; Cosenza, V.; Ogawa, Y.; Jeacomine, I.; Vallet, A.; Ortega, S.; Michel, R.; Olsson, J.D.M.; Gerfaud, T.; Boiteau, J.G.; et al. Boronic acid and diol-containing polymers: How to choose the correct couple to form “strong” hydrogels at physiological pH. Soft Matter 2020, 16, 3628–3641. [Google Scholar] [CrossRef] [PubMed]
- Abdi, F.; Michel, R.; Poirot, R.; Dakir, M.; Sancey, L.; Ravaine, V.; Auzély-Velty, R. Dynamic Covalent Chemistry Enables Reconfigurable All-Polysaccharide Nanogels. Macromol. Rapid Commun. 2020, 41, 2000213. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Liu, Y.; Hsu, S.H. Hydrogels based on schiff base linkages for biomedical applications. Molecules 2019, 24, 3005. [Google Scholar] [CrossRef] [Green Version]
- Apostolides, D.E.; Patrickios, C.S. Dynamic covalent polymer hydrogels and organogels crosslinked through acylhydrazone bonds: Synthesis, characterization and applications. Polym. Int. 2018, 67, 627–649. [Google Scholar] [CrossRef]
- Li, S.; Pei, M.; Wan, T.; Yang, H.; Gu, S.; Tao, Y.; Liu, X.; Zhou, Y.; Xu, W.; Xiao, P. Self-healing hyaluronic acid hydrogels based on dynamic Schiff base linkages as biomaterials. Carbohydr. Polym. 2020, 250, 116922. [Google Scholar] [CrossRef]
- Qian, C.; Zhang, T.; Gravesande, J.; Baysah, C.; Song, X.; Xing, J. Injectable and self-healing polysaccharide-based hydrogel for pH-responsive drug release. Int. J. Biol. Macromol. 2019, 123, 140–148. [Google Scholar] [CrossRef]
- Li, L.; Wang, N.; Jin, X.; Deng, R.; Nie, S.; Sun, L.; Wu, Q.; Wei, Y.; Gong, C. Biodegradable and injectable in situ cross-linking chitosan-hyaluronic acid based hydrogels for postoperative adhesion prevention. Biomaterials 2014, 35, 3903–3917. [Google Scholar] [CrossRef]
- Kim, D.Y.; Park, H.; Kim, S.W.; Lee, J.W.; Lee, K.Y. Injectable hydrogels prepared from partially oxidized hyaluronate and glycol chitosan for chondrocyte encapsulation. Carbohydr. Polym. 2017, 157, 1281–1287. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Nah, H.; Heo, D.N.; Kim, K.H.; Seok, J.M.; Heo, M.; Moon, H.J.; Lee, D.; Lee, J.S.; An, S.Y.; et al. Induction of osteogenic differentiation in a rat calvarial bone defect model using an In situ forming graphene oxide incorporated glycol chitosan/oxidized hyaluronic acid injectable hydrogel. Carbon N. Y. 2020, 168, 264–277. [Google Scholar] [CrossRef]
- Kim, S.W.; Kim, D.Y.; Roh, H.H.; Kim, H.S.; Lee, J.W.; Lee, K.Y. Three-Dimensional Bioprinting of Cell-Laden Constructs Using Polysaccharide-Based Self-Healing Hydrogels. Biomacromolecules 2019, 20, 1860–1866. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cheng, C.; Gao, X.; Wang, S.; Ye, H.; Han, X. Aminoglycoside hydrogels based on dynamic covalent bonds with pH sensitivity, biocompatibility, self-healing, and antibacterial ability. J. Appl. Polym. Sci. 2020, 137, 49250. [Google Scholar] [CrossRef]
- Kirker, K.R.; Luo, Y.; Nielson, J.H.; Shelby, J.; Prestwich, G.D. Glycosaminoglycan hydrogel films as bio-interactive dressings for wound healing. Biomaterials 2002, 23, 3661–3671. [Google Scholar] [CrossRef]
- Liu, X.; Wang, L.; Song, X.; Song, H.; Zhao, J.R.; Wang, S. A kinetic model for oxidative degradation of bagasse pulp fiber by sodium periodate. Carbohydr. Polym. 2012, 90, 218–223. [Google Scholar] [CrossRef]
- Hozumi, T.; Kageyama, T.; Ohta, S.; Fukuda, J.; Ito, T. Injectable Hydrogel with Slow Degradability Composed of Gelatin and Hyaluronic Acid Cross-Linked by Schiff’s Base Formation. Biomacromolecules 2018, 19, 288–297. [Google Scholar] [CrossRef]
- Wang, S.; Nawale, G.N.; Oommen, O.P.; Hilborn, J.; Varghese, O.P. Influence of ions to modulate hydrazone and oxime reaction kinetics to obtain dynamically cross-linked hyaluronic acid hydrogels. Polym. Chem. 2019, 10, 4322–4327. [Google Scholar] [CrossRef] [Green Version]
- Baker, A.E.G.; Cui, H.; Ballios, B.G.; Ing, S.; Yan, P.; Wolfer, J.; Wright, T.; Dang, M.; Gan, N.Y.; Cooke, M.J.; et al. Stable oxime-crosslinked hyaluronan-based hydrogel as a biomimetic vitreous substitute. Biomaterials 2021, 271, 120750. [Google Scholar] [CrossRef]
- Shu, X.Z.; Liu, Y.; Luo, Y.; Roberts, M.C.; Prestwich, G.D. Disulfide cross-linked hyaluronan hydrogels. Biomacromolecules 2002, 3, 1304–1311. [Google Scholar] [CrossRef]
- Bian, S.; He, M.; Sui, J.; Cai, H.; Sun, Y.; Liang, J.; Fan, Y.; Zhang, X. The self-crosslinking smart hyaluronic acid hydrogels as injectable three-dimensional scaffolds for cells culture. Colloids Surfaces B Biointerfaces 2016, 140, 392–402. [Google Scholar] [CrossRef] [Green Version]
- Shu, X.Z.; Liu, Y.; Palumbo, F.; Prestwich, G.D. Disulfide-crosslinked hyaluronan-gelatin hydrogel films: A covalent mimic of the extracellular matrix for in vitro cell growth. Biomaterials 2003, 24, 3825–3834. [Google Scholar] [CrossRef]
- Bermejo-Velasco, D.; Azémar, A.; Oommen, O.P.; Hilborn, J.; Varghese, O.P. Modulating Thiol pKa Promotes Disulfide Formation at Physiological pH: An Elegant Strategy to Design Disulfide Cross-Linked Hyaluronic Acid Hydrogels. Biomacromolecules 2019, 20, 1412–1420. [Google Scholar] [CrossRef]
- Xu, K.; Yao, H.; Fan, D.; Zhou, L.; Wei, S. Hyaluronic acid thiol modified injectable hydrogel: Synthesis, characterization, drug release, cellular drug uptake and anticancer activity. Carbohydr. Polym. 2021, 254, 117286. [Google Scholar] [CrossRef] [PubMed]
- Laffleur, F.; Netsomboon, K.; Erman, L.; Partenhauser, A. Evaluation of modified hyaluronic acid in terms of rheology, enzymatic degradation and mucoadhesion. Int. J. Biol. Macromol. 2019, 123, 1204–1210. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Docampo, Z.; Otto, S. Orthogonal or simultaneous use of disulfide and hydrazone exchange in dynamic covalent chemistry in aqueous solution. Chem. Commun. 2008, 41, 5301–5303. [Google Scholar] [CrossRef]
- Deng, G.; Li, F.; Yu, H.; Liu, F.; Liu, C.; Sun, W.; Jiang, H.; Chen, Y. Dynamic hydrogels with an environmental adaptive self-healing ability and dual responsive Sol-Gel transitions. ACS Macro Lett. 2012, 1, 275–279. [Google Scholar] [CrossRef]
- Wu, L.; Di Cio, S.; Azevedo, H.S.; Gautrot, J.E. Photoconfigurable, Cell-Remodelable Disulfide Cross-linked Hyaluronic Acid Hydrogels. Biomacromolecules 2020, 21, 4663–4672. [Google Scholar] [CrossRef]
- Summonte, S.; Racaniello, G.F.; Lopedota, A.; Denora, N.; Bernkop-Schnürch, A. Thiolated polymeric hydrogels for biomedical application: Cross-linking mechanisms. J. Control. Release 2021, 330, 470–482. [Google Scholar] [CrossRef] [PubMed]
- Mehra, T.D.; Ghosh, K.; Shu, X.Z.; Prestwich, G.D.; Clark, R.A.F. Molecular Stenting with a Crosslinked Hyaluronan Derivative Inhibits Collagen Gel Contraction. J. Investig. Dermatol. 2006, 126, 2202–2209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanderhooft, J.L.; Alcoutlabi, M.; Magda, J.J.; Prestwich, G.D. Rheological Properties of Cross-Linked Hyaluronan–Gelatin Hydrogels for Tissue Engineering. Macromol. Biosci. 2009, 9, 20–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, K.; Shu, X.Z.; Mou, R.; Lombardi, J.; Prestwich, G.D.; Rafailovich, M.H.; Clark, R.A.F. Rheological characterization of in situ cross-linkable hysluronan hydrogels. Biomacromolecules 2005, 6, 2857–2865. [Google Scholar] [CrossRef] [PubMed]
- Godesky, M.D.; Shreiber, D.I. Hyaluronic acid-based hydrogels with independently tunable mechanical and bioactive signaling features. Biointerphases 2019, 14, 061005. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Sui, J.; Ma, M.; Xu, Y.; Lin, W.; Chen, Y.; Man, Y.; Sun, Y.; Fan, Y.; Zhang, X. The preparation and biocompatible evaluation of injectable dual crosslinking hyaluronic acid hydrogels as cytoprotective agents. J. Mater. Chem. B 2019, 7, 4413–4423. [Google Scholar] [CrossRef]
- Shu, X.Z.; Liu, Y.; Palumbo, F.S.; Luo, Y.; Prestwich, G.D. In situ crosslinkable hyaluronan hydrogels for tissue engineering. Biomaterials 2004, 25, 1339–1348. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, P.; Li, X.; Xu, Y.; Lu, G.; Jiang, Q.; Sun, Y.; Fan, Y.; Zhang, X. A di-self-crosslinking hyaluronan-based hydrogel combined with type I collagen to construct a biomimetic injectable cartilage-filling scaffold. Acta Biomater. 2020, 111, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Zhu, M.; Wei, K.; Bian, L. Cell-Mediated Degradation Regulates Human Mesenchymal Stem Cell Chondrogenesis and Hypertrophy in MMP-Sensitive Hyaluronic Acid Hydrogels. PLoS ONE 2014, 9, e99587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Y.; Chau, Y. One-step “click” method for generating vinyl sulfone groups on hydroxyl-containing water-soluble polymers. Biomacromolecules 2012, 13, 937–942. [Google Scholar] [CrossRef]
- Jin, R.; Teixeira, L.S.M.; Krouwels, A.; Dijkstra, P.J.; Van Blitterswijk, C.A.; Karperien, M.; Feijen, J. Synthesis and characterization of hyaluronic acid-poly(ethylene glycol) hydrogels via Michael addition: An injectable biomaterial for cartilage repair. Acta Biomater. 2010, 6, 1968–1977. [Google Scholar] [CrossRef]
- Daglar, O.; Luleburgaz, S.; Baysak, E.; Gunay, U.S.; Hizal, G.; Tunca, U.; Durmaz, H. Nucleophilic Thiol-yne reaction in Macromolecular Engineering: From synthesis to applications. Eur. Polym. J. 2020, 137, 109926. [Google Scholar] [CrossRef]
- Pérez-Madrigal, M.M.; Shaw, J.E.; Arno, M.C.; Hoyland, J.A.; Richardson, S.M.; Dove, A.P. Robust alginate/hyaluronic acid thiol-yne click-hydrogel scaffolds with superior mechanical performance and stability for load-bearing soft tissue engineering. Biomater. Sci. 2020, 8, 405–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Sigen, A.; Xu, Q.; Alshehri, F.; Zeng, M.; Zhou, D.; Li, J.; Zhou, G.; Wang, W. Cartilage-Derived Progenitor Cell-Laden Injectable Hydrogel-An Approach for Cartilage Tissue Regeneration. ACS Appl. Bio Mater. 2020, 3, 4756–4765. [Google Scholar] [CrossRef]
- Zhong, Y.; Zhao, X.; Li, G.; Zhang, D.; Wang, D. Mussel-inspired hydrogels as tissue adhesives for hemostasis with fast-forming and self-healing properties. Eur. Polym. J. 2021, 148, 110361. [Google Scholar] [CrossRef]
- Van Dijk, M.; Rijkers, D.T.S.; Liskamp, R.M.J.; Van Nostrum, C.F.; Hennink, W.E. Synthesis and applications of biomedical and pharmaceutical polymers via click chemistry methodologies. Bioconjug. Chem. 2009, 20, 2001–2016. [Google Scholar] [CrossRef] [PubMed]
- Piluso, S.; Hiebl, B.; Gorb, S.N.; Kovalev, A.; Lendlein, A.; Neffe, A.T. Hyaluronic acid-based hydrogels crosslinked by copper-catalyzed azide-alkyne cycloaddition with tailorable mechanical properties. Int. J. Artif. Organs 2011, 34, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Truong, V.X.; Ablett, M.P.; Gilbert, H.T.J.; Bowen, J.; Richardson, S.M.; Hoyland, J.A.; Dove, A.P. In situ-forming robust chitosan-poly(ethylene glycol) hydrogels prepared by copper-free azide-alkyne click reaction for tissue engineering. Biomater. Sci. 2014, 2, 167–175. [Google Scholar] [CrossRef] [Green Version]
- Fan, M.; Ma, Y.; Mao, J.; Zhang, Z.; Tan, H. Cytocompatible in situ forming chitosan/hyaluronan hydrogels via a metal-free click chemistry for soft tissue engineering. Acta Biomater. 2015, 20, 60–68. [Google Scholar] [CrossRef]
- Fu, S.; Dong, H.; Deng, X.; Zhuo, R.; Zhong, Z. Injectable hyaluronic acid/poly(ethylene glycol) hydrogels crosslinked via strain-promoted azide-alkyne cycloaddition click reaction. Carbohydr. Polym. 2017, 169, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Han, S.S.; Yoon, H.Y.; Yhee, J.Y.; Cho, M.O.; Shim, H.E.; Jeong, J.E.; Lee, D.E.; Kim, K.; Guim, H.; Lee, J.H.; et al. In situ cross-linkable hyaluronic acid hydrogels using copper free click chemistry for cartilage tissue engineering. Polym. Chem. 2018, 9, 20–27. [Google Scholar] [CrossRef]
- Tasdelen, M.A. Diels-Alder “click” reactions: Recent applications in polymer and material science. Polym. Chem. 2011, 2, 2133–2145. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, R.; Luo, G.; Wu, J.; Xia, H. A self-healing, re-moldable and biocompatible crosslinked polysiloxane elastomer. J. Mater. Chem. B 2016, 4, 982–989. [Google Scholar] [CrossRef]
- Nimmo, C.M.; Owen, S.C.; Shoichet, M.S. Diels-alder click cross-linked hyaluronic acid hydrogels for tissue engineering. Biomacromolecules 2011, 12, 824–830. [Google Scholar] [CrossRef] [Green Version]
- Tam, R.Y.; Fisher, S.A.; Baker, A.E.G.; Shoichet, M.S. Transparent Porous Polysaccharide Cryogels Provide Biochemically Defined, Biomimetic Matrices for Tunable 3D Cell Culture. Chem. Mater. 2016, 28, 3762–3770. [Google Scholar] [CrossRef]
- Owen, S.C.; Fisher, S.A.; Tam, R.Y.; Nimmo, C.M.; Shoichet, M.S. Hyaluronic acid click hydrogels emulate the extracellular matrix. Langmuir 2013, 29, 7393–7400. [Google Scholar] [CrossRef]
- Smith, L.J.; Taimoory, S.M.; Tam, R.Y.; Baker, A.E.G.; Binth Mohammad, N.; Trant, J.F.; Shoichet, M.S. Diels-Alder Click-Cross-Linked Hydrogels with Increased Reactivity Enable 3D Cell Encapsulation. Biomacromolecules 2018, 19, 926–935. [Google Scholar] [CrossRef]
- Heo, J.Y.; Noh, J.H.; Park, S.H.; Ji, Y.B.; Ju, H.J.; Kim, D.Y.; Lee, B.; Kim, M.S. An injectable click-crosslinked hydrogel that prolongs dexamethasone release from dexamethasone-loaded microspheres. Pharmaceutics 2019, 11, 438. [Google Scholar] [CrossRef] [Green Version]
- Seo, J.; Park, S.H.; Kim, M.J.; Ju, H.J.; Yin, X.Y.; Min, B.H.; Kim, M.S. Injectable Click-Crosslinked Hyaluronic Acid Depot to Prolong Therapeutic Activity in Articular Joints Affected by Rheumatoid Arthritis. ACS Appl. Mater. Interfaces 2019, 11, 24984–24998. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Park, J.Y.; Ji, Y.B.; Ju, H.J.; Min, B.H.; Kim, M.S. An injectable click-crosslinked hyaluronic acid hydrogel modified with a BMP-2 mimetic peptide as a bone tissue engineering scaffold. Acta Biomater. 2020, 117, 108–120. [Google Scholar] [CrossRef]
- Famili, A.; Rajagopal, K. Bio-Orthogonal Cross-Linking Chemistry Enables in Situ Protein Encapsulation and Provides Sustained Release from Hyaluronic Acid Based Hydrogels. Mol. Pharm. 2017, 14, 1961–1968. [Google Scholar] [CrossRef] [PubMed]
- Delplace, V.; Nickerson, P.E.B.; Ortin-Martinez, A.; Baker, A.E.G.; Wallace, V.A.; Shoichet, M.S. Nonswelling, Ultralow Content Inverse Electron-Demand Diels–Alder Hyaluronan Hydrogels with Tunable Gelation Time: Synthesis and In Vitro Evaluation. Adv. Funct. Mater. 2020, 30, 1903978. [Google Scholar] [CrossRef]
- Yu, C.; Gao, H.; Li, Q.; Cao, X. Injectable dual cross-linked adhesive hyaluronic acid multifunctional hydrogel scaffolds for potential applications in cartilage repair. Polym. Chem. 2020, 11, 3169–3178. [Google Scholar] [CrossRef]
- Jeong, B.; Kim, S.W.; Bae, Y.H. Thermosensitive sol-gel reversible hydrogels. Adv. Drug Deliv. Rev. 2002, 54, 37–51. [Google Scholar] [CrossRef]
- Mathew, A.P.; Uthaman, S.; Cho, K.H.; Cho, C.S.; Park, I.K. Injectable hydrogels for delivering biotherapeutic molecules. Int. J. Biol. Macromol. 2018, 110, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Ha, D.I.; Lee, S.B.; Chong, M.S.; Lee, Y.M.; Kim, S.Y.; Park, Y.H. Preparation of thermo-responsive and injectable hydrogels based on hyaluronic acid and poly(N-isopropylacrylamide) and their drug release behaviors. Macromol. Res. 2006, 14, 87–93. [Google Scholar] [CrossRef]
- Fong, Y.T.; Chen, C.H.; Chen, J.P. Intratumoral delivery of doxorubicin on folate-conjugated graphene oxide by in-situ forming thermo-sensitive hydrogel for breast cancer therapy. Nanomaterials 2017, 7, 388. [Google Scholar] [CrossRef] [Green Version]
- Coronado, R.; Pekerar, S.; Lorenzo, A.T.; Sabino, M.A. Characterization of thermo-sensitive hydrogels based on poly(N-isopropylacrylamide)/hyaluronic acid. Polym. Bull. 2011, 67, 101–124. [Google Scholar] [CrossRef]
- Jung, Y.s.; Park, W.; Park, H.; Lee, D.K.; Na, K. Thermo-sensitive injectable hydrogel based on the physical mixing of hyaluronic acid and Pluronic F-127 for sustained NSAID delivery. Carbohydr. Polym. 2017, 156, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Chung, H.J.; Yeo, S.; Ahn, C.H.; Lee, H.; Messersmith, P.B.; Park, T.G. Thermo-sensitive, injectable, and tissue adhesive sol-gel transition hyaluronic acid/pluronic composite hydrogels prepared from bio-inspired catechol-thiol reaction. Soft Matter 2010, 6, 977–983. [Google Scholar] [CrossRef]
- Lee, H.; Park, T.G. Photo-crosslinkable, biomimetic, and thermo-sensitive pluronic grafted hyaluronic acid copolymers for injectable delivery of chondrocytes. J. Biomed. Mater. Res.-Part A 2009, 88, 797–806. [Google Scholar] [CrossRef]
- Chenite, A.; Chaput, C.; Wang, D.; Combes, C.; Buschmann, M.D.; Hoemann, C.D.; Leroux, J.C.; Atkinson, B.L.; Binette, F.; Selmani, A. Novel injectable neutral solutions of chitosan form biodegradable gels in situ. Biomaterials 2000, 21, 2155–2161. [Google Scholar] [CrossRef]
- Lejardi, A.; Hernández, R.; Criado, M.; Santos, J.I.; Etxeberria, A.; Sarasua, J.R.; Mijangos, C. Novel hydrogels of chitosan and poly(vinyl alcohol)-g-glycolic acid copolymer with enhanced rheological properties. Carbohydr. Polym. 2014, 103, 267–273. [Google Scholar] [CrossRef]
- Zhang, W.; Jin, X.; Li, H.; Zhang, R.-R.; Wu, C.-W. Injectable and body temperature sensitive hydrogels based on chitosan and hyaluronic acid for pH sensitive drug release. Carbohydr. Polym. 2018, 186, 82–90. [Google Scholar] [CrossRef]
- Yu, P.; Xie, J.; Chen, Y.; Liu, J.; Liu, Y.; Bi, B.; Luo, J.; Li, S.; Jiang, X.; Li, J. A thermo-sensitive injectable hydroxypropyl chitin hydrogel for sustained salmon calcitonin release with enhanced osteogenesis and hypocalcemic effects. J. Mater. Chem. B 2020, 8, 270–281. [Google Scholar] [CrossRef]
- Sultana, T.; Gwon, J.G.; Lee, B.T. Thermal stimuli-responsive hyaluronic acid loaded cellulose based physical hydrogel for post-surgical de novo peritoneal adhesion prevention. Mater. Sci. Eng. C 2020, 110, 110661. [Google Scholar] [CrossRef] [PubMed]
- Djabourov, M. Chapter 1:Gels. In NMR and MRI of Gels; The Royal Society of Chemistry: Cambridge, UK, 2020; pp. 1–44. [Google Scholar]
- Gonzalez, J.S.; Mijangos, C.; Hernandez, R. Polysaccharide coating of gelatin gels for controlled BSA release. Polymers 2019, 11, 702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanmartín-Masiá, E.; Poveda-Reyes, S.; Gallego Ferrer, G. Extracellular matrix–inspired gelatin/hyaluronic acid injectable hydrogels. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 280–288. [Google Scholar] [CrossRef]
- Moura, M.J.; Figueiredo, M.M.; Gil, M.H. Rheological study of genipin cross-linked chitosan hydrogels. Biomacromolecules 2007, 8, 3823–3829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilarska, A.; Lewandowska-Łańcucka, J.; Guzdek-Zając, K.; Karewicz, A.; Horak, W.; Lach, R.; Wójcik, K.; Nowakowska, M. Bioactive yet antimicrobial structurally stable collagen/chitosan/lysine functionalized hyaluronic acid–based injectable hydrogels for potential bone tissue engineering applications. Int. J. Biol. Macromol. 2020, 155, 938–950. [Google Scholar] [CrossRef]
- Chang, K.C.; Lin, D.J.; Wu, Y.R.; Chang, C.W.; Chen, C.H.; Ko, C.L.; Chen, W.C. Characterization of genipin-crosslinked gelatin/hyaluronic acid-based hydrogel membranes and loaded with hinokitiol: In vitro evaluation of antibacterial activity and biocompatibility. Mater. Sci. Eng. C 2019, 105, 110074. [Google Scholar] [CrossRef]
- Meng, X.; Lu, Y.; Gao, Y.; Cheng, S.; Tian, F.; Xiao, Y.; Li, F. Chitosan/alginate/hyaluronic acid polyelectrolyte composite sponges crosslinked with genipin for wound dressing application. Int. J. Biol. Macromol. 2021, 182, 512–523. [Google Scholar] [CrossRef]
- Gilarska, A.; Lewandowska-Łańcucka, J.; Horak, W.; Nowakowska, M. Collagen/chitosan/hyaluronic acid–based injectable hydrogels for tissue engineering applications–design, physicochemical and biological characterization. Colloids Surfaces B Biointerfaces 2018, 170, 152–162. [Google Scholar] [CrossRef] [PubMed]










| Cross-Linking | Complementary Groups | HA Chemical Modification | Biomedical Applications |
|---|---|---|---|
| Boronic–ester formation | Boronic acid + amine/hydroxyl [36,42,43,45,47] | Amidation [36,42,43,45,47]; | Injectable [36,45]; biomaterial inks [36]; tissue engineering [36]: bone; ROS-responsive properties [36]; drug release [43] |
| Schiff base formation | Amine + Aldehyde [51,53,54,55,59] | Oxidation [51,53,54,55]; Amidation [59,61]; | Injectable [51,53,54,55,59,61]; tissue engineering [59]: bone [54], cartilage [53,55]; Bioprinting [55]; pH responsive property [51]; drug release [51]; vitreous substitute [61] |
| Dihydrazide + Aldehyde + [56,59] | |||
| Oxyamine + aldehyde/Ketone [61] | |||
| Thiol Chemistry | |||
| Disulfide formation/exchange | Thiol-thiol [62,63,64,74,75] | Amidation [62,63,64,74] | Tissue engineering [62,63,64] |
| Michael addition | Thiol + (metha)/acrylate [72,73,74,75,76,77,84] | Amidation [72,74,77,78,79,81,85]; ether formation [80] | Injectable [77,78,81,84]; tissue engineering [72,73,77,84]: cartilage [79,81]; cell encapsulation [84]; wound healing [72]; hemostasis [85] |
| Thiol + Melaimide [78,79] | |||
| Thiol + Vinyl-sulfone [80,81] | |||
| Thiol + Catechol [85] | |||
| Thiol-yne coupling | Thiol/yne [83] | Amidation [83] | Injectable [83]; cell encapsulation [83]; tissue engineering: cartilage [83] |
| Cycloaddition reaction | |||
| Azide–alkyne cycloaddition reaction | Azide + Oxanorbornadiene [89] | Amidation [89,90,91] | Injectable [89,90,91]; tissue engineering [89]; cell encapsulation [90,91] |
| Azide + Cyclooctyne [90,91] | |||
| Diels–Alder formation | Furan + Maleimide [97,103] | Amidation [97,98,99,100,101,102,103] | Injectable [98,99,100,103]; tissue engineering [99,103]: bone [100]; rheumatoid arthritis [99]; cell encapsulation [97]; protein encapsulation [101]; drug release [98,99] |
| Tetrazine + Trans-cyclooctene [98,99,100] | |||
| Tetrazine + norbornene [101,102] | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez, L.A.; Hernández, R.; Alonso, J.M.; Pérez-González, R.; Sáez-Martínez, V. Hyaluronic Acid Hydrogels Crosslinked in Physiological Conditions: Synthesis and Biomedical Applications. Biomedicines 2021, 9, 1113. https://doi.org/10.3390/biomedicines9091113
Pérez LA, Hernández R, Alonso JM, Pérez-González R, Sáez-Martínez V. Hyaluronic Acid Hydrogels Crosslinked in Physiological Conditions: Synthesis and Biomedical Applications. Biomedicines. 2021; 9(9):1113. https://doi.org/10.3390/biomedicines9091113
Chicago/Turabian StylePérez, Luis Andrés, Rebeca Hernández, José María Alonso, Raúl Pérez-González, and Virginia Sáez-Martínez. 2021. "Hyaluronic Acid Hydrogels Crosslinked in Physiological Conditions: Synthesis and Biomedical Applications" Biomedicines 9, no. 9: 1113. https://doi.org/10.3390/biomedicines9091113