Figure 1.
Fundamental structure of the adult newt skin. Images were obtained from transverse sections of the dorsal skin of the forearm unless otherwise mentioned. (a) A representative image of the skin. Masson’s trichrome stain. Blue/gray area: collagen-rich connective tissue. (b) A representative image of the epidermis. Hematoxylin-eosin stain. Cells in both the basal layer and the transitional layer contained melanin pigments. (c–f) Sample images showing cell division along the basal layer. DAPI stain of nuclei or chromosomes. White arrowheads: spindle pole. The basal stem cells divided either horizontally (c) or vertically (e). (g,h) Representative images of the dendritic melanophore. DAPI stain of nuclei is shown in (h). The cell had a cell body (arrows) located at the innermost region of the transitional layer, and extended dendritic fibers along the border between the transitional layer and the basal layer. (i) A representative image of lateral skin with a black and orange color pattern. V: ventral side. (j) A representative image of the plane orange area of ventral skin. The top is the ventral side. Note that the epidermis was transparent in lateral and ventral skin with a color pattern. We could not detect dendritic melanophores or pigment granules in cells of the basal and transitional layers. BL: basal layer; D: dermis; Ep: epidermis; GG: granular gland; M: muscle; MG: mucous gland; Mp: melanophore; PLC: pigment cell layer; SC: stratum corneum; TL: transitional layer; Xp: xanthophore. Asterisks: blood capillaries. Scale bars: 100 μm (a,i); 40 μm (b,g,h,j); 50 μm (c,e); 10 μm (d,f).
Figure 1.
Fundamental structure of the adult newt skin. Images were obtained from transverse sections of the dorsal skin of the forearm unless otherwise mentioned. (a) A representative image of the skin. Masson’s trichrome stain. Blue/gray area: collagen-rich connective tissue. (b) A representative image of the epidermis. Hematoxylin-eosin stain. Cells in both the basal layer and the transitional layer contained melanin pigments. (c–f) Sample images showing cell division along the basal layer. DAPI stain of nuclei or chromosomes. White arrowheads: spindle pole. The basal stem cells divided either horizontally (c) or vertically (e). (g,h) Representative images of the dendritic melanophore. DAPI stain of nuclei is shown in (h). The cell had a cell body (arrows) located at the innermost region of the transitional layer, and extended dendritic fibers along the border between the transitional layer and the basal layer. (i) A representative image of lateral skin with a black and orange color pattern. V: ventral side. (j) A representative image of the plane orange area of ventral skin. The top is the ventral side. Note that the epidermis was transparent in lateral and ventral skin with a color pattern. We could not detect dendritic melanophores or pigment granules in cells of the basal and transitional layers. BL: basal layer; D: dermis; Ep: epidermis; GG: granular gland; M: muscle; MG: mucous gland; Mp: melanophore; PLC: pigment cell layer; SC: stratum corneum; TL: transitional layer; Xp: xanthophore. Asterisks: blood capillaries. Scale bars: 100 μm (a,i); 40 μm (b,g,h,j); 50 μm (c,e); 10 μm (d,f).
![Biomedicines 09 01892 g001]()
Figure 2.
Abdominal skin of the adult newt. (a,b) Representative images of transverse sections of the border of black and orange areas. Ep: epidermis; GG: granular gland; M: muscle; MG: mucous gland; Mp: melanophore; PLC: pigment cell layer; Xp: xanthophore. Arrow heads: pigmented fiber layers. Scale bars: 100 μm.
Figure 2.
Abdominal skin of the adult newt. (a,b) Representative images of transverse sections of the border of black and orange areas. Ep: epidermis; GG: granular gland; M: muscle; MG: mucous gland; Mp: melanophore; PLC: pigment cell layer; Xp: xanthophore. Arrow heads: pigmented fiber layers. Scale bars: 100 μm.
Figure 3.
An evaluation of regenerative capacity of skin at various locations on the body of the adult newt. (a) Schematic drawing of the site of skin removal. A 4–9 mm2 square-to-oval shaped piece was excised from the dorsal skin of the head, trunk and limbs (forelimbs and hind limbs), as well as from abdominal skin, including skin around the cloaca (white squares). In the dorsal skin of the trunk, an excision was made within the area between the top (dorsal midline) and the boundary with orange abdominal skin. In the abdominal skin (i.e., belly skin), the plain orange area and the black-orange patterned area were separately examined. (b) Summary of results at 180 days after operation. * and ** show the results at 720 days after operation; each was 100% (n = 2) (for details, see the main text). N: not applicable.
Figure 3.
An evaluation of regenerative capacity of skin at various locations on the body of the adult newt. (a) Schematic drawing of the site of skin removal. A 4–9 mm2 square-to-oval shaped piece was excised from the dorsal skin of the head, trunk and limbs (forelimbs and hind limbs), as well as from abdominal skin, including skin around the cloaca (white squares). In the dorsal skin of the trunk, an excision was made within the area between the top (dorsal midline) and the boundary with orange abdominal skin. In the abdominal skin (i.e., belly skin), the plain orange area and the black-orange patterned area were separately examined. (b) Summary of results at 180 days after operation. * and ** show the results at 720 days after operation; each was 100% (n = 2) (for details, see the main text). N: not applicable.
Figure 4.
Sample images of regenerating skin at different locations on the body of the adult newt. The left- and right-hand columns show the images immediately (Day 0) and at 180 days (Day 180) after operation, respectively. (
a,
b) Dorsal skin of the head. (
c,
d) Dorsal skin of the forearm. (
e,
f) Dorsal skin of the shin of a hind limb. In these three cases, regenerating area had almost blended into the surroundings by 180 days. The conditions in (
b,
d,
f) were almost acceptable according to the requirements of reconstructive surgery and cosmetic studies. (
g,
h) Dorsal skin of the trunk. In this case, the area near the dorsal midline was excised (see
Figure 3a). Note that the texture and color tone in this area had not fully recovered by 180 days. (
i,
j) Color patterned area of the ventral skin of the trunk (i.e., the belly skin). In this particular case, the shape of the black area was altered although the skin structure had recovered well by 180 days. Note that the orange area surrounding the black area had not been restored to its original tone by 180 days. The conditions in (
h,
j) were not acceptable from a reconstructive surgical or cosmetic standpoint. For these animals, the wound healing process continued to be monitored for as long as 720 days after surgery (see following sections). Scale bars: 2 mm (
a,
b); 1 mm (
c–
j).
Figure 4.
Sample images of regenerating skin at different locations on the body of the adult newt. The left- and right-hand columns show the images immediately (Day 0) and at 180 days (Day 180) after operation, respectively. (
a,
b) Dorsal skin of the head. (
c,
d) Dorsal skin of the forearm. (
e,
f) Dorsal skin of the shin of a hind limb. In these three cases, regenerating area had almost blended into the surroundings by 180 days. The conditions in (
b,
d,
f) were almost acceptable according to the requirements of reconstructive surgery and cosmetic studies. (
g,
h) Dorsal skin of the trunk. In this case, the area near the dorsal midline was excised (see
Figure 3a). Note that the texture and color tone in this area had not fully recovered by 180 days. (
i,
j) Color patterned area of the ventral skin of the trunk (i.e., the belly skin). In this particular case, the shape of the black area was altered although the skin structure had recovered well by 180 days. Note that the orange area surrounding the black area had not been restored to its original tone by 180 days. The conditions in (
h,
j) were not acceptable from a reconstructive surgical or cosmetic standpoint. For these animals, the wound healing process continued to be monitored for as long as 720 days after surgery (see following sections). Scale bars: 2 mm (
a,
b); 1 mm (
c–
j).
![Biomedicines 09 01892 g004]()
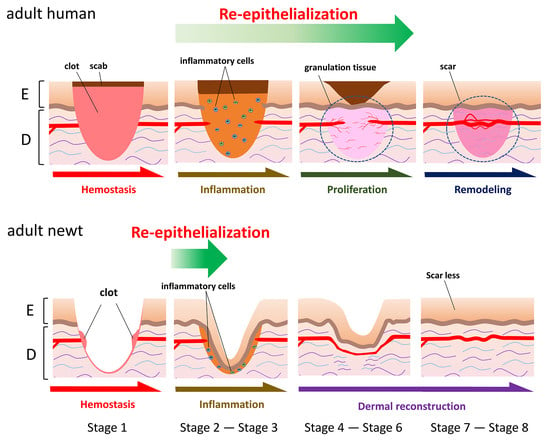
Figure 5.
Morphological stages of skin regeneration in the adult newt.
Figure 5.
Morphological stages of skin regeneration in the adult newt.
Figure 6.
Morphological changes of the skin wound during its regeneration in the adult newt. A representative data set was obtained from the dorsal skin of the forearms. The full-thickness skin was excised from the dorsal surface as in
Figure 4c, and allowed to regenerate for as long as 2 years. Images were obtained from transverse sections. Masson’s trichrome stain was used, unless mentioned otherwise. Blue or gray: collagen-rich extracellular matrix (ECM). (
a) Stage 1: Immediately after the excision of full-thickness skin. Hematoxylin-eosin stain. (
b,
c) Stage 2: 12 h after operation. The wound epidermis appeared on the wound bed. Black arrow: leading end of the wound epidermis. Note that it was multilayered. Asterisks: coagulated blood; red blood cells are dark yellow. (
d) Stage 3: 3 days after operation. The wound bed was completely covered by the wound epidermis. (
e) Stage 4: 4 days after operation. The collagen-rich dermal tissue obviously started to expand from the wound margin. Blue arrow: top of the collagen-rich dermal tissue. (
f) Stage 5: 7 days after operation. Melanophores obviously started to migrate from the wound margin. White arrowhead: top of the migrating melanophores. By this stage, the space in between the wound bed and wound epidermis was entirely filled by collagen-rich dermal tissue, as suggested by the blue or gray color by Masson’s trichrome stain. (
g) Stage 6: 120 days after operation. Exocrine glands appeared. In this section, a granular gland was recognized. Repetition of the thinner region corresponding to the sulcus cutis became recognizable along the regenerating epidermis. (
h) Stage 7: 180 days after operation. Exocrine glands grew. By this stage, the collagen-rich dermal tissue thickened to the level of the surrounding, and the pigment cell layer was restored. (
i) Stage 8: 720 days after operation. The skin regenerated. The regenerating skin had almost blended into its surroundings. By this stage, different types of exocrine glands differentiated, and the crista cutis was also restored. In this section, a mucous gland as well as a granular gland were recognized. Yellow arrowheads: location of wound margin. D: dermis; Ep: epidermis; rGG: regenerating granular gland; rMG: regenerating mucous gland. Scale bars, 100 μm.
Figure 6.
Morphological changes of the skin wound during its regeneration in the adult newt. A representative data set was obtained from the dorsal skin of the forearms. The full-thickness skin was excised from the dorsal surface as in
Figure 4c, and allowed to regenerate for as long as 2 years. Images were obtained from transverse sections. Masson’s trichrome stain was used, unless mentioned otherwise. Blue or gray: collagen-rich extracellular matrix (ECM). (
a) Stage 1: Immediately after the excision of full-thickness skin. Hematoxylin-eosin stain. (
b,
c) Stage 2: 12 h after operation. The wound epidermis appeared on the wound bed. Black arrow: leading end of the wound epidermis. Note that it was multilayered. Asterisks: coagulated blood; red blood cells are dark yellow. (
d) Stage 3: 3 days after operation. The wound bed was completely covered by the wound epidermis. (
e) Stage 4: 4 days after operation. The collagen-rich dermal tissue obviously started to expand from the wound margin. Blue arrow: top of the collagen-rich dermal tissue. (
f) Stage 5: 7 days after operation. Melanophores obviously started to migrate from the wound margin. White arrowhead: top of the migrating melanophores. By this stage, the space in between the wound bed and wound epidermis was entirely filled by collagen-rich dermal tissue, as suggested by the blue or gray color by Masson’s trichrome stain. (
g) Stage 6: 120 days after operation. Exocrine glands appeared. In this section, a granular gland was recognized. Repetition of the thinner region corresponding to the sulcus cutis became recognizable along the regenerating epidermis. (
h) Stage 7: 180 days after operation. Exocrine glands grew. By this stage, the collagen-rich dermal tissue thickened to the level of the surrounding, and the pigment cell layer was restored. (
i) Stage 8: 720 days after operation. The skin regenerated. The regenerating skin had almost blended into its surroundings. By this stage, different types of exocrine glands differentiated, and the crista cutis was also restored. In this section, a mucous gland as well as a granular gland were recognized. Yellow arrowheads: location of wound margin. D: dermis; Ep: epidermis; rGG: regenerating granular gland; rMG: regenerating mucous gland. Scale bars, 100 μm.
![Biomedicines 09 01892 g006]()
Figure 7.
The process of re-epithelialization. A representative data set was obtained from the dorsal skin of the forearms. (a) Wound margin immediately after operation (Stage 1). (b) Wound epidermis which appeared 6 h after operation. Blue in (a,b): DAPI staining of nuclei. (c,d) Neighboring sections showing the wound epidermis at Stage 2 (12 h after operation). Masson’s trichrome stain. As shown in (d), the region composed of the stratum corneum and a part of the transitional layer was sometimes detached from the extending wound epidermis during sectioning. (e–h) Dendritic melanophores (magenta arrow) in the extending wound epidermis. (e,f) 6 h after operation. (f) is a magnification of a part of (e). (g,h) Stage 2 (12 h after operation). (h) is a magnification of a part of (g). (i) The extending epidermal tongue (EET) 6 h after operation. A view from above. The EET was brown because its constituent cells contained melanin pigments. Following a delay after the wound epidermis started to extend, differentiation of the stratum corneum began from the circumference of the wound. (j,k) Wound epidermis closing around the center of the wound bed 24 h after operation. (k) is a magnification of a part of (j). This is a sagittal section of the forearm. The wave of differentiation of the stratum corneum caught up with the closure of the wound epidermis; however, in this particular case, the stratum corneum and the EET were not integrated into one continuous epithelium. The central hole surrounded by the wound epidermis was filled with white blood cells containing neutrophils and monocytes (arrowheads in the inset). BL: basal layer; GG: granular gland; PCL: pigment cell layer; SC: stratum corneum; TL: transitional layer. Black and white arrows: location of the wound margin. Arrowheads: margin of the stratum corneum and a part of the transitional layer that were left behind the wound epidermis. Asterisks: leading end of the wound epidermis. Scale bars, 50 μm (a–h,k); 100 μm (i,j).
Figure 7.
The process of re-epithelialization. A representative data set was obtained from the dorsal skin of the forearms. (a) Wound margin immediately after operation (Stage 1). (b) Wound epidermis which appeared 6 h after operation. Blue in (a,b): DAPI staining of nuclei. (c,d) Neighboring sections showing the wound epidermis at Stage 2 (12 h after operation). Masson’s trichrome stain. As shown in (d), the region composed of the stratum corneum and a part of the transitional layer was sometimes detached from the extending wound epidermis during sectioning. (e–h) Dendritic melanophores (magenta arrow) in the extending wound epidermis. (e,f) 6 h after operation. (f) is a magnification of a part of (e). (g,h) Stage 2 (12 h after operation). (h) is a magnification of a part of (g). (i) The extending epidermal tongue (EET) 6 h after operation. A view from above. The EET was brown because its constituent cells contained melanin pigments. Following a delay after the wound epidermis started to extend, differentiation of the stratum corneum began from the circumference of the wound. (j,k) Wound epidermis closing around the center of the wound bed 24 h after operation. (k) is a magnification of a part of (j). This is a sagittal section of the forearm. The wave of differentiation of the stratum corneum caught up with the closure of the wound epidermis; however, in this particular case, the stratum corneum and the EET were not integrated into one continuous epithelium. The central hole surrounded by the wound epidermis was filled with white blood cells containing neutrophils and monocytes (arrowheads in the inset). BL: basal layer; GG: granular gland; PCL: pigment cell layer; SC: stratum corneum; TL: transitional layer. Black and white arrows: location of the wound margin. Arrowheads: margin of the stratum corneum and a part of the transitional layer that were left behind the wound epidermis. Asterisks: leading end of the wound epidermis. Scale bars, 50 μm (a–h,k); 100 μm (i,j).
![Biomedicines 09 01892 g007]()
Figure 8.
PCNA immunoreactivity in intact skin. (a) A representative image showing PCNA immunoreactivity in a transverse section of an intact forelimb. (b) Enlargement of the skin in the area indicated by the bracket in (a). Almost all the cell nuclei along the basal layer (BL) showed immunoreactivity (light brown). Nuclei in the tissue were counterstained purple with hematoxylin. (c) Control. A section next to the one shown in (a) was stained as in (a), but without primary antibody. (d) Enlargement of the skin in the area indicated by the bracket in (c). PCL: pigment cell layer; SC: stratum corneum; TL: transitional layer. Scale bars: 300 μm (a,c); 100 μm (b,d).
Figure 8.
PCNA immunoreactivity in intact skin. (a) A representative image showing PCNA immunoreactivity in a transverse section of an intact forelimb. (b) Enlargement of the skin in the area indicated by the bracket in (a). Almost all the cell nuclei along the basal layer (BL) showed immunoreactivity (light brown). Nuclei in the tissue were counterstained purple with hematoxylin. (c) Control. A section next to the one shown in (a) was stained as in (a), but without primary antibody. (d) Enlargement of the skin in the area indicated by the bracket in (c). PCL: pigment cell layer; SC: stratum corneum; TL: transitional layer. Scale bars: 300 μm (a,c); 100 μm (b,d).
Figure 9.
Cell sources for extension of the wound epidermis. (a) A schematic drawing of a transverse section of a forearm, illustrating the terms of the regions in the skin (WE, Lateral and Ventral). In WE, the wound epidermis/extending epidermal tongue (EET) from both sides (light green) was examined. (b) Changes in the number of M-phase cells in defined regions during re-epithelialization. Forearms at Stage 1 (immediately after operation), 6 h, Stage 2 (12 h), 18 h, and Stage 3 (48–70 h) were examined. For Lateral, the number of cells on both sides of the forearm were summed. Data were collected from four sections per forearm, and the data obtained from three forearms (three newts) were compared between stages (see Methods). In Lateral, the number of M-phase cells, or dividing basal stem cells, significantly increased at 6 h (Shirley-Williams’ multiple comparison test, **: p < 0.025), and then returned to a normal level as re-epithelialization proceeded. The wound epidermis started cell division as soon as re-epithelialization was completed (Stage 3). (c–f) Sample images showing mitotic figures in Lateral at 6 and 18 h. The boxes in (c,e) were enlarged in (d,f), respectively. Green lines: position of the wound margin. Asterisks: leading end of the wound epidermis and the EET. The stratum corneum (SC) in (e) was folded (the white arrow points to its distal end). Green arrowheads: spindle pole. The cells in (d,f) were dividing vertically and horizontally, respectively. In (d), two sets of daughter chromosomes had slid horizontally, probably because this cell got caught in collective cell migration. It must be noted that observation of dividing cells in this region was occasional. (g–i) Sample transmitted and DAPI images showing a mitotic figure in the closed wound epidermis (Stage 3). Seventy hours after operation. The dotted line in (g) indicates the border between the wound epidermis and the wound bed. The box in (h) was enlarged in (i). Scale bars: 100 μm (c,e,g,h); 20 μm (d,f,i).
Figure 9.
Cell sources for extension of the wound epidermis. (a) A schematic drawing of a transverse section of a forearm, illustrating the terms of the regions in the skin (WE, Lateral and Ventral). In WE, the wound epidermis/extending epidermal tongue (EET) from both sides (light green) was examined. (b) Changes in the number of M-phase cells in defined regions during re-epithelialization. Forearms at Stage 1 (immediately after operation), 6 h, Stage 2 (12 h), 18 h, and Stage 3 (48–70 h) were examined. For Lateral, the number of cells on both sides of the forearm were summed. Data were collected from four sections per forearm, and the data obtained from three forearms (three newts) were compared between stages (see Methods). In Lateral, the number of M-phase cells, or dividing basal stem cells, significantly increased at 6 h (Shirley-Williams’ multiple comparison test, **: p < 0.025), and then returned to a normal level as re-epithelialization proceeded. The wound epidermis started cell division as soon as re-epithelialization was completed (Stage 3). (c–f) Sample images showing mitotic figures in Lateral at 6 and 18 h. The boxes in (c,e) were enlarged in (d,f), respectively. Green lines: position of the wound margin. Asterisks: leading end of the wound epidermis and the EET. The stratum corneum (SC) in (e) was folded (the white arrow points to its distal end). Green arrowheads: spindle pole. The cells in (d,f) were dividing vertically and horizontally, respectively. In (d), two sets of daughter chromosomes had slid horizontally, probably because this cell got caught in collective cell migration. It must be noted that observation of dividing cells in this region was occasional. (g–i) Sample transmitted and DAPI images showing a mitotic figure in the closed wound epidermis (Stage 3). Seventy hours after operation. The dotted line in (g) indicates the border between the wound epidermis and the wound bed. The box in (h) was enlarged in (i). Scale bars: 100 μm (c,e,g,h); 20 μm (d,f,i).
![Biomedicines 09 01892 g009]()
Figure 10.
Tracking of the epidermis around the wound margin during skin regeneration. A mosaic pattern of a transgenic adult newt (CAGGs > mCherry (I-SceI)) was used in which a red fluorescent protein, mCherry, was expressed in spots on the back skin. (a) A magnified view of the stratum corneum in the fluorescent spot under a dissecting microscope. The mCherry fluorescence of cobble stone-shaped epithelial cells made them visible. (b–e) Tracking of the fluorescent epidermis. A central part of the fluorescent spot was excised and then the fluorescent epidermis around the wound was monitored. (b) Before surgery (intact). (c) Immediately after surgery. The wound bed fluoresced because muscles intensely expressed mCherry in this animal. (d) 35 days after surgery. (e) 129 days after surgery. Left-hand column: bright light image. Central column: mCherry fluorescence. Right-hand column: Merge. Arrows in the right-hand column indicate secretion glands as landmarks. We waited for the stratum corneum and the upper part of the transitional layer of the fluorescent epidermis to be renewed (the stratum corneum was shed every two to three weeks) allowing the fluorescent area of the basal layer and the lower part of the transitional layer to be tracked, both of which should slide toward the wound bed according to our hypothesis. Consistently, at 35 days after surgery, the fluorescent area had shrunk compared to the landmarks. The fluorescence of the wound epidermis became recognizable as the wound bed was covered by the pigment cell layer, which blocked off fluorescence from the muscles (129 days). At 129 days, the fluorescent area seemed to have expanded slightly, probably because the skin had relaxed. Note that this experiment was conducted on a single newt, so the same experiment needs to be repeated on multiple individuals to corroborate the results. Scale bars: 40 μm (a); 5 mm (b–e).
Figure 10.
Tracking of the epidermis around the wound margin during skin regeneration. A mosaic pattern of a transgenic adult newt (CAGGs > mCherry (I-SceI)) was used in which a red fluorescent protein, mCherry, was expressed in spots on the back skin. (a) A magnified view of the stratum corneum in the fluorescent spot under a dissecting microscope. The mCherry fluorescence of cobble stone-shaped epithelial cells made them visible. (b–e) Tracking of the fluorescent epidermis. A central part of the fluorescent spot was excised and then the fluorescent epidermis around the wound was monitored. (b) Before surgery (intact). (c) Immediately after surgery. The wound bed fluoresced because muscles intensely expressed mCherry in this animal. (d) 35 days after surgery. (e) 129 days after surgery. Left-hand column: bright light image. Central column: mCherry fluorescence. Right-hand column: Merge. Arrows in the right-hand column indicate secretion glands as landmarks. We waited for the stratum corneum and the upper part of the transitional layer of the fluorescent epidermis to be renewed (the stratum corneum was shed every two to three weeks) allowing the fluorescent area of the basal layer and the lower part of the transitional layer to be tracked, both of which should slide toward the wound bed according to our hypothesis. Consistently, at 35 days after surgery, the fluorescent area had shrunk compared to the landmarks. The fluorescence of the wound epidermis became recognizable as the wound bed was covered by the pigment cell layer, which blocked off fluorescence from the muscles (129 days). At 129 days, the fluorescent area seemed to have expanded slightly, probably because the skin had relaxed. Note that this experiment was conducted on a single newt, so the same experiment needs to be repeated on multiple individuals to corroborate the results. Scale bars: 40 μm (a); 5 mm (b–e).
![Biomedicines 09 01892 g010]()
Figure 11.
Wound surface after the excision of full-thickness skin from the dorsal surface of the forearms. (a,b) Sample images showing the wound surface (i.e., the muscle layer) immediately after operation (Stage 1). These images were obtained from different animals. Wright-Giemsa stain. In this staining condition, cell nuclei were stained in dark blue, and muscle fibers were in light blue. On the other hand, connective tissues and blood capillaries were not stained. Therefore, the thickness of connective tissues or kinds of blood cells could be examined under Nomarski optics. In (a), the connective tissue (the epimysium) forming the surface of the muscle layer was mostly removed together with the skin, and therefore the muscle fibers were sometimes exposed to air. In (b), the epimysium remained. Arrowheads point to the nuclei of cells embedded in the epimysium. (c) Sample image of the wound surface at 3 h after operation. View under a dissecting microscope. Slight bleeding occurred along the wound margin but the blood immediately coagulated, leading to hemostasis. A thin membrane-like structure (possibly a fibrin membrane) covered the surface of the wound bed. (d) Representative image of the wound surface at 6 h after operation. In all 48 sections obtained from three forearms (16 sections each) whose wound surface suffered varying degrees of damage, the wound surface at 6 h was, as shown here, always smooth with the epimysium tissue, suggesting that the surface of the muscle layer repaired itself very quickly. In the repaired epimysium, nuclei were sometimes recognizable (arrowheads). (e) A view of the early wound epidermis (6 h after operation) from diagonally above. Cell nuclei were visualized by DAPI fluorescence. The wound epidermis at this stage extended, but was never attached, to the surface of the wound bed (double arrow). Scale bars: 100 μm (a,b,d,e); 2 mm (c).
Figure 11.
Wound surface after the excision of full-thickness skin from the dorsal surface of the forearms. (a,b) Sample images showing the wound surface (i.e., the muscle layer) immediately after operation (Stage 1). These images were obtained from different animals. Wright-Giemsa stain. In this staining condition, cell nuclei were stained in dark blue, and muscle fibers were in light blue. On the other hand, connective tissues and blood capillaries were not stained. Therefore, the thickness of connective tissues or kinds of blood cells could be examined under Nomarski optics. In (a), the connective tissue (the epimysium) forming the surface of the muscle layer was mostly removed together with the skin, and therefore the muscle fibers were sometimes exposed to air. In (b), the epimysium remained. Arrowheads point to the nuclei of cells embedded in the epimysium. (c) Sample image of the wound surface at 3 h after operation. View under a dissecting microscope. Slight bleeding occurred along the wound margin but the blood immediately coagulated, leading to hemostasis. A thin membrane-like structure (possibly a fibrin membrane) covered the surface of the wound bed. (d) Representative image of the wound surface at 6 h after operation. In all 48 sections obtained from three forearms (16 sections each) whose wound surface suffered varying degrees of damage, the wound surface at 6 h was, as shown here, always smooth with the epimysium tissue, suggesting that the surface of the muscle layer repaired itself very quickly. In the repaired epimysium, nuclei were sometimes recognizable (arrowheads). (e) A view of the early wound epidermis (6 h after operation) from diagonally above. Cell nuclei were visualized by DAPI fluorescence. The wound epidermis at this stage extended, but was never attached, to the surface of the wound bed (double arrow). Scale bars: 100 μm (a,b,d,e); 2 mm (c).
![Biomedicines 09 01892 g011]()
Figure 12.
Wound surface on which the wound epidermis extends. (a) Representative image showing the wound surface under an extending epidermal tongue (EET) at 18 h after operation. Asterisk: leading end. SC: stratum corneum. Line: position of the wound margin. The EET lay over either the fibrin-like membrane or the epimysium tissue with a space containing white blood cells. A part of the EET is enlarged in inset (b). Blue: DAPI stain of nuclei. Dotted line in green: the innermost margin of the EET. Dotted line in magenta: fibrin-like membrane. Nuclei of white blood cells were recognized on the fibrin-like membrane. A break of the fibrin membrane near the leading end of the EET, as indicated by an arrow in (a), is enlarged in inset c. Scale bars: 50 μm (a); 20 μm (b,c).
Figure 12.
Wound surface on which the wound epidermis extends. (a) Representative image showing the wound surface under an extending epidermal tongue (EET) at 18 h after operation. Asterisk: leading end. SC: stratum corneum. Line: position of the wound margin. The EET lay over either the fibrin-like membrane or the epimysium tissue with a space containing white blood cells. A part of the EET is enlarged in inset (b). Blue: DAPI stain of nuclei. Dotted line in green: the innermost margin of the EET. Dotted line in magenta: fibrin-like membrane. Nuclei of white blood cells were recognized on the fibrin-like membrane. A break of the fibrin membrane near the leading end of the EET, as indicated by an arrow in (a), is enlarged in inset c. Scale bars: 50 μm (a); 20 μm (b,c).
Figure 13.
Inflammatory reaction in the wound bed. Representative images were obtained from transverse sections of the forearms at Stage 1 (immediately after operation), 6 h, Stage 2 (12 h), 18 h, and Stage 3 (48–70 h). Wright-Giemsa stain. (a–d) Mesenchymal cells in the wound bed at Stage 1 and 18 h. The boxes in (a,c) were enlarged in (b,d), respectively. EET: extending epidermal tongue. Arrows in (a,c): position of the wound margin. Dotted lines between (b,d) show the increase of the space between the wound surface (epimysium) and muscle layer at 18 h, in which mesenchymal cells obviously gathered. (e) A schematic drawing of a section, illustrating the region in which the number of mesenchymal cells was counted. (f) Changes in the number of mesenchymal cells in the defined region during re-epithelialization. Data were collected from four sections per forearm, and the data obtained from three forearms (three newts) were compared between stages (see Methods). EM(B)N: eosinophils, mast cells (or basophils) and neutrophils; RBC: mature erythrocytes. (g) Changes in the number of eosinophils. (h,i) Sample images of eosinophils. The cells characteristically had cytoplasm stained pink or orange, and sometimes had a lobated nucleus. (j) Changes in the number of mast cells (basophils). (k,l) Sample images of mast cells (basophils). The cells characteristically had granules stained in dark purple in their cytoplasm. Note that in this study mast cells and basophils could not be differentiated. (m) Changes in the number of neutrophils. (n,o) Sample images of neutrophils. The cells characteristically had a multi-lobed nucleus. (p) Sample image of neutrophils (arrowheads) gathering in the wound bed at 18 h after operation. In this study, statistical significance of the increase against the value at Stage 1 was analyzed by Shirley-Williams’ multiple comparison test (**: p < 0.025; *: p < 0.05). Scale bars: 200 μm (a,c); 40 μm (b,d); 20 μm (h,i,k,l,n–p).
Figure 13.
Inflammatory reaction in the wound bed. Representative images were obtained from transverse sections of the forearms at Stage 1 (immediately after operation), 6 h, Stage 2 (12 h), 18 h, and Stage 3 (48–70 h). Wright-Giemsa stain. (a–d) Mesenchymal cells in the wound bed at Stage 1 and 18 h. The boxes in (a,c) were enlarged in (b,d), respectively. EET: extending epidermal tongue. Arrows in (a,c): position of the wound margin. Dotted lines between (b,d) show the increase of the space between the wound surface (epimysium) and muscle layer at 18 h, in which mesenchymal cells obviously gathered. (e) A schematic drawing of a section, illustrating the region in which the number of mesenchymal cells was counted. (f) Changes in the number of mesenchymal cells in the defined region during re-epithelialization. Data were collected from four sections per forearm, and the data obtained from three forearms (three newts) were compared between stages (see Methods). EM(B)N: eosinophils, mast cells (or basophils) and neutrophils; RBC: mature erythrocytes. (g) Changes in the number of eosinophils. (h,i) Sample images of eosinophils. The cells characteristically had cytoplasm stained pink or orange, and sometimes had a lobated nucleus. (j) Changes in the number of mast cells (basophils). (k,l) Sample images of mast cells (basophils). The cells characteristically had granules stained in dark purple in their cytoplasm. Note that in this study mast cells and basophils could not be differentiated. (m) Changes in the number of neutrophils. (n,o) Sample images of neutrophils. The cells characteristically had a multi-lobed nucleus. (p) Sample image of neutrophils (arrowheads) gathering in the wound bed at 18 h after operation. In this study, statistical significance of the increase against the value at Stage 1 was analyzed by Shirley-Williams’ multiple comparison test (**: p < 0.025; *: p < 0.05). Scale bars: 200 μm (a,c); 40 μm (b,d); 20 μm (h,i,k,l,n–p).
![Biomedicines 09 01892 g013]()
Figure 14.
Changes in the relative expression levels of cytokines during re-epithelialization. Semi-quantitative PCR analysis was carried out with samples of the wound at Stage 1 (immediately after operation), 6 h, Stage 2 (12 h), 18 h, Stage 3 (96 h). The samples contained tissues in a range between the surface (containing the wound epidermis, if present) and a few millimeters depth within the wound area. IL-1β: interleukin-1 beta; IL-6: interleukin 6; iNOS: inducible nitric oxide synthase; TGFβ: transforming growth factor beta; IL-10: interleukin 10; Arg1: arginase 1; EF1α: elongation factor 1 alpha. The statistical significance among stages (
n = 3 each) was analyzed by Tukey’s HSD multiple comparison test, and
p-values less than 0.05 are illustrated. Sample images of electrophoresis of PCR products are shown in
Supplementary Figure S2.
Figure 14.
Changes in the relative expression levels of cytokines during re-epithelialization. Semi-quantitative PCR analysis was carried out with samples of the wound at Stage 1 (immediately after operation), 6 h, Stage 2 (12 h), 18 h, Stage 3 (96 h). The samples contained tissues in a range between the surface (containing the wound epidermis, if present) and a few millimeters depth within the wound area. IL-1β: interleukin-1 beta; IL-6: interleukin 6; iNOS: inducible nitric oxide synthase; TGFβ: transforming growth factor beta; IL-10: interleukin 10; Arg1: arginase 1; EF1α: elongation factor 1 alpha. The statistical significance among stages (
n = 3 each) was analyzed by Tukey’s HSD multiple comparison test, and
p-values less than 0.05 are illustrated. Sample images of electrophoresis of PCR products are shown in
Supplementary Figure S2.
Figure 15.
A representative image showing extension of blood capillaries from the wound margin. This image was taken at 28 days after full-thickness skin was excised from the patterned area on abdominal skin (see
Supplementary Video S2). Blood capillaries for cutaneous respiration, which surrounded exocrine glands, extended from the wound margin, accompanying the migration of melanophores. Interestingly, capillaries were located under the region where single melanophores covered them. Scale bars: 500 μm.
Figure 15.
A representative image showing extension of blood capillaries from the wound margin. This image was taken at 28 days after full-thickness skin was excised from the patterned area on abdominal skin (see
Supplementary Video S2). Blood capillaries for cutaneous respiration, which surrounded exocrine glands, extended from the wound margin, accompanying the migration of melanophores. Interestingly, capillaries were located under the region where single melanophores covered them. Scale bars: 500 μm.
Figure 16.
Restoration of skin texture and appendages. (
a–
d) Skin texture of normal skin. The surface of
C. pyrrhogaster skin had structures such as the crista cutis and sulcus cutis. (
a) Head. N: nasal side. (
b) Dorsal skin of the trunk. One crista cutis surrounded by sulcus cutis (dotted line) is shown. An opening of the exocrine gland can be recognized on the top of the crista cutis. (
c) Forelimb. (
d) Ventral skin of the trunk. (
e–
g) A representative data set showing restoration of grooves on the dorsal skin of the trunk (
n = 9). The dorsal skin near the border with the ventral skin (i.e., the dorsal-lateral skin) was excised. V: ventral side. (
f,
g) Enlargement of images before operation (Pre-op) and at 180 days after operation (Day 180). Thick and thin grooves were traced as blue and white lines, respectively. Note that the color pattern (orange spots) on lateral skin did not recover. (
h) Representative data showing the appearance of the sulcus cutis on the wound (
n = 9).
Figure 1h was reproduced here. DM: dorsal midline. (
i) Representative data showing recovery of skin texture (
n = 2). We operated on adult newts as in (
h) and selected those that exhibited incomplete recovery of skin texture at 180 days. Their skin wound was traced for as long as two years. In this animal, the wounded area near the DM blended into its surroundings by 720 days. Thick grooves had become restored across the wounded area. (
j) A representative histological section of the wound in which the sulcus cutis had just appeared. Masson’s trichrome stain. At this stage, the layer of collagen-rich extracellular matrix (or reconstructing dermal layer) along the wound bed was still thinner than the dermis relative to the surroundings. Immature exocrine glands (arrows) were recognized under the presumptive crista cutis, and the epidermal region was flanked by thinner regions corresponding to the sulcus cutis. The characteristics of this tissue corresponded to Stage 6. Scale bars: 1 mm (
a–
i); 100 μm (upper panel in (
j)); 400 μm (lower panel in (
j)).
Figure 16.
Restoration of skin texture and appendages. (
a–
d) Skin texture of normal skin. The surface of
C. pyrrhogaster skin had structures such as the crista cutis and sulcus cutis. (
a) Head. N: nasal side. (
b) Dorsal skin of the trunk. One crista cutis surrounded by sulcus cutis (dotted line) is shown. An opening of the exocrine gland can be recognized on the top of the crista cutis. (
c) Forelimb. (
d) Ventral skin of the trunk. (
e–
g) A representative data set showing restoration of grooves on the dorsal skin of the trunk (
n = 9). The dorsal skin near the border with the ventral skin (i.e., the dorsal-lateral skin) was excised. V: ventral side. (
f,
g) Enlargement of images before operation (Pre-op) and at 180 days after operation (Day 180). Thick and thin grooves were traced as blue and white lines, respectively. Note that the color pattern (orange spots) on lateral skin did not recover. (
h) Representative data showing the appearance of the sulcus cutis on the wound (
n = 9).
Figure 1h was reproduced here. DM: dorsal midline. (
i) Representative data showing recovery of skin texture (
n = 2). We operated on adult newts as in (
h) and selected those that exhibited incomplete recovery of skin texture at 180 days. Their skin wound was traced for as long as two years. In this animal, the wounded area near the DM blended into its surroundings by 720 days. Thick grooves had become restored across the wounded area. (
j) A representative histological section of the wound in which the sulcus cutis had just appeared. Masson’s trichrome stain. At this stage, the layer of collagen-rich extracellular matrix (or reconstructing dermal layer) along the wound bed was still thinner than the dermis relative to the surroundings. Immature exocrine glands (arrows) were recognized under the presumptive crista cutis, and the epidermal region was flanked by thinner regions corresponding to the sulcus cutis. The characteristics of this tissue corresponded to Stage 6. Scale bars: 1 mm (
a–
i); 100 μm (upper panel in (
j)); 400 μm (lower panel in (
j)).
![Biomedicines 09 01892 g016]()
Figure 17.
Restoration of skin color. (
a) Sample image of dorsal-lateral skin (patterned area) of the trunk. This area contained a line of small orange spots (asterisks). The skin structure was almost restored by 180 days after operation (Day 180), whereas the orange spots on the excised skin were never restored; instead, the black area expanded into the wound region. V: ventral side. (
b–
d) Sample images of ventral skin (patterned area) of the trunk. A part of the ventral skin was excised across the black area (Day 0). Melanophores started collective migration from the wound margin (Day 20). This phase corresponds to Stage 5 in which collagen-rich extracellular matrix (reconstructing dermis) has filled the space between the wound epidermis and the wound bed. Collective migration of xanthophores started on Day 40. Melanophores detached from each other by this stage. Skin structure was almost restored by Day 180 whereas melanophores occupied most of the area of the wound region, resulting in an alteration of color pattern. (
e) Sample image of ventral skin (plain orange area) of the trunk. A part of the orange area was excised without wounding the black area. Even though the structure of the skin had almost recovered by Day 180, the wound had not blended into its surroundings in terms of color tone (white arrow). (
f,
g) Sample image showing the collective migration of xanthophores at Day 10. Black arrows: a top of the extending orange area. (
h,
i) Sample images showing the recovery of the orange color tone in ventral skin. The color tone was monitored for longer than 2 years. The data in (
h) was obtained from the animal shown in
Figure 1i,j. In these two cases, the orange area created by Day 180 still had a pale tone (white arrow), but by Day 720 it became sufficiently dark to blend into its surroundings. Scale bars: 1 mm.
Figure 17.
Restoration of skin color. (
a) Sample image of dorsal-lateral skin (patterned area) of the trunk. This area contained a line of small orange spots (asterisks). The skin structure was almost restored by 180 days after operation (Day 180), whereas the orange spots on the excised skin were never restored; instead, the black area expanded into the wound region. V: ventral side. (
b–
d) Sample images of ventral skin (patterned area) of the trunk. A part of the ventral skin was excised across the black area (Day 0). Melanophores started collective migration from the wound margin (Day 20). This phase corresponds to Stage 5 in which collagen-rich extracellular matrix (reconstructing dermis) has filled the space between the wound epidermis and the wound bed. Collective migration of xanthophores started on Day 40. Melanophores detached from each other by this stage. Skin structure was almost restored by Day 180 whereas melanophores occupied most of the area of the wound region, resulting in an alteration of color pattern. (
e) Sample image of ventral skin (plain orange area) of the trunk. A part of the orange area was excised without wounding the black area. Even though the structure of the skin had almost recovered by Day 180, the wound had not blended into its surroundings in terms of color tone (white arrow). (
f,
g) Sample image showing the collective migration of xanthophores at Day 10. Black arrows: a top of the extending orange area. (
h,
i) Sample images showing the recovery of the orange color tone in ventral skin. The color tone was monitored for longer than 2 years. The data in (
h) was obtained from the animal shown in
Figure 1i,j. In these two cases, the orange area created by Day 180 still had a pale tone (white arrow), but by Day 720 it became sufficiently dark to blend into its surroundings. Scale bars: 1 mm.
![Biomedicines 09 01892 g017]()