ROR1 is Expressed in Diffuse Large B-Cell Lymphoma (DLBCL) and a Small Molecule Inhibitor of ROR1 (KAN0441571C) Induced Apoptosis of Lymphoma Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Tissue Microarray (TMA) and Immunohistochemical (IHC) Assays
2.3. Cell Lines
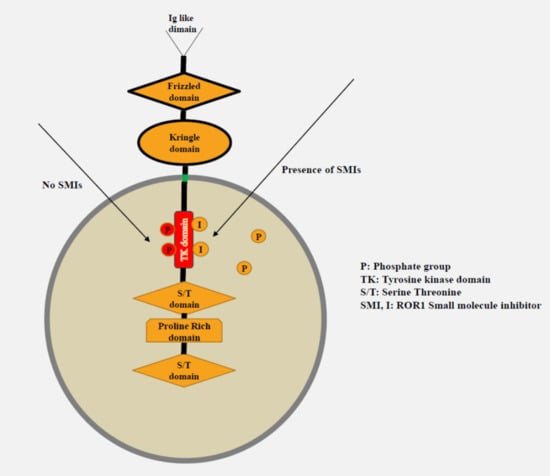
2.4. Small Molecule ROR1 Tyrosine Kinase Inhibitors (KAN0439834 and KAN0441571C)
2.5. Cell Surface Markers (Flow Cytometry)
2.6. SDS-PAGE and Western Blot
2.7. MTT Cytotoxicity Assay
2.8. Apoptosis Assay (Flow Cytometry)
2.9. Apoptosis of DLBCL Cells Co-Cultured with Stromal Cells
2.10. Proximity Ligation Assay (PLA)
2.11. Zebrafish Transplantable Tumor Growth Model
2.12. Statistical Analysis
3. Results
3.1. Expression of ROR1 in Relation to Clinical Subgroups and Prognosis
3.2. Cytotoxicity of KAN0441571C in DLBCL Cell Lines
3.3. Effects on Signaling in DLBCL Cells
3.4. Effects of KAN0441571C in Zebra Fish Transplanted with DLBCL Cells
3.5. Effects of KAN0441571C in Combination with Venetoclax or Ibrutinib in ROR1+ DLBCL Cell Lines
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hojjat-Farsangi, M. Small-Molecule Inhibitors of the Receptor Tyrosine Kinases: Promising Tools for Targeted Cancer Therapies. Int. J. Mol. Sci. 2014, 15, 13768–13801. [Google Scholar] [CrossRef] [Green Version]
- Stricker, S.; Rauschenberger, V.; Schambony, A. ROR-Family Receptor Tyrosine Kinases; Elsevier BV: Amsterdam, The Netherlands, 2017; Volume 123, pp. 105–142. [Google Scholar]
- Billiard, J.; Way, D.S.; Seestaller-Wehr, L.M.; Moran, R.A.; Bodine, P.V.; Mangine, A. The Orphan Receptor Tyrosine Kinase Ror2 Modulates Canonical Wnt Signaling in Osteoblastic Cells. Mol. Endocrinol. 2005, 19, 90–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoda, A.; Oishi, I.; Minami, Y. Expression and Function of the Ror? Family Receptor Tyrosine Kinases During Development: Lessons from Genetic Analyses of Nematodes, Mice, and Humans. J. Recept. Signal Transduct. 2003, 23, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, A.; Goodpaster, T.; Randolph-Habecker, J.; Hoffstrom, B.G.; Jalikis, F.G.; Koch, L.K.; Berger, C.; Kosasih, P.L.; Rajan, A.; Sommermeyer, D.; et al. Analysis of ROR1 protein expression in human cancer and normal tissues. Clin. Cancer Res. 2016, 23, 3061–3071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daneshmanesh, A.H.; Mikaelsson, E.; Jeddi-Tehrani, M.; Bayat, A.A.; Ghods, R.; Ostadkarampour, M.; Akhondi, M.; Lagercrantz, S.; Larsson, C.; Österborg, A.; et al. Ror1, a cell surface receptor tyrosine kinase is expressed in chronic lymphocytic leukemia and may serve as a putative target for therapy. Int. J. Cancer 2008, 123, 1190–1195. [Google Scholar] [CrossRef]
- Fukuda, T.; Chen, L.; Endo, T.; Tang, L.; Lu, D.; Castro, J.E.; Widhopf, G.F.; Rassenti, L.Z.; Cantwell, M.J.; Prussak, C.E.; et al. Antisera induced by infusions of autologous Ad-CD154-leukemia B cells identify ROR1 as an oncofetal antigen and receptor for Wnt5a. Proc. Natl. Acad. Sci. USA 2008, 105, 3047–3052. [Google Scholar] [CrossRef] [Green Version]
- Rabbani, H.; Ostadkarampour, M.; Manesh, A.H.D.; Basiri, A.; Jeddi-Tehrani, M.; Forouzesh, F. Expression of ROR1 in Patients with Renal Cancer-A Potential Diagnostic Marker. Iran. Biomed. J. 2010, 14, 77–82. [Google Scholar]
- Shabani, M.; Omran, H.A.; Jeddi-Tehrani, M.; Vossough, P.; Faranoush, M.; Sharifian, R.A.; Toughe, G.R.; Kordmahin, M.; Khoshnoodi, J.; Roohi, A.; et al. Overexpression of Orphan Receptor Tyrosine Kinase Ror1 as a Putative Tumor-Associated Antigen in Iranian Patients with Acute Lymphoblastic Leukemia. Tumor Boil. 2007, 28, 318–326. [Google Scholar] [CrossRef]
- Hojjat-Farsangi, M.; Moshfegh, A.; Daneshmanesh, A.H.; Khan, S.; Mikaelsson, E.; Österborg, A.; Mellstedt, H. The receptor tyrosine kinase ROR1 – An oncofetal antigen for targeted cancer therapy. Semin. Cancer Boil. 2014, 29, 21–31. [Google Scholar] [CrossRef]
- Bicocca, V.; Chang, B.H.; Masouleh, B.K.; Müschen, M.; Loriaux, M.M.; Druker, B.J.; Tyner, J.W. Crosstalk between ROR1 and the Pre-B cell receptor promotes survival of t(1;19) acute lymphoblastic leukemia. Cancer Cell 2012, 22, 656–667. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, T.; Yanagisawa, K.; Sugiyama, R.; Hosono, Y.; Shimada, Y.; Arima, C.; Kato, S.; Tomida, S.; Suzuki, M.; Osada, H.; et al. NKX2-1/TITF1/TTF-1-Induced ROR1 is required to sustain EGFR survival signaling in lung adenocarcinoma. Cancer Cell 2012, 21, 348–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.; Chen, L.; Cui, B.; Widhopf, G.F., 2nd; Shen, Z.; Wu, R.; Zhang, L.; Zhang, S.; Briggs, S.P.; Kipps, T.J. Wnt5a induces ROR1/ROR2 heterooligomerization to enhance leukemia chemotaxis and proliferation. J. Clin. Invest. 2016, 126, 585–598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, B.; Zhang, S.; Chen, L.; Yu, J.; Widhopf, G.F., 2nd; Fecteau, J.F.; Rassenti, L.Z.; Kipps, T.J. Targeting ROR1 inhibits epithelial-mesenchymal transition and metastasis. Cancer Res. 2013, 73, 3649–3660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, M.Y.; Widhopf, G.F.; Ghia, E.M.; Kidwell, R.L.; Hasan, K.; Yu, J.; Rassenti, L.Z.; Chen, L.; Chen, Y.; Pittman, E.; et al. Phase I Trial: Cirmtuzumab Inhibits ROR1 Signaling and Stemness Signatures in Patients with Chronic Lymphocytic Leukemia. Cell Stem Cell 2018, 22, 951–959.e3. [Google Scholar] [CrossRef] [Green Version]
- Jung, E.; Lee, H.; Han, G.; Kim, M. Targeting ROR1 inhibits the self-renewal and invasive ability of glioblastoma stem cells. Cell Biochem. Funct. 2016, 34, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Cui, B.; Lai, S.; Liu, G.; Ghia, E.M.; Widhopf, G.F.; Zhang, Z.; Wu, C.C.N.; Chen, L.; Wu, R.; et al. Ovarian cancer stem cells express ROR1, which can be targeted for anti–cancer-stem-cell therapy. Proc. Natl. Acad. Sci. USA 2014, 111, 17266–17271. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Chen, L.; Cui, B.; Chuang, H.-Y.; Yu, J.; Wang-Rodriguez, J.; Tang, L.; Chen, G.; Basak, G.W.; Kipps, T.J. ROR1 Is Expressed in Human Breast Cancer and Associated with Enhanced Tumor-Cell Growth. PLoS ONE 2012, 7, e31127. [Google Scholar] [CrossRef] [Green Version]
- Hojjat-Farsangi, M.; Khan, S.; Daneshmanesh, A.H.; Moshfegh, A.; Sandin, Å.; Mansouri, L.; Palma, M.; Lundin, J.; Österborg, A.; Mellstedt, H. The Tyrosine Kinase Receptor ROR1 Is Constitutively Phosphorylated in Chronic Lymphocytic Leukemia (CLL) Cells. PLoS ONE 2013, 8, e78339. [Google Scholar] [CrossRef] [Green Version]
- Mikels, A.J.; Nusse, R. Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Boil. 2006, 4, e115. [Google Scholar] [CrossRef]
- Nomachi, A.; Nishita, M.; Inaba, D.; Enomoto, M.; Hamasaki, M.; Minami, Y. Receptor Tyrosine Kinase Ror2 Mediates Wnt5a-induced Polarized Cell Migration by Activating c-Jun N-terminal Kinase via Actin-binding Protein Filamin A. J. Boil. Chem. 2008, 283, 27973–27981. [Google Scholar] [CrossRef] [Green Version]
- Zhuo, W.; Kang, Y. Lnc-ing ROR1–HER3 and Hippo signalling in metastasis. Nature 2017, 19, 81–83. [Google Scholar] [CrossRef] [PubMed]
- Daneshmanesh, A.H.; Porwit, A.; Hojjat-Farsangi, M.; Jeddi-Tehrani, M.; Tamm, K.P.; Grander, D.; Lehmann, S.; Norin, S.; Shokri, F.; Rabbani, H.; et al. Orphan receptor tyrosine kinases ROR1 and ROR2 in hematological malignancies. Leuk. Lymphoma 2012, 54, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, A.; Derkow, K.; Daneshmanesh, A.H.; Mikaelsson, E.; Kiaii, S.; Kokhaei, P.; Österborg, A.; Mellstedt, H. Silencing of ROR1 and FMOD with siRNA results in apoptosis of CLL cells. Br. J. Haematol. 2010, 151, 327–335. [Google Scholar] [CrossRef]
- Mao, Y.; Xu, L.; Wang, J.; Zhang, L.; Hou, N.; Xu, J.; Wang, L.; Yang, S.; Chen, Y.; Xiong, L.; et al. ROR1 associates unfavorable prognosis and promotes lymphoma growth in DLBCL by affecting PI3K/Akt/mTOR signaling pathway. Bio. Factors 2019. [Google Scholar] [CrossRef]
- Cui, B.; Ghia, E.M.; Chen, L.; Rassenti, L.Z.; DeBoever, C.; Widhopf, G.F.; Yu, J.; Neuberg, D.S.; Wierda, W.G.; Rai, K.; et al. High-level ROR1 associates with accelerated disease progression in chronic lymphocytic leukemia. Blood 2016, 128, 2931–2940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scielzo, C.; Ghia, P.; Conti, A.; Bachi, A.; Guida, G.; Geuna, M.; Alessio, M.; Caligaris-Cappio, F. HS1 protein is differentially expressed in chronic lymphocytic leukemia patient subsets with good or poor prognoses. J. Clin. Investig. 2005, 115, 1644–1650. [Google Scholar] [CrossRef]
- Hacken, E.T.; Scielzo, C.; Bertilaccio, M.T.S.; Scarfò, L.; Apollonio, B.; Barbaglio, F.; Stamatopoulos, K.; Ponzoni, M.; Ghia, P.; Caligaris-Cappio, F. Targeting the LYN/HS1 signaling axis in chronic lymphocytic leukemia. Blood 2013, 121, 2264–2273. [Google Scholar] [CrossRef] [Green Version]
- Hojjat-Farsangi, M.; Daneshmanesh, A.H.; Khan, A.S.; Shetye, J.; Mozaffari, F.; Kharaziha, P.; Rathje, L.-S.; Kokhaei, P.; Hansson, L.; Vagberg, J.; et al. First-in-class oral small molecule inhibitor of the tyrosine kinase ROR1 (KAN0439834) induced significant apoptosis of chronic lymphocytic leukemia cells. Leuk. 2018, 32, 2291–2295. [Google Scholar] [CrossRef] [Green Version]
- Cabanillas, F.; Shah, B. Advances in Diagnosis and Management of Diffuse Large B-cell Lymphoma. Clin. Lymphoma Myeloma Leuk. 2017, 17, 783–796. [Google Scholar] [CrossRef]
- Jiang, M.; Bennani, N.N.; Feldman, A.L.; Bennani-Baiti, N. Lymphoma classification update: B-cell non-Hodgkin lymphomas. Expert Rev. Hematol. 2017, 10, 405–415. [Google Scholar] [CrossRef]
- Akyurek, N.; Drakos, E.; Giaslakiotis, K.; Knoblock, R.J.; Abruzzo, L.V.; Ning, Y.; Rassidakis, G.Z.; Medeiros, L.J. Differential expression of CKS-1B in typical and blastoid variants of mantle cell lymphoma. Hum. Pathol. 2010, 41, 1448–1455. [Google Scholar] [CrossRef] [PubMed]
- Janovska, P.; Poppova, L.; Plevova, K.; Plesingerova, H.; Behal, M.; Kaucka, M.; Ovesna, P.; Hlozkova, M.; Borsky, M.; Stehlikova, O.; et al. Autocrine Signaling by Wnt-5a Deregulates Chemotaxis of Leukemic Cells and Predicts Clinical Outcome in Chronic Lymphocytic Leukemia. Clin. Cancer Res. 2015, 22, 459–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdou, A.G.; Asaad, N.; Kandil, M.; Shabaan, M.; Shams, A. Significance of stromal-1 and stromal-2 signatures and biologic prognostic model in diffuse large B-cell lymphoma. Cancer Boil. Med. 2017, 14, 151–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velentza, L.; Zerdes, I.; Daneshmanesh, A.H.; Tsesmetzis, N.; Hojjat-Frarsangi, M.; Ghaderi, A.; Drakos, E.; Palma, M.; Österborg, A. Targeting ROR-1 receptor in classical Hodgkin lymphoma: Effects on apoptosis and proliferation and its emerging therapeutic implications. Abstract PF422. HemaSphere 2018, 2, 164–165. [Google Scholar]
- Liu, Y.; Barta, S. Diffuse large B-cell lymphoma: 2019 update on diagnosis, risk stratification, and treatment. Am. J. Hematol. 2019, 94, 604–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy, A.; Zhang, J.; Davis, N.S.; Moffitt, R.A.; Love, C.L.; Waldrop, A.; Leppä, S.; Pasanen, A.; Meriranta, L.; Karjalainen-Lindsberg, M.-L.; et al. Genetic and Functional Drivers of Diffuse Large B Cell Lymphoma. Cell 2017, 171, 481–494.e15. [Google Scholar] [CrossRef] [Green Version]
- Saleh, R.R.; Fuentes-Antrás, J.; Peinado, P.; Pérez-Segura, P.; Pandiella, A.; Amir, E.; Ocaña, A. Prognostic value of receptor tyrosine kinase-like orphan receptor (ROR) family in cancer: A meta-analysis. Cancer Treat. Rev. 2019, 77, 11–19. [Google Scholar] [CrossRef]
- Wallstabe, L.; Göttlich, C.; Nelke, L.C.; Kühnemundt, J.; Schwarz, T.; Nerreter, T.; Einsele, H.; Walles, H.; Dandekar, G.; Nietzer, S.L.; et al. ROR1-CAR T cells are effective against lung and breast cancer in advanced microphysiologic 3D tumor models. JCI. Insight 2019, 4, 4. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Park, H.; Greene, J.; Pao, J.; Mulvey, E.; Zhou, S.X.; Albert, C.M.; Moy, F.; Sachdev, D.; Yee, D.; et al. IGF1R- and ROR1-Specific CAR T Cells as a Potential Therapy for High Risk Sarcomas. PLOS ONE 2015, 10, e0133152. [Google Scholar] [CrossRef]
- Gohil, S.H.; Paredes-Moscosso, S.; Harrasser, M.; Vezzalini, M.; Scarpa, A.; Morris, E.; Davidoff, A.M.; Sorio, C.; Nathwani, A.C.; Della Peruta, M. An ROR1 bi-specific T-cell engager provides effective targeting and cytotoxicity against a range of solid tumors. Oncoimmunology 2017, 6, e1326437. [Google Scholar] [CrossRef]
- Daneshmanesh, A.H.; Hojjat-Farsangi, M.; Ghaderi, A.; Moshfegh, A.; Hansson, L.; Schultz, J.; Vågberg, J.; Byström, S.; Olsson, E.; Olin, T.; et al. A receptor tyrosine kinase ROR1 inhibitor (KAN0439834) induced significant apoptosis of pancreatic cells which was enhanced by erlotinib and ibrutinib. PLoS ONE 2018, 13, e0198038. [Google Scholar] [CrossRef] [PubMed]
- Fultang, N.; Illendula, A.; Chen, B.; Wu, C.; Jonnalagadda, S.; Baird, N.; Klase, Z.; Peethambaran, B. Strictinin, a novel ROR1-inhibitor, represses triple negative breast cancer survival and migration via modulation of PI3K/AKT/GSK3ß activity. PLoS ONE 2019, 14, e0217789. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Pu, W.; He, H.; Fan, X.; Zheng, Y.; Zhou, J.-K.; Ma, R.; He, J.; Zheng, Y.; Wu, K.; et al. Novel ROR1 inhibitor ARI-1 suppresses the development of non-small cell lung cancer. Cancer Lett. 2019, 458, 76–85. [Google Scholar] [CrossRef] [PubMed]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghaderi, A.; Daneshmanesh, A.H.; Moshfegh, A.; Kokhaei, P.; Vågberg, J.; Schultz, J.; Olin, T.; Harrysson, S.; Smedby, K.E.; Drakos, E.; et al. ROR1 is Expressed in Diffuse Large B-Cell Lymphoma (DLBCL) and a Small Molecule Inhibitor of ROR1 (KAN0441571C) Induced Apoptosis of Lymphoma Cells. Biomedicines 2020, 8, 170. https://doi.org/10.3390/biomedicines8060170
Ghaderi A, Daneshmanesh AH, Moshfegh A, Kokhaei P, Vågberg J, Schultz J, Olin T, Harrysson S, Smedby KE, Drakos E, et al. ROR1 is Expressed in Diffuse Large B-Cell Lymphoma (DLBCL) and a Small Molecule Inhibitor of ROR1 (KAN0441571C) Induced Apoptosis of Lymphoma Cells. Biomedicines. 2020; 8(6):170. https://doi.org/10.3390/biomedicines8060170
Chicago/Turabian StyleGhaderi, Amineh, Amir Hossein Daneshmanesh, Ali Moshfegh, Parviz Kokhaei, Jan Vågberg, Johan Schultz, Thomas Olin, Sara Harrysson, Karin E Smedby, Elias Drakos, and et al. 2020. "ROR1 is Expressed in Diffuse Large B-Cell Lymphoma (DLBCL) and a Small Molecule Inhibitor of ROR1 (KAN0441571C) Induced Apoptosis of Lymphoma Cells" Biomedicines 8, no. 6: 170. https://doi.org/10.3390/biomedicines8060170