Human Adipose-Derived Stem Cells Reduce Cellular Damage after Experimental Spinal Cord Injury in Rats
Abstract
:1. Introduction
2. Materials and Methods
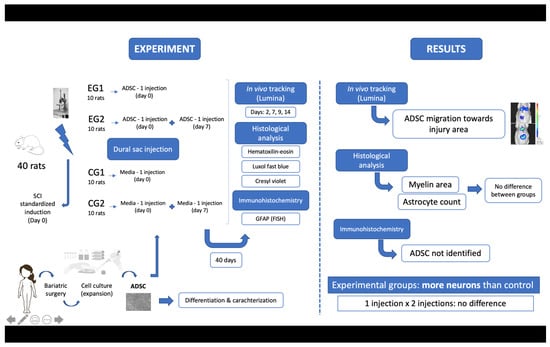
2.1. Study Design
2.2. Adipose-Derived Stem Cells Isolation and Expansion
2.3. Adipose-Derived Stem Cells Characterization
2.4. Adipose-Derived Stem Cells Transduction
2.5. Surgical Procedure
2.6. Cell Transplantation
2.7. In Vivo Cell Tracking
2.8. Euthanasia and Tissue Collection
2.9. Anatomopathological Study—Histology and Immunohistochemistry
2.10. Statistical Analysis
3. Results
3.1. Animal Care and Surgical Procedures
3.2. ADSC Characterization
3.3. Histomophometric Analysis of Myelin and Neurons
3.4. Immunohistochemical Astrocyte Evaluation
3.5. In Vivo Cell Tracking with Bioluminescence Imaging
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cripps, R.A.; Lee, B.B.; Wing, P.; Weerts, E.; Mackay, J.; Brown, D. A global map for traumatic spinal cord injury epidemiology: Towards a living data repository for injury prevention. Spinal Cord 2011, 49, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Noonan, V.K.; Fingas, M.; Farry, A.; Baxter, D.; Singh, A.; Fehlings, M.G.; Dvorak, M.F. Incidence and prevalence of spinal cord injury in Canada: A national perspective. Neuroepidemiology 2012, 38, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Choo, A.M.; Liu, J.; Lam, C.K.; Dvorak, M.; Tetzlaff, W.; Oxland, T.R. Contusion, dislocation, and distraction: Primary hemorrhage and membrane permeability in distinct mechanisms of spinal cord injury. J. Neurosurg. Spine 2007, 6, 255–266. [Google Scholar] [CrossRef] [PubMed]
- LaPlaca, M.C.; Simon, C.M.; Prado, G.R.; Cullen, D.K. CNS injury biomechanics and experimental models. Prog. Brain Res. 2007, 161, 13–26. [Google Scholar] [CrossRef]
- Fehlings, M.G.; Perrin, R.G. The role and timing of early decompression for cervical spinal cord injury: Update with a review of recent clinical evidence. Injury 2005, 36 (Suppl. S2), B13–B26. [Google Scholar] [CrossRef]
- Hausmann, O.N. Post-traumatic inflammation following spinal cord injury. Spinal Cord 2003, 41, 369–378. [Google Scholar] [CrossRef]
- Kwon, B.K.; Tetzlaff, W.; Grauer, J.N.; Beiner, J.; Vaccaro, A.R. Pathophysiology and pharmacologic treatment of acute spinal cord injury. Spine J. 2004, 4, 451–464. [Google Scholar] [CrossRef]
- Ahuja, C.S.; Martin, A.R.; Fehlings, M. Recent advances in managing a spinal cord injury secondary to trauma. F1000Research 2016, 5, 1017. [Google Scholar] [CrossRef]
- Chen, D.; Zeng, W.; Fu, Y.; Gao, M.; Lv, G. Bone marrow mesenchymal stem cells combined with minocycline improve spinal cord injury in a rat model. Int. J. Clin. Exp. Pathol. 2015, 8, 11957–11969. [Google Scholar]
- Carvalho, K.A.; Cunha, R.C.; Vialle, E.N.; Osiecki, R.; Moreira, G.H.; Simeoni, R.B.; Francisco, J.C.; Guarita-Souza, L.C.; Oliveira, L.; Zocche, L.; et al. Functional outcome of bone marrow stem cells (CD45(+)/CD34(-)) after cell therapy in acute spinal cord injury: In exercise training and in sedentary rats. Transplant. Proc. 2008, 40, 847–849. [Google Scholar] [CrossRef]
- Fonseca, A.F.B.; Scheffer, J.P.; Giraldi-Guimarães, A.; Coelho, B.P.; Medina, R.M.; Oliveira, A.L.A. Comparison among bone marrow mesenchymal stem and mononuclear cells to promote functional recovery after spinal cord injury in rabbits. Acta Cir. Bras. 2017, 32, 1026–1035. [Google Scholar] [CrossRef]
- Lv, C.; Zhang, T.; Li, K.; Gao, K. Bone marrow mesenchymal stem cells improve spinal function of spinal cord injury in rats via TGF-β/Smads signaling pathway. Exp. Ther. Med. 2020, 19, 3657–3663. [Google Scholar] [CrossRef]
- Caplan, A.I. Why are MSCs therapeutic? New data: New insight. J. Pathol. 2009, 217, 318–324. [Google Scholar] [CrossRef]
- Fang, B.; Song, Y.; Zhao, R.C.; Han, Q.; Cao, Y. Treatment of resistant pure red cell aplasia after major ABO-incompatible bone marrow transplantation with human adipose tissue-derived mesenchymal stem cells. Am. J. Hematol. 2007, 82, 772–773. [Google Scholar] [CrossRef]
- Fraser, J.K.; Wulur, I.; Alfonso, Z.; Hedrick, M.H. Fat tissue: An underappreciated source of stem cells for biotechnology. Trends Biotechnol. 2006, 24, 150–154. [Google Scholar] [CrossRef]
- Vialle, E.; Vialle, L.R.G.; Rasera, E.; Cechinel, C.; Leonel, I.; Seyboth, C. Avaliação da recuperação motora em ratos submetidos a lesão medular experimental. Rev. Bras. Ortop. 2002, 37, 83–91. [Google Scholar]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Rebelatto, C.K.; Aguiar, A.M.; Moretão, M.P.; Senegaglia, A.C.; Hansen, P.; Barchiki, F.; Oliveira, J.; Martins, J.; Kuligovski, C.; Mansur, F.; et al. Dissimilar differentiation of mesenchymal stem cells from bone marrow, umbilical cord blood, and adipose tissue. Exp. Biol. Med. 2008, 233, 901–913. [Google Scholar] [CrossRef]
- Vialle, L.R.G.; Fischer, S.; Marcon, J.C.; Vialle, E.; Luzzi, R.; Bleggi-Torres, L.F. Estudo histológico da lesão medular experimental em ratos. Rev. Bras. Ortop. 1999, 34, 85–89. [Google Scholar]
- Meyer, F.; Vialle, L.R.; Vialle, E.N.; Bleggi-Torres, L.F.; Rasera, E.; Leonel, I. Alterações vesicais na lesão medular experimental em ratos. Acta Cirúrgica Bras. 2003, 18, 203–208. [Google Scholar] [CrossRef]
- Vialle, E.N.; Vialle, L.R.; Arruda Ade, O. Histomorphometric analysis of experimental disc degeneration. Global Spine J. 2012, 2, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Talac, R.; Friedman, J.A.; Moore, M.J.; Lu, L.; Jabbari, E.; Windebank, A.J.; Currier, B.L.; Yaszemski, M.J. Animal models of spinal cord injury for evaluation of tissue engineering treatment strategies. Biomaterials 2004, 25, 1505–1510. [Google Scholar] [CrossRef] [PubMed]
- Sharif-Alhoseini, M.; Khormali, M.; Rezaei, M.; Safdarian, M.; Hajighadery, A.; Khalatbari, M.M.; Meknatkhah, S.; Rezvan, M.; Chalangari, M.; Derakhshan, P.; et al. Animal models of spinal cord injury: A systematic review. Spinal Cord 2017, 55, 714–721. [Google Scholar] [CrossRef] [PubMed]
- Villanova Junior, J.A.; Fracaro, L.; Rebelatto, C.L.K.; da Silva, A.J.; Barchiki, F.; Senegaglia, A.C.; Dominguez, A.C.; de Moura, S.A.B.; Pimpão, C.T.; Brofman, P.R.S.; et al. Recovery of motricity and micturition after transplantation of mesenchymal stem cells in rats subjected to spinal cord injury. Neurosci. Lett. 2020, 734, 135134. [Google Scholar] [CrossRef]
- Chen, J.; Wang, L.; Liu, M.; Gao, G.; Zhao, W.; Fu, Q.; Wang, Y. Implantation of adipose-derived mesenchymal stem cell sheets promotes axonal regeneration and restores bladder function after spinal cord injury. Stem. Cell Res. Ther. 2022, 13, 503. [Google Scholar] [CrossRef]
- Dave, S.D.; Patel, C.N.; Vanikar, A.V.; Trivedi, H.L. In vitro differentiation of neural cells from human adipose tissue derived stromal cells. Neurol. India 2018, 66, 716–721. [Google Scholar] [CrossRef]
- Anjum, A.; Yazid, M.D.; Fauzi Daud, M.; Idris, J.; Ng, A.M.H.; Selvi Naicker, A.; Ismail, O.H.R.; Athi Kumar, R.K.; Lokanathan, Y. Spinal Cord Injury: Pathophysiology, Multimolecular Interactions, and Underlying Recovery Mechanisms. Int. J. Mol. Sci. 2020, 21, 7533. [Google Scholar] [CrossRef]
- Kim, T.J.; Tuerkcan, S.; Ceballos, A.; Pratx, G. Modular platform for low-light microscopy. Biomed Opt. Express. 2015, 6, 4585–4598. [Google Scholar] [CrossRef]
- Zaminy, A.; Shokrgozar, M.A.; Sadeghi, Y.; Norouzian, M.; Heidari, M.H.; Piryaei, A. Transplantation of schwann cells differentiated from adipose stem cells improves functional recovery in rat spinal cord injury. Arch. Iran. Med. 2013, 16, 533–541. [Google Scholar]
- Callera, F.; de Melo, C.M. Magnetic resonance tracking of magnetically labeled autologous bone marrow CD34+ cells transplanted into the spinal cord via lumbar puncture technique in patients with chronic spinal cord injury: CD34+ cells’ migration into the injured site. Stem. Cells Dev. 2007, 16, 461–466. [Google Scholar] [CrossRef]
- Hubertus, V.; Meyer, L.; Roolfs, L.; Waldmann, L.; Nieminen-Kelhä, M.; Fehlings, M.G.; Vajkoczy, P. In vivo imaging in experimental spinal cord injury–Techniques and trends. Brain Spine 2022, 2, 100859. [Google Scholar] [CrossRef]
- Geoffroy, C.G.; Zheng, B. Myelin-associated inhibitors in axonal growth after CNS injury. Curr. Opin. Neurobiol. 2014, 27, 31–38. [Google Scholar] [CrossRef]
- Lee, J.K.; Geoffroy, C.G.; Chan, A.F.; Tolentino, K.E.; Crawford, M.J.; Leal, M.A.; Kang, B.; Zheng, B. Assessing spinal axon regeneration and sprouting in Nogo-, MAG-, and OMgp-deficient mice. Neuron 2010, 66, 663–670. [Google Scholar] [CrossRef]
- Ashammakhi, N.; Kim, H.J.; Ehsanipour, A.; Bierman, R.D.; Kaarela, O.; Xue, C.; Khademhosseini, A.; Seidlits, S.K. Regenerative Therapies for Spinal Cord Injury. Tissue Eng. Part B Rev. 2019, 25, 471–491. [Google Scholar] [CrossRef]
- Ohta, Y.; Hamaguchi, A.; Ootaki, M.; Watanabe, M.; Takeba, Y.; Iiri, T.; Matsumoto, N.; Takenaga, M. Intravenous infusion of adipose-derived stem/stromal cells improves functional recovery of rats with spinal cord injury. Cytotherapy 2017, 19, 839–848. [Google Scholar] [CrossRef]
- Menezes, K.; Nascimento, M.A.; Gonçalves, J.P.; Cruz, A.S.; Lopes, D.V.; Curzio, B.; Bonamino, M.; de Menezes, J.R.; Borojevic, R.; Rossi, M.I.; et al. Human mesenchymal cells from adipose tissue deposit laminin and promote regeneration of injured spinal cord in rats. PLoS ONE 2014, 9, e96020. [Google Scholar] [CrossRef]
- Faulkner, J.R.; Herrmann, J.E.; Woo, M.J.; Tansey, K.E.; Doan, N.B.; Sofroniew, M.V. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J. Neurosci. 2004, 24, 2143–2155. [Google Scholar] [CrossRef]








| Area | Group | N | Average | Median | Min | Max | SD |
|---|---|---|---|---|---|---|---|
| AR | CG1 | 9 | 19,738 | 18,760 | 8690 | 28,785 | 6500 |
| CG2 | 10 | 16,778 | 15,287 | 7870 | 36,177 | 8326 | |
| EG1 | 8 | 21,547 | 21,702 | 15,805 | 26,511 | 3597 | |
| EG2 | 9 | 20,042 | 17,650 | 10,312 | 34,553 | 8369 | |
| AL | CG1 | 9 | 19,882 | 21,492 | 7922 | 27,080 | 6766 |
| CG2 | 10 | 13,586 | 12,688 | 5525 | 25,587 | 6325 | |
| EG1 | 8 | 21,256 | 20,114 | 15,863 | 29,074 | 4339 | |
| EG2 | 9 | 18,629 | 18,453 | 9779 | 37,710 | 9531 | |
| PR | CG1 | 9 | 20,139 | 20,121 | 8302 | 30,488 | 6559 |
| CG2 | 10 | 18,034 | 16,628 | 8737 | 32,206 | 7045 | |
| EG1 | 8 | 24,795 | 24,927 | 15,770 | 29,270 | 4107 | |
| EG2 | 9 | 19,064 | 21,752 | 10,207 | 31,100 | 7589 | |
| PL | CG1 | 9 | 21,411 | 21,664 | 10,541 | 29,412 | 5929 |
| CG2 | 10 | 15,765 | 16,124 | 8161 | 22,956 | 4404 | |
| EG1 | 8 | 23,672 | 23,148 | 16,040 | 31,780 | 5776 | |
| EG2 | 9 | 19,058 | 13,830 | 11,131 | 38,737 | 10,120 |
| p * Value | ||||
|---|---|---|---|---|
| Groups | AR | AL | PR | PL |
| EG1 × EG2 | 0.633 | 0.486 | 0.077 | 0.275 |
| CG1 × CG2 | 0.404 | 0.051 | 0.511 | 0.033 |
| EG1 × CG1 | 0.497 | 0.631 | 0.105 | 0.439 |
| EG2 × CG2 | 0.407 | 0.188 | 0.763 | 0.387 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vialle, E.N.; Fracaro, L.; Barchiki, F.; Dominguez, A.C.; Arruda, A.d.O.; Olandoski, M.; Brofman, P.R.S.; Kuniyoshi Rebelatto, C.L. Human Adipose-Derived Stem Cells Reduce Cellular Damage after Experimental Spinal Cord Injury in Rats. Biomedicines 2023, 11, 1394. https://doi.org/10.3390/biomedicines11051394
Vialle EN, Fracaro L, Barchiki F, Dominguez AC, Arruda AdO, Olandoski M, Brofman PRS, Kuniyoshi Rebelatto CL. Human Adipose-Derived Stem Cells Reduce Cellular Damage after Experimental Spinal Cord Injury in Rats. Biomedicines. 2023; 11(5):1394. https://doi.org/10.3390/biomedicines11051394
Chicago/Turabian StyleVialle, Emiliano Neves, Letícia Fracaro, Fabiane Barchiki, Alejandro Correa Dominguez, André de Oliveira Arruda, Marcia Olandoski, Paulo Roberto Slud Brofman, and Carmen Lúcia Kuniyoshi Rebelatto. 2023. "Human Adipose-Derived Stem Cells Reduce Cellular Damage after Experimental Spinal Cord Injury in Rats" Biomedicines 11, no. 5: 1394. https://doi.org/10.3390/biomedicines11051394