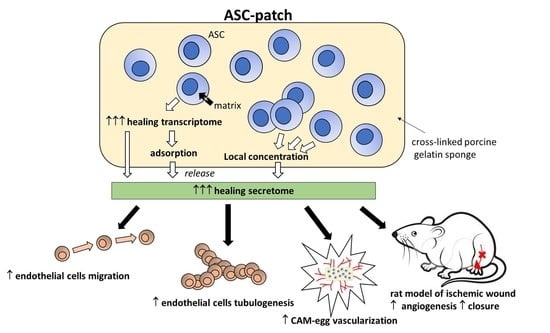
Adipose-Derived Stromal Cells within a Gelatin Matrix Acquire Enhanced Regenerative and Angiogenic Properties: A Pre-Clinical Study for Application to Chronic Wounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. ASC Culture and Engineering of an ASC-Enriched Patch
2.2. Flow Cytometry and Multipotent Differentiation of ASC
2.3. Immunocytochemistry and Immunofluorescence on Tissue Sections
2.4. Cytokine Measurements
2.5. Transcriptomic
2.6. Mass Spectrometry
2.7. Chick Chorioallantoic Membrane Model
2.8. Migration and Tubulogenesis of HUVEC
2.9. Animal Experiments
2.10. Statistical Analysis
3. Results
3.1. Culture of ASC in a Sponge Made of Porcine Crosslinked Gelatin
3.2. ASC in a Gelatin Sponge Enhanced Their Regenerative Transcriptome
3.3. The Secreted Proteome of ASC Is Absorbed by Gelatin
3.4. The ASC Gelatin Sponge Promotes Angiogenesis
3.5. ASC in Gelatin Sponge Stability and Tumorigenicity In Vivo
3.6. ASC in Gelatin Sponge Accelerates the Healing and Induces Early Neo-Angiogenesis in a Rat Model of Ischemic Wound
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guest, J.F.; Vowden, K.; Vowden, P. The health economic burden that acute and chronic wounds impose on an average clinical commissioning group/health board in the UK. J. Wound Care 2017, 26, 292–303. [Google Scholar] [CrossRef] [Green Version]
- Sen, C.K.; Gordillo, G.M.; Roy, S.; Kirsner, R.; Lambert, L.; Hunt, T.K.; Gottrup, F.; Gurtner, G.C.; Longaker, M.T. Human skin wounds: A major and snowballing threat to public health and the economy. Wound Repair Regen. 2009, 17, 763–771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, G.; Ceilley, R. Chronic Wound Healing: A Review of Current Management and Treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piaggesi, A.; Lauchli, S.; Bassetto, F.; Biedermann, T.; Marques, A.; Najafi, B.; Palla, I.; Scarpa, C.; Seimetz, D.; Triulzi, I.; et al. Advanced therapies in wound management: Cell and tissue based therapies, physical and bio-physical therapies smart and IT based technologies. J. Wound Care 2018, 27, S1–S137. [Google Scholar] [CrossRef] [PubMed]
- Brembilla, N.C.; Vuagnat, H.; Boehncke, W.H.; Krause, K.H.; Preynat-Seauve, O. Adipose-Derived Stromal Cells for Chronic Wounds: Scientific Evidence and Roadmap Toward Clinical Practice. Stem Cells Transl. Med. 2023, 12, 17–25. [Google Scholar] [CrossRef]
- DesJardins-Park, H.E.; Gurtner, G.C.; Wan, D.C.; Longaker, M.T. From Chronic Wounds to Scarring: The Growing Health Care Burden of Under- and Over-Healing Wounds. Adv. Wound Care 2022, 11, 496–510. [Google Scholar] [CrossRef]
- Czerwiec, K.; Zawrzykraj, M.; Deptula, M.; Skoniecka, A.; Tyminska, A.; Zielinski, J.; Kosinski, A.; Pikula, M. Adipose-Derived Mesenchymal Stromal Cells in Basic Research and Clinical Applications. Int. J. Mol. Sci. 2023, 24, 3888. [Google Scholar] [CrossRef]
- Gadelkarim, M.; Abushouk, A.I.; Ghanem, E.; Hamaad, A.M.; Saad, A.M.; Abdel-Daim, M.M. Adipose-derived stem cells: Effectiveness and advances in delivery in diabetic wound healing. Biomed. Pharmacother. 2018, 107, 625–633. [Google Scholar] [CrossRef]
- Henriksen, J.L.; Sorensen, N.B.; Fink, T.; Zachar, V.; Porsborg, S.R. Systematic Review of Stem-Cell-Based Therapy of Burn Wounds: Lessons Learned from Animal and Clinical Studies. Cells 2020, 9, 2545. [Google Scholar] [CrossRef]
- Mamsen, F.P.; Munthe-Fog, L.; Kring, M.K.M.; Duscher, D.; Taudorf, M.; Katz, A.J.; Kolle, S.T. Differences of embedding adipose-derived stromal cells in natural and synthetic scaffolds for dermal and subcutaneous delivery. Stem Cell Res. Ther. 2021, 12, 68. [Google Scholar] [CrossRef] [PubMed]
- Silachev, D.N.; Goryunov, K.V.; Shpilyuk, M.A.; Beznoschenko, O.S.; Morozova, N.Y.; Kraevaya, E.E.; Popkov, V.A.; Pevzner, I.B.; Zorova, L.D.; Evtushenko, E.A.; et al. Effect of MSCs and MSC-Derived Extracellular Vesicles on Human Blood Coagulation. Cells 2019, 8, 258. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Tredget, E.E.; Wu, P.Y.; Wu, Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS ONE 2008, 3, e1886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stout, R.D. Editorial: Macrophage functional phenotypes: No alternatives in dermal wound healing? J. Leukoc. Biol. 2010, 87, 19–21. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.Z.; Su, W.R.; Shi, S.H.; Wilder-Smith, P.; Xiang, A.P.; Wong, A.; Nguyen, A.L.; Kwon, C.W.; Le, A.D. Human gingiva-derived mesenchymal stem cells elicit polarization of m2 macrophages and enhance cutaneous wound healing. Stem Cells 2010, 28, 1856–1868. [Google Scholar] [CrossRef] [Green Version]
- Smith, A.N.; Willis, E.; Chan, V.T.; Muffley, L.A.; Isik, F.F.; Gibran, N.S.; Hocking, A.M. Mesenchymal stem cells induce dermal fibroblast responses to injury. Exp. Cell Res. 2010, 316, 48–54. [Google Scholar] [CrossRef] [Green Version]
- Yates, C.C.; Rodrigues, M.; Nuschke, A.; Johnson, Z.I.; Whaley, D.; Stolz, D.; Newsome, J.; Wells, A. Multipotent stromal cells/mesenchymal stem cells and fibroblasts combine to minimize skin hypertrophic scarring. Stem Cell Res. Ther. 2017, 8, 193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, M.; Hua, T.; Ouyang, T.; Qian, H.; Yu, B. Applications of Mesenchymal Stem Cells in Liver Fibrosis: Novel Strategies, Mechanisms, and Clinical Practice. Stem Cells Int. 2021, 2021, 6546780. [Google Scholar] [CrossRef]
- Maacha, S.; Sidahmed, H.; Jacob, S.; Gentilcore, G.; Calzone, R.; Grivel, J.C.; Cugno, C. Paracrine Mechanisms of Mesenchymal Stromal Cells in Angiogenesis. Stem Cells Int. 2020, 2020, 4356359. [Google Scholar] [CrossRef]
- Guillamat-Prats, R. The Role of MSC in Wound Healing, Scarring and Regeneration. Cells 2021, 10, 1729. [Google Scholar] [CrossRef]
- Yu, B.; Zhang, X.; Li, X. Exosomes derived from mesenchymal stem cells. Int. J. Mol. Sci. 2014, 15, 4142–4157. [Google Scholar] [CrossRef] [Green Version]
- Holm, J.S.; Toyserkani, N.M.; Sorensen, J.A. Adipose-derived stem cells for treatment of chronic ulcers: Current status. Stem Cell Res. Ther. 2018, 9, 142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.Z.; Gou, M.; Da, L.C.; Zhang, W.Q.; Xie, H.Q. Mesenchymal Stem Cells for Chronic Wound Healing: Current Status of Preclinical and Clinical Studies. Tissue Eng. Part B Rev. 2020, 26, 555–570. [Google Scholar] [CrossRef]
- Krasilnikova, O.A.; Baranovskii, D.S.; Lyundup, A.V.; Shegay, P.V.; Kaprin, A.D.; Klabukov, I.D. Stem and Somatic Cell Monotherapy for the Treatment of Diabetic Foot Ulcers: Review of Clinical Studies and Mechanisms of Action. Stem Cell Rev. Rep. 2022, 18, 1974–1985. [Google Scholar] [CrossRef] [PubMed]
- Rustad, K.C.; Gurtner, G.C. Mesenchymal Stem Cells Home to Sites of Injury and Inflammation. Adv. Wound Care 2012, 1, 147–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kallmeyer, K.; Andre-Levigne, D.; Baquie, M.; Krause, K.H.; Pepper, M.S.; Pittet-Cuenod, B.; Modarressi, A. Fate of systemically and locally administered adipose-derived mesenchymal stromal cells and their effect on wound healing. Stem Cells Transl. Med. 2020, 9, 131–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, E.J.; Lee, H.W.; Kalimuthu, S.; Kim, T.J.; Kim, H.M.; Baek, S.H.; Zhu, L.; Oh, J.M.; Son, S.H.; Chung, H.Y.; et al. In vivo migration of mesenchymal stem cells to burn injury sites and their therapeutic effects in a living mouse model. J. Control. Release 2018, 279, 79–88. [Google Scholar] [CrossRef]
- Tobalem, M.; Levigne, D.; Modarressi, A.; Atashi, F.; Villard, F.; Hinz, B.; Pittet-Cuenod, B. Hyperglycemia Interacts with Ischemia in a Synergistic Way on Wound Repair and Myofibroblast Differentiation. Plast. Reconstr. Surg. Glob. Open 2015, 3, e471. [Google Scholar] [CrossRef]
- Shan, Y.; Li, C.; Wu, Y.; Li, Q.; Liao, J. Hybrid cellulose nanocrystal/alginate/gelatin scaffold with improved mechanical properties and guided wound healing. RSC Adv. 2019, 9, 22966–22979. [Google Scholar] [CrossRef] [Green Version]
- Bourin, P.; Bunnell, B.A.; Casteilla, L.; Dominici, M.; Katz, A.J.; March, K.L.; Redl, H.; Rubin, J.P.; Yoshimura, K.; Gimble, J.M. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 2013, 15, 641–648. [Google Scholar] [CrossRef] [Green Version]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Patten, J.; Wang, K. Fibronectin in development and wound healing. Adv. Drug Deliv. Rev. 2021, 170, 353–368. [Google Scholar] [CrossRef] [PubMed]
- Ajit, A.; Ambika Gopalankutty, I. Adipose-derived stem cell secretome as a cell-free product for cutaneous wound healing. 3 Biotech 2021, 11, 413. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Garcovich, S. Systematic Review: Adipose-Derived Mesenchymal Stem Cells, Platelet-Rich Plasma and Biomaterials as New Regenerative Strategies in Chronic Skin Wounds and Soft Tissue Defects. Int. J. Mol. Sci. 2021, 22, 1538. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, F.; Palumbo, P.; Augello, F.R.; Cifone, M.G.; Cinque, B.; Giuliani, M. Secretome of Adipose Tissue-Derived Stem Cells (ASCs) as a Novel Trend in Chronic Non-Healing Wounds: An Overview of Experimental In Vitro and In Vivo Studies and Methodological Variables. Int. J. Mol. Sci. 2019, 20, 3721. [Google Scholar] [CrossRef] [Green Version]
- Zou, M.L.; Liu, S.Y.; Sun, Z.L.; Wu, J.J.; Yuan, Z.D.; Teng, Y.Y.; Feng, Y.; Yuan, F.L. Insights into the role of adipose-derived stem cells: Wound healing and clinical regenerative potential. J. Cell. Physiol. 2021, 236, 2290–2297. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhu, L.; Han, Q.; Liu, W.; Mao, X.; Li, Y.; Yu, N.; Feng, S.; Fu, Q.; Wang, X.; et al. Preformed gelatin microcryogels as injectable cell carriers for enhanced skin wound healing. Acta Biomater. 2015, 25, 291–303. [Google Scholar] [CrossRef]
- Petrenko, Y.; Sykova, E.; Kubinova, S. The therapeutic potential of three-dimensional multipotent mesenchymal stromal cell spheroids. Stem Cell Res. Ther. 2017, 8, 94. [Google Scholar] [CrossRef] [Green Version]
- Mytsyk, M.; Cerino, G.; Reid, G.; Sole, L.G.; Eckstein, F.S.; Santer, D.; Marsano, A. Long-Term Severe In Vitro Hypoxia Exposure Enhances the Vascularization Potential of Human Adipose Tissue-Derived Stromal Vascular Fraction Cell Engineered Tissues. Int. J. Mol. Sci. 2021, 22, 7920. [Google Scholar] [CrossRef]
- Naomi, R.; Bahari, H.; Ridzuan, P.M.; Othman, F. Natural-Based Biomaterial for Skin Wound Healing (Gelatin vs. Collagen): Expert Review. Polymers 2021, 13, 2319. [Google Scholar] [CrossRef]
- Gonzalez, A.C.; Costa, T.F.; Andrade, Z.A.; Medrado, A.R. Wound healing—A literature review. An. Bras. Dermatol. 2016, 91, 614–620. [Google Scholar] [CrossRef] [Green Version]
- Gaspar-Pintiliescu, A.; Stanciuc, A.M.; Craciunescu, O. Natural composite dressings based on collagen, gelatin and plant bioactive compounds for wound healing: A review. Int. J. Biol. Macromol. 2019, 138, 854–865. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Orue, I.; Santos-Vizcaino, E.; Etxabide, A.; Uranga, J.; Bayat, A.; Guerrero, P.; Igartua, M.; de la Caba, K.; Hernandez, R.M. Development of Bioinspired Gelatin and Gelatin/Chitosan Bilayer Hydrofilms for Wound Healing. Pharmaceutics 2019, 11, 314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikpasand, A.; Parvizi, M.R. Evaluation of the Effect of Titatnium Dioxide Nanoparticles/Gelatin Composite on Infected Skin Wound Healing; An Animal Model Study. Bull. Emerg. Trauma 2019, 7, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Jackson, C.J. Extracellular Matrix Reorganization During Wound Healing and Its Impact on Abnormal Scarring. Adv. Wound Care 2015, 4, 119–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulakov, A.; Kogan, E.; Brailovskaya, T.; Vedyaeva, A.; Zharkov, N.; Krasilnikova, O.; Krasheninnikov, M.; Baranovskii, D.; Rasulov, T.; Klabukov, I. Mesenchymal Stromal Cells Enhance Vascularization and Epithelialization within 7 Days after Gingival Augmentation with Collagen Matrices in Rabbits. Dent. J. 2021, 9, 101. [Google Scholar] [CrossRef]
- Wang, K.; Chen, Z.; Jin, L.; Zhao, L.; Meng, L.; Kong, F.; He, C.; Kong, F.; Zheng, L.; Liang, F. LPS-pretreatment adipose-derived mesenchymal stromal cells promote wound healing in diabetic rats by improving angiogenesis. Injury 2022, 53, 3920–3929. [Google Scholar] [CrossRef]
- Chen, J.; Gao, K.; Liu, S.; Wang, S.; Elango, J.; Bao, B.; Dong, J.; Liu, N.; Wu, W. Fish Collagen Surgical Compress Repairing Characteristics on Wound Healing Process In Vivo. Mar. Drugs 2019, 17, 33. [Google Scholar] [CrossRef] [Green Version]
- Dang, Q.F.; Liu, H.; Yan, J.Q.; Liu, C.S.; Liu, Y.; Li, J.; Li, J.J. Characterization of collagen from haddock skin and wound healing properties of its hydrolysates. Biomed. Mater. 2015, 10, 015022. [Google Scholar] [CrossRef] [PubMed]
- El Masry, M.S.; Chaffee, S.; Das Ghatak, P.; Mathew-Steiner, S.S.; Das, A.; Higuita-Castro, N.; Roy, S.; Anani, R.A.; Sen, C.K. Stabilized collagen matrix dressing improves wound macrophage function and epithelialization. FASEB J. 2019, 33, 2144–2155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jridi, M.; Bardaa, S.; Moalla, D.; Rebaii, T.; Souissi, N.; Sahnoun, Z.; Nasri, M. Microstructure, rheological and wound healing properties of collagen-based gel from cuttlefish skin. Int. J. Biol. Macromol. 2015, 77, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Pal, P.; Srivas, P.K.; Dadhich, P.; Das, B.; Maity, P.P.; Moulik, D.; Dhara, S. Accelerating full thickness wound healing using collagen sponge of mrigal fish (Cirrhinus cirrhosus) scale origin. Int. J. Biol. Macromol. 2016, 93, 1507–1518. [Google Scholar] [CrossRef]
- Wiegand, C.; Buhren, B.A.; Bunemann, E.; Schrumpf, H.; Homey, B.; Frykberg, R.G.; Lurie, F.; Gerber, P.A. A novel native collagen dressing with advantageous properties to promote physiological wound healing. J. Wound Care 2016, 25, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Feroz, S.; Muhammad, N.; Ranayake, J.; Dias, G. Keratin—Based materials for biomedical applications. Bioact. Mater. 2020, 5, 496–509. [Google Scholar] [CrossRef] [PubMed]
- Ye, D.; Wu, S.; Zhang, B.; Hong, C.; Yang, L. Characteristics and clinical potential of a cellularly modified gelatin sponge. J. Appl. Biomater. Funct. Mater. 2021, 19, 22808000211035061. [Google Scholar] [CrossRef]
- Markowicz, M.; Koellensperger, E.; Neuss, S.; Koenigschulte, S.; Bindler, C.; Pallua, N. Human bone marrow mesenchymal stem cells seeded on modified collagen improved dermal regeneration in vivo. Cell Transplant. 2006, 15, 723–732. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Mitsuno, H.; Nonaka, I.; Sen, Y.; Kawanishi, K.; Inada, Y.; Takakura, Y.; Okuchi, K.; Nonomura, A. Wound therapy by marrow mesenchymal cell transplantation. Plast. Reconstr. Surg. 2008, 121, 860–877. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.Q.; Chen, Y.; Chai, M.; Tao, R.; Lei, Y.H.; Jia, Y.Q.; Shu, J.; Ren, J.; Li, G.; Wei, W.X.; et al. Adipose extracellular matrix promotes skin wound healing by inducing the differentiation of adiposederived stem cells into fibroblasts. Int. J. Mol. Med. 2019, 43, 890–900. [Google Scholar] [CrossRef] [Green Version]
- Borowczyk, J.; Buerger, C.; Tadjrischi, N.; Drukala, J.; Wolnicki, M.; Wnuk, D.; Modarressi, A.; Boehncke, W.H.; Brembilla, N.C. IL-17E (IL-25) and IL-17A Differentially Affect the Functions of Human Keratinocytes. J. Investig. Dermatol. 2020, 140, 1379–1389.e2. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brembilla, N.C.; Modarressi, A.; André-Lévigne, D.; Brioudes, E.; Lanza, F.; Vuagnat, H.; Durual, S.; Marger, L.; Boehncke, W.-H.; Krause, K.-H.; et al. Adipose-Derived Stromal Cells within a Gelatin Matrix Acquire Enhanced Regenerative and Angiogenic Properties: A Pre-Clinical Study for Application to Chronic Wounds. Biomedicines 2023, 11, 987. https://doi.org/10.3390/biomedicines11030987
Brembilla NC, Modarressi A, André-Lévigne D, Brioudes E, Lanza F, Vuagnat H, Durual S, Marger L, Boehncke W-H, Krause K-H, et al. Adipose-Derived Stromal Cells within a Gelatin Matrix Acquire Enhanced Regenerative and Angiogenic Properties: A Pre-Clinical Study for Application to Chronic Wounds. Biomedicines. 2023; 11(3):987. https://doi.org/10.3390/biomedicines11030987
Chicago/Turabian StyleBrembilla, Nicolo Costantino, Ali Modarressi, Dominik André-Lévigne, Estelle Brioudes, Florian Lanza, Hubert Vuagnat, Stéphane Durual, Laurine Marger, Wolf-Henning Boehncke, Karl-Heinz Krause, and et al. 2023. "Adipose-Derived Stromal Cells within a Gelatin Matrix Acquire Enhanced Regenerative and Angiogenic Properties: A Pre-Clinical Study for Application to Chronic Wounds" Biomedicines 11, no. 3: 987. https://doi.org/10.3390/biomedicines11030987