Mechanical Characterization of 3D-Printed Patterned Membranes for Cardiac Tissue Engineering: An Experimental and Numerical Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation and Characterization of 3D-Printed Membranes
2.1.1. Materials
2.1.2. Preparation of the Biomaterial Ink
2.1.3. Rheological Properties of the Biomaterial Ink
2.1.4. 3D Printing and Evaluation of the Printability
2.1.5. Degradation Profile of 3D-Printed Constructs
2.1.6. Dimensional Changes of the 3D-Printed Constructs after Gelation
2.2. Experimental Evaluation of the Mechanical Properties of the 3D-Printed Membranes
2.2.1. Creation of the Internal Pattern of Membranes by 3D Printing
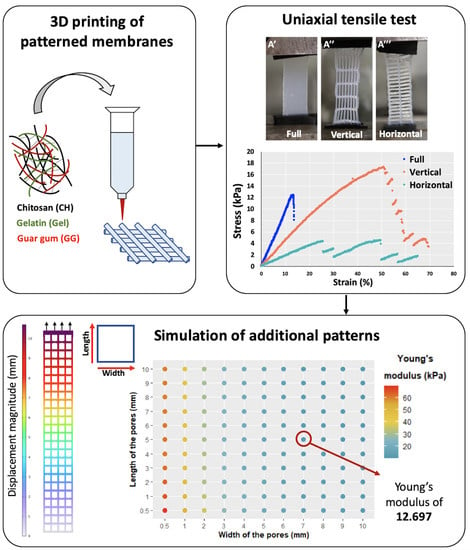
2.2.2. Uniaxial Tensile Tests
2.3. Numerical Simulation of Tensile Tests and Modeling of Additional Membranes
2.3.1. Geometrical Models of Membranes
2.3.2. Modeling of the Tensile Tests
2.3.3. Simulation of the Tensile Tests to Determine the Young’s Modulus of the Membranes and of the Material
2.3.4. Statistical Analysis
3. Results
3.1. Characterization of the Biomaterial Ink and the Resulting 3D-Printed Constructs
3.1.1. Viscosity, Printability, and Degradation Profile
3.1.2. Dimensional Changes of the Membranes after Gelation
3.2. Evaluation of the Mechanical Properties of CH–Gel–GG Membranes by the Tensile Test and Numerical Simulations
3.2.1. Experimental Uniaxial Tensile Test
3.2.2. Validation of the Young’s Modulus of the Membranes and Material
3.2.3. Modeling the Impact of the Geometry on the Young’s Modulus of the Membrane
3.2.4. Validation of the Model through Experimental Confirmation
4. Discussion
5. Limitations of the Study
6. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Varela, C.E.; Fan, Y.; Roche, E.T. Optimizing epicardial restraint and reinforcement following myocardial infarction: Moving towards localized, biomimetic, and multitherapeutic options. Biomimetics 2019, 4, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clarke, S.A.; Ghanta, R.K.; Ailawadi, G.; Holmes, J.W. Cardiac restraint and support following myocardial infarction. In Cardiovascular and Cardiac Therapeutic Devices; Springer: Berlin/Heidelberg, Germany, 2013; pp. 169–206. [Google Scholar]
- Kalhori, D.; Zakeri, N.; Zafar-Jafarzadeh, M.; Moroni, L.; Solati-Hashjin, M. Cardiovascular 3D bioprinting: A review on cardiac tissue development. Bioprinting 2022, 28, e00221. [Google Scholar] [CrossRef]
- Dwyer, K.D.; Coulombe, K.L. Cardiac mechanostructure: Using mechanics and anisotropy as inspiration for developing epicardial therapies in treating myocardial infarction. Bioact. Mater. 2021, 6, 2198–2220. [Google Scholar] [CrossRef]
- Kwon, M.H.; Cevasco, M.; Schmitto, J.D.; Chen, F.Y. Ventricular restraint therapy for heart failure: A review, summary of state of the art, and future directions. J. Thorac. Cardiovasc. Surg. 2012, 144, 771–777. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Mu, W.; Zhang, Y.; He, X.; Wang, Y.; Ma, H.; Zhu, T.; Li, A.; Hou, Q.; Yang, W.; et al. Recent Advances in Cardiac Patches: Materials, Preparations, and Properties. ACS Biomater. Sci. Eng. 2022, 8, 3659–3675. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, L.; Li, T.; Liu, S.; Guo, B.; Huang, W.; Wu, Y. 3D bioprinting in cardiac tissue engineering. Theranostics 2021, 11, 7948. [Google Scholar] [CrossRef] [PubMed]
- Ghosal, K.; Chandra, A.; Roy, S.; Agatemor, C.; Thomas, S.; Provaznik, I. Electrospinning over solvent casting: Tuning of mechanical properties of membranes. Sci. Rep. 2018, 8, 5058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martins, A.M.; Eng, G.; Caridade, S.G.; Mano, J.F.; Reis, R.L.; Vunjak-Novakovic, G. Electrically conductive chitosan/carbon scaffolds for cardiac tissue engineering. Biomacromolecules 2014, 15, 635–643. [Google Scholar] [CrossRef]
- Groll, J.; Burdick, J.A.; Cho, D.W.; Derby, B.; Gelinsky, M.; Heilshorn, S.C.; Juengst, T.; Malda, J.; Mironov, V.A.; Nakayama, K.; et al. A definition of bioinks and their distinction from biomaterial inks. Biofabrication 2018, 11, 013001. [Google Scholar] [CrossRef] [PubMed]
- Atala, A.; Yoo, J.J. Essentials of 3D Biofabrication and Translation; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef]
- Domengé, O.; Ragot, H.; Deloux, R.; Crépet, A.; Revet, G.; Boitard, S.E.; Simon, A.; Mougenot, N.; David, L.; Delair, T.; et al. Efficacy of epicardial implantation of acellular chitosan hydrogels in ischemic and nonischemic heart failure: Impact of the acetylation degree of chitosan. Acta Biomater. 2021, 119, 125–139. [Google Scholar] [CrossRef]
- Mora Boza, A.; Wlodarczyk-Biegun, M.K.; Del Campo, A.; Vázquez-Lasal, B.; San Román, J. Chitosan-based inks: 3D printing and bioprinting strategies to improve shape fidelity, mechanical properties, and biocompatibility of 3D scaffolds. Biomecánica 2019, 27, 7–16. [Google Scholar] [CrossRef]
- Ouyang, L.; Yao, R.; Zhao, Y.; Sun, W. Effect of bioink properties on printability and cell viability for 3D bioplotting of embryonic stem cells. Biofabrication 2016, 8, 035020. [Google Scholar] [CrossRef]
- Paxton, N.; Smolan, W.; Böck, T.; Melchels, F.; Groll, J.; Jungst, T. Proposal to assess printability of bioinks for extrusion-based bioprinting and evaluation of rheological properties governing bioprintability. Biofabrication 2017, 9, 044107. [Google Scholar] [CrossRef] [Green Version]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guide, I. Comsol Multiphysics. 5.6, COMSOL AB. 1998, pp. 204–208. Available online: https://www.comsol.com (accessed on 15 March 2023).
- Javanmardi, Y.; Colin-York, H.; Szita, N.; Fritzsche, M.; Moeendarbary, E. Quantifying cell-generated forces: Poisson’s ratio matters. Commun. Phys. 2021, 4, 237. [Google Scholar] [CrossRef] [PubMed]
- Anseth, K.S.; Bowman, C.N.; Brannon-Peppas, L. Mechanical properties of hydrogels and their experimental determination. Biomaterials 1996, 17, 1647–1657. [Google Scholar] [CrossRef]
- Carrow, J.K.; Kerativitayanan, P.; Jaiswal, M.K.; Lokhande, G.; Gaharwar, A.K. Polymers for bioprinting. In Essentials of 3D Biofabrication and Translation; Elsevier: Amsterdam, The Netherlands, 2015; pp. 229–248. [Google Scholar]
- Hankiewicz, J.; Swierczek, E. Lysozyme in human body fluids. Clin. Chim. Acta 1974, 57, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Kitahara, M.; Miyagawa, S.; Fukushima, S.; Saito, A.; Shintani, A.; Akita, T.; Sawa, Y. Biodegradable vs nonbiodegradable cardiac support device for treating ischemic cardiomyopathy in a canine heart. In Seminars in Thoracic and Cardiovascular Surgery; Elsevier: Amsterdam, The Netherlands, 2017; Volume 29, pp. 51–61. [Google Scholar]
- Chatterjee, S.; Hui, P.C.l. Stimuli-responsive hydrogels: An interdisciplinary overview. In Hydrogels—Smart Materials for Biomedical Applications; BoD–Books on Demand: Norderstedt, Germany, 2018; pp. 1–23. [Google Scholar]
- Mohammadinejad, R.; Kumar, A.; Ranjbar-Mohammadi, M.; Ashrafizadeh, M.; Han, S.S.; Khang, G.; Roveimiab, Z. Recent advances in natural gum-based biomaterials for tissue engineering and regenerative medicine: A review. Polymers 2020, 12, 176. [Google Scholar] [CrossRef] [Green Version]
- Mano, J.; Silva, G.; Azevedo, H.S.; Malafaya, P.; Sousa, R.; Silva, S.S.; Boesel, L.; Oliveira, J.M.; Santos, T.; Marques, A.; et al. Natural origin biodegradable systems in tissue engineering and regenerative medicine: Present status and some moving trends. J. R. Soc. Interface 2007, 4, 999–1030. [Google Scholar] [CrossRef] [Green Version]
- Engelmayr, G.C.; Cheng, M.; Bettinger, C.J.; Borenstein, J.T.; Langer, R.; Freed, L.E. Accordion-like honeycombs for tissue engineering of cardiac anisotropy. Nat. Mater. 2008, 7, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Jehl, J.P.; Dan, P.; Voignier, A.; Tran, N.; Bastogne, T.; Maureira, P.; Cleymand, F. Transverse isotropic modelling of left-ventricle passive filling: Mechanical characterization for epicardial biomaterial manufacturing. J. Mech. Behav. Biomed. Mater. 2021, 119, 104492. [Google Scholar] [CrossRef] [PubMed]







| Young’s Modulus (kPa) | |||||
|---|---|---|---|---|---|
| Type of | Membrane | Material | |||
| Membrane | Sample | In Silico | Experimental | In Silico | Experimental |
| 1 | 45.2 | 49.6 | 95.8 | ||
| 2 | 43.9 | 44.7 | 91.5 | ||
| Vertical | 3 | 44.2 | 44.3 | 91.2 | |
| 4 | 45.0 | 44.1 | 91.1 | ||
| 5 | 45.9 | 48.0 | 94.8 | ||
| 1 | 18.6 | 18.3 | 89.7 | ||
| 2 | 18.3 | 21.2 | 107.6 | ||
| Horizontal | 3 | 18.9 | 18.3 | 88.3 | |
| 4 | 17.4 | 18.1 | 90.4 | ||
| 5 | 17.5 | 22.9 | 117.7 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poerio, A.; Guibert, B.; Leroux, M.M.; Mano, J.F.; Cleymand, F.; Jehl, J.-P. Mechanical Characterization of 3D-Printed Patterned Membranes for Cardiac Tissue Engineering: An Experimental and Numerical Study. Biomedicines 2023, 11, 963. https://doi.org/10.3390/biomedicines11030963
Poerio A, Guibert B, Leroux MM, Mano JF, Cleymand F, Jehl J-P. Mechanical Characterization of 3D-Printed Patterned Membranes for Cardiac Tissue Engineering: An Experimental and Numerical Study. Biomedicines. 2023; 11(3):963. https://doi.org/10.3390/biomedicines11030963
Chicago/Turabian StylePoerio, Aurelia, Bertrand Guibert, Mélanie M. Leroux, João F. Mano, Franck Cleymand, and Jean-Philippe Jehl. 2023. "Mechanical Characterization of 3D-Printed Patterned Membranes for Cardiac Tissue Engineering: An Experimental and Numerical Study" Biomedicines 11, no. 3: 963. https://doi.org/10.3390/biomedicines11030963