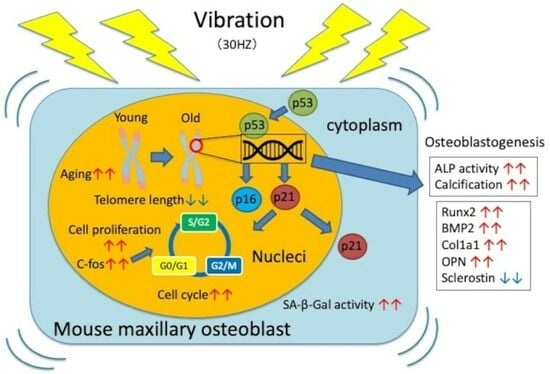
Vertical Vibration of Mouse Osteoblasts Promotes Cellular Differentiation and Cell Cycle Progression and Induces Aging In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Cell Counts
2.3. Vibration System
2.4. Cell Proliferation Assay
2.5. Cell-Clock Cell Cycle Assay
2.6. Evaluation of ALP Activity in Osteoblasts
2.7. Evaluation of Calcification Ability in Osteoblasts
2.8. Analysis of mRNA Expression via Real-Time PCR
2.9. Evaluation of SA-β-Gal Activity for Cell Aging
2.10. Telomere Length Assay (qPCR)
2.11. Statistical Analysis
3. Results
3.1. Effects of Vibration on Cell Proliferation
3.2. Effects of Vibration on the Cell Cycle
3.3. Vibration Accelerates Osteoblast Differentiation
3.4. Vibrations Cause Changes in Osteoblast Differentiation Marker Genes
3.5. Vibration Accelerates Cellular Aging
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Kenwright, J.; Goodship, A.E. Controlled mechanical stimulation in the treatment of tibial fractures. Clin. Orthop. Relat. Res. 1989, 241, 36–47. [Google Scholar] [CrossRef]
- Rubin, C.; Turner, A.S.; Bain, S.; Mallinckrodt, C.; McLeod, K. Low mechanical signals strengthen long bones. Nature 2001, 412, 603–604. [Google Scholar] [CrossRef] [PubMed]
- Rubin, C.; Turner, A.S.; Müller, R.; Mittra, E.; McLeod, K.; Lin, W.; Qin, X.Y. Quantity and quality of trabecular bone in the femur are enhanced by a strongly anabolic, noninvasive mechanical intervention. J. Bone Miner. Res. 2002, 17, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Judex, S.; Lei, X.; Han, D.; Rubin, C. Low-magnitude mechanical signals that stimulate bone formation in the ovariectomized rat are dependent on the applied frequency but not on the strain magnitude. J. Biomech. 2007, 40, 1333–1339. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.M.; Alam, I.M.; Turner, C.H. Stochastic resonance in osteogenic response to mechanical loading. FASEB J. 2003, 17, 313–314. [Google Scholar] [CrossRef]
- Ward, K.; Alsop, C.; Caulton, J.; Rubin, C.; Adams, J.; Mughal, Z. Low magnitude mechanical loading is osteogenic in children with disabling conditions. J. Bone Miner. Res. 2004, 19, 360–369. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, Y.; Liu, Y.; Wang, H.; Guo, C.; Zhang, X. Effect of the same mechanical loading on osteogenesis and osteoclastogenesis in vitro. Chin. J. Traumatol. 2015, 18, 150–156. [Google Scholar] [CrossRef]
- Lackner, I.; Liedert, A.; Fischer, V.; Ignatius, A. Effects of low-magnitude high-frequency vibration on osteoblasts are dependent on estrogen receptor a signaling and cytoskeletal remodeling. Biochem. Biophys. Res. Commun. 2018, 503, 2678–2684. [Google Scholar] [CrossRef]
- Nishimura, M.; Chiba, M.; Ohashi, T.; Sato, M.; Shimizu, Y.; Igarashi, K.; Mitani, H. Periodontal tissue activation by vibration: Intermittent stimulation by resonance vibration accelerates experimental tooth movement in rats. Am. J. Orthod. Dentofac. Orthop. 2008, 133, 572–583. [Google Scholar] [CrossRef]
- Pavlin, D.; Anthony, R.; Raj, V.; Gakunga, P.T. Cyclic loading (vibration) accelerates tooth movement in orthodontic patients: A double-blind, randomized controlled trial. Semin. Orthod. 2015, 21, 187–194. [Google Scholar] [CrossRef]
- DiBiase, A.T.; Woodhouse, N.R.; Papageorgiou, S.N.; Johnson, N.; Slipper, C.; Grant, J.; Alsaleh, M.; Khaja, Y.; Cobourne, T.M. Effects of supplemental vibrational force on space closure, treatment duration, and occlusal outcome: A multicenter randomized clinical trial. Am. J. Orthod. Dentofac. Orthop. 2018, 153, 469–480.e4. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Takahashi, Y.; Tabata, Y. Controlled release by biodegradable hydrogels enhances the ectopic bone formation of bone morphogenetic protein. Biomatterials 2003, 24, 4375–4383. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Miyauchi, S.; Tawada, A.; Anada, T.; Matsuzaka, S.; Suzuki, O. Oversulfated chondroitin sulfate-E binds to BMP-4 and enhances osteoblast differentiation. J. Cell Physiol. 2008, 217, 768–777. [Google Scholar] [CrossRef]
- Peck, W.A.; Birge, S.J.; Fedak, S.A. Bone cells: Biochemical and biological studies after enzymatic isolation. Science 1964, 146, 1476–1477. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.M.; Johnston, C.C.; Severson, A.R. Studies of the metabolism of separated bone cells. I. Techniques of separation and identification. Calcif. Tissue Res. 1973, 11, 56–69. [Google Scholar] [CrossRef] [PubMed]
- Dziak, R.; Brand, J.S. Calcium transport in isolated bone cells. I. Bone cell isolation procedures. J. Cell Physiol. 1974, 84, 75–83. [Google Scholar] [CrossRef]
- Hefley, T.; Cushing, J.; Brand, J.S. Enzymatic isolation of cells from bone: Cytotoxic enzymes of bacterial collagenase. Am. J. Physiol. 1981, 240, C234–C238. [Google Scholar] [CrossRef]
- Sarsour, E.H.; Kumar, M.G.; Chaudhuri, L.; Kalen, A.L.; Goswami, P.C. Redox control of the cell cycle in health and disease. Antioxid. Redox Signal. 2009, 11, 2985–3011. [Google Scholar] [CrossRef]
- Stein, G.S.; Lian, J.B. Molecular mechanisms mediating proliferation/differentiation interrelationships during progressive development of the osteoblast phenotype. Endocrine 1993, 14, 424–442. [Google Scholar] [CrossRef]
- Zhang, C.; Li, J.; Zhang, L.; Zhou, Y.; Hou, W.; Quan, H.; Li, X.; Chen, Y.; Yu, H. Effects of mechanical vibration on proliferation and osteogenic differentiation of human periodontal ligament stem cells. Arch. Oral Biol. 2012, 57, 1395–1407. [Google Scholar] [CrossRef]
- Zhang, C.; Lu, Y.; Zhang, L.; Liu, Y.; Zhou, Y.; Chen, Y.; Yu, H. Influence of different intensities of vibration on proliferation and differentiation of human periodontal ligament stem cells. Arch. Med. Sci. 2015, 11, 638–646. [Google Scholar] [CrossRef]
- Bianco, P.; Fisher, L.W.; Young, M.F.; Termine, J.D.; Robey, P.G. Expression and localization of the two small proteoglycans biglycan and decorin in developing human skeletal and non-skeletal tissues. J. Histochem. Cytochem. 1990, 38, 1549–1563. [Google Scholar] [CrossRef] [PubMed]
- Ingram, R.T.; Clarke, B.L.; Fisher, L.W.; Fitzpatrick, L.A. Distribution of noncollagenous proteins in the matrix of adult human bone: Evidence of anatomic and functional heterogeneity. J. Bone Miner. Res. 1993, 8, 1019–1029. [Google Scholar] [CrossRef] [PubMed]
- Wilda, M.; Bächner, D.; Just, W.; Geerkens, C.; Kraus, P.; Vogel, W.; Hameister, H. A comparison of the expression pattern of five genes of the family of small leucine-rich proteoglycans during mouse development. J. Bone Miner. Res. 2000, 15, 2187–2196. [Google Scholar] [CrossRef]
- Kamiya, N.; Shigemasa, K.; Takagi, M. Gene expression and immunohistochemical localization of decorin and biglycan in association with early bone formation in the developing mandible. J. Oral Sci. 2001, 43, 179–188. [Google Scholar] [CrossRef]
- Ferraro, V.; Gaillard-Martinie, B.; Sayd, T.; Chambon, C.; Anton, M.; Santé-Lhoutellier, V. Collagen type I from bovine bone. Effect of animal age, bone anatomy and drying methodology on extraction yield, self-assembly, thermal behaviour and electrokinetic potential. Int. J. Biol. Macromol. 2017, 97, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Yang, S.; Shao, J.; Li, Y.P. Signaling and transcriptional regulation in osteoblast commitment and differentiation. Front. Biosci. 2007, 12, 3068–3092. [Google Scholar] [CrossRef]
- Harris, S.L.; Levine, A.J. The p53 pathway: Positive and negative feedback loops. Oncogene 2005, 24, 2899–2908. [Google Scholar] [CrossRef]
- Tyner, S.D.; Venkatachalam, S.; Choi, J.; Jones, S.; Ghebranious, N.; Igelmann, H.; Lu, X.; Gabrielle, S.; Benjamin, C.; Cory, B.; et al. P53 mutant mice that display early ageing-associated phenotypes. Nature 2002, 415, 45–53. [Google Scholar] [CrossRef]
- Rayess, H.; Wang, M.B.; Srivatsan, E.S. Cellular senescence and tumor suppressor gene p16. Int. J. Cancer 2012, 130, 1715–1725. [Google Scholar] [CrossRef]
- Johmura, Y.; Shimada, M.; Misaki, T.; Naiki-Ito, A.; Miyoshi, H.; Motoyama, N.; Ohtani, N.; Hara, E.; Nakamura, M.; Morita, A.; et al. Necessary and sufficient role for a mitosis skip in senescence induction. Mol. Cell 2014, 55, 73–84. [Google Scholar] [CrossRef]
- Dimri, G.P.; Lee, X.; Basile, G.; Acosta, M.; Scott, G.; Roskelley, C.; Medrano, E.E.; Linskens, M.; Rubelj, I.; Pereira-Smith, O. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA 1995, 92, 9363–9367. [Google Scholar] [CrossRef]
- Kurz, D.J.; Decary, S.; Hong, Y.; Erusalimsky, J.D. Senescence-associated (beta)-galactosidase reflects an increase in lysosomal mass during replicative ageing of human endothelial cells. J. Cell Sci. 2000, 113, 3613–3622. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.Y.; Han, J.A.; Im, J.S.; Morrone, A.; Johung, K.; Goodwin, E.C.; Kleijer, W.J.; DiMaio, D.; Hwang, E.S. Senescence-associated β-galactosidase is lysosomal β-galactosidase. Aging Cell 2006, 5, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Yin, X.; Zhang, X.; Jing, D.; Feng, X. Cyclic stretch enhances bone morphogenetic protein-2-induced osteoblastic differentiation through the inhibition of Hey1. Int. J. Mol. Med. 2015, 36, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Bacabac, R.G.; Smit, T.H.; Van Loon, J.J.W.A.; Doulabi, B.Z.; Helder, M.; Klein-Nulend, J. Bone cell responses to high-frequency vibration stress: Does the nucleus oscillate within the cytoplasm? FASEB J. 2006, 20, 858–864. [Google Scholar] [CrossRef]
- Uzer, G.; Manske, S.L.; Chan, M.E.; Chiang, F.P.; Rubin, C.T.; Frame, M.D.; Judex, S. Separating fluid shear stress from acceleration during vibrations in vitro: Identification of mechanical signals modulating the cellular response. Cell Mol. Bioeng. 2012, 5, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Ota, T.; Chiba, M.; Hayashi, H. Vibrational stimulation induces osteoblast differentiation and the upregulation of osteogenic gene expression in vitro. Cytotechnology 2016, 68, 2287–2299. [Google Scholar] [CrossRef]
- Fu, L.; Patel, M.S.; Bradley, A.; Wagner, E.F.; Karsenty, G. The molecular clock mediates leptin-regulated bone formation. Cell 2005, 122, 803–815. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Sui, B.D.; Hu, C.H.; Cao, J.; Zheng, C.X.; Hou, R.; Yang, K.Z.; Zhao, P.; Chen, Q.; Yang, J.Q.; et al. MicroRNA-21 contributes to orthodontic tooth movement. J. Dent. Res. 2016, 95, 1425–1433. [Google Scholar] [CrossRef]
- Alikhani, M.; Alansari, S.; Hamidaddin, M.A.; Sangsuwon, C.; Alyami, B.; Thirumoorthy, S.N.; Oliveira, M.S.; Nervina, M.J.; Teixeira, C.C. Vibration paradox in orthodontics: Anabolic and catabolic effects. PLoS ONE 2018, 13, e0196540. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Gu, Y.; Bai, Y.; Xia, S.; Zhang, Y.; Lou, Y.; Zhu, Y.; Dai, Y.; Tsoi, J.K.; Wang, S. Does Low-Magnitude High-Frequency Vibration (LMHFV) Worth for Clinical Trial on Dental Implant? A Systematic Review and Meta-Analysis on Animal Studies. Front. Bioeng. Biotechnol. 2021, 9, 626892. [Google Scholar] [CrossRef] [PubMed]
- D’Orto, B.; Polizzi, E.; Nagni, M.; Tetè, G.; Capparè, P. Full Arch Implant-Prosthetic Rehabilitation in Patients with Type I Diabetes Mellitus: Retrospective Clinical Study with 10 Year Follow-Up. Int. J. Environ. Res. Public Health 2022, 19, 11735. [Google Scholar] [CrossRef] [PubMed]
- Carossa, M.; Cavagnetto, D.; Mancini, F.; Mosca Balma, A.; Mussano, F. Plasma of Argon Treatment of the Implant Surface, Systematic Review of In Vitro Studies. Biomolecules 2022, 12, 1219. [Google Scholar] [CrossRef] [PubMed]
- Shobara, K.; Ogawa, T.; Shibamoto, A.; Miyashita, M.; Ito, A.; Sitalaksmi, R.M. Osteogenic effect of low-intensity pulsed ultrasound and whole-body vibration on peri-implant bone. An experimental in vivo study. Clin. Oral Implants Res. 2021, 32, 641–650. [Google Scholar] [CrossRef]
- Ferrari, C.E.; Carboncini, F.; Parrini, S.; Doldo, T.; Nagni, M.; Uti, N.; Ferrari, M. Functional Implant Prosthodontic Score of a one-year prospective study on three different connections for single-implant restorations. J. Osseointegr. 2018, 10, 130–135. [Google Scholar] [CrossRef]






| Gene | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| β-actin | CGGTTCCGATGCCCTGAGGCTCTT | CGTCACACTTCATGATGGAATTGA |
| Runx2 | AATTAACGCCAGTCGGAGCA | CACTTCTCGGTCTGACGACG |
| BMP2 | CTCTCTCAATGGACGTGCCC | AACACTAGAAGACAGCGGGTC |
| Col1a1 | TTCTCCTGGCAAAGACGGAC | CGGCCACCATCTTGAGACTT |
| OPN | GGCTGAATTCTGAGGGACTAACT | ACAGCATTCTGTGGCGCAAG |
| OCN | GTATGGCTTGAAGACCGCCTA | AGGGAGGATCAAGTCCCG |
| Sclerostin | GCCTTCAGGAATGATGCCAC | CTGTCAGGAAGCGGGTGTAG |
| p53 | AAACGCTTCGAGATGTTCCG | CAAGGCTTGGAAGGCTCTAGG |
| p21 | TAAGGACGTCCCACTTTGCC | AAAGTTCCACCGTTCTCGGG |
| p16 | CGAACTCGAGGAGAGCCATC | TACGTGAACGTTGCCCATCA |
| c-fos | TACTACCATTCCCCAGCCGA | GCTGTCACCGTGGGGATAAA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, D.; Ishii, T.; Ishikawa, M.; Ootake, T.; Kamei, H.; Nagai, K.; Sueishi, K. Vertical Vibration of Mouse Osteoblasts Promotes Cellular Differentiation and Cell Cycle Progression and Induces Aging In Vitro. Biomedicines 2023, 11, 444. https://doi.org/10.3390/biomedicines11020444
Choi D, Ishii T, Ishikawa M, Ootake T, Kamei H, Nagai K, Sueishi K. Vertical Vibration of Mouse Osteoblasts Promotes Cellular Differentiation and Cell Cycle Progression and Induces Aging In Vitro. Biomedicines. 2023; 11(2):444. https://doi.org/10.3390/biomedicines11020444
Chicago/Turabian StyleChoi, Daehwan, Takenobu Ishii, Munetada Ishikawa, Tomohisa Ootake, Hirokazu Kamei, Kohei Nagai, and Kenji Sueishi. 2023. "Vertical Vibration of Mouse Osteoblasts Promotes Cellular Differentiation and Cell Cycle Progression and Induces Aging In Vitro" Biomedicines 11, no. 2: 444. https://doi.org/10.3390/biomedicines11020444