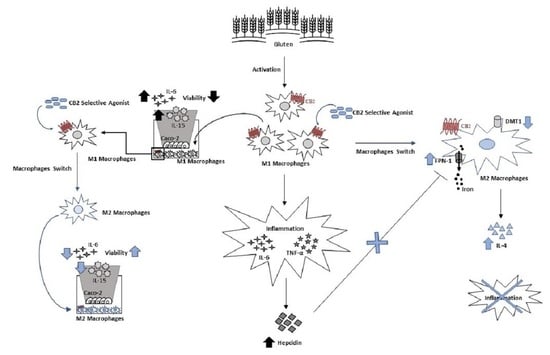
Effects of CB2 Receptor Modulation on Macrophage Polarization in Pediatric Celiac Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Macrophages Cell Cultures
2.3. Caco-2 Cells Culture
2.4. Macrophages and Caco-2 Cells Coculture in a Transwell System
2.5. Drugs and Treatments
2.6. Protein Isolation, Western Blot
2.7. ELISA
2.8. Iron Assay
2.9. Count and Viability Assay Kit
2.10. Statistical Analysis
3. Results
3.1. Characterization of Macrophages Derived from CD Patients
3.2. Evaluation of Iron Metabolism in Macrophages Derived from CD Patients
3.3. Effects of CB2 Modulation on CD Macrophage Polarization
3.4. Effects of CB2 Modulation on CD Macrophages’ Iron Metabolism
3.5. Effect of CB2 Modulation on IL-6 and IL-15 Release by Caco-2 Cells Alone and in Coculture with CD Macrophages
3.6. Effect of CB2 Modulation on Caco-2 Viability Alone and in Coculture with CD Macrophages
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jimenez, J.; Loveridge-Lenza, B.; Horvath, K. Celiac Disease in Children. Pediatric Clin. N. Am. 2021, 68, 1205–1219. [Google Scholar] [CrossRef] [PubMed]
- Kamada, N.; Rogler, G. The Innate Immune System: A Trigger for Many Chronic Inflammatory Intestinal Diseases. Inflamm. Intest. Dis. 2016, 1, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Serena, G.; Yan, S.; Camhi, S.; Patel, S.; Lima, R.S.; Sapone, A.; Leonard, M.M.; Mukherjee, R.; Nath, B.J.; Lammers, K.M.; et al. Proinflammatory cytokine interferon-gamma and microbiome-derived metabolites dictate epigenetic switch between forkhead box protein 3 isoforms in coeliac disease. Clin. Exp. Immunol. 2017, 187, 490–506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aljada, B.; Zohni, A.; El-Matary, W. The Gluten-Free Diet for Celiac Disease and Beyond. Nutrients 2021, 13, 3993. [Google Scholar] [CrossRef] [PubMed]
- Arranz, E.; De Prado, A.; Fiz-Lopez, A.; Arribas, E.; Garrote, J.A.; Bernardo, D. Human intestinal dendritic cell and macrophage subsets in coeliac disease. Int. Rev. Cell Mol. Biol. 2021, 358, 85–104. [Google Scholar] [PubMed]
- Farina, F.; Pisapia, L.; Laezza, M.; Serena, G.; Rispo, A.; Ricciolino, S.; Gianfrani, C.; Fasano, A.; Del Pozzo, G. Effect of Gliadin Stimulation on HLA-DQ2.5 Gene Expression in Macrophages from Adult Celiac Disease Patients. Biomedicines 2021, 10, 63. [Google Scholar] [CrossRef]
- Barilli, A.; Gaiani, F.; Prandi, B.; Cirlini, M.; Ingoglia, F.; Visigalli, R.; Rotoli, B.M.; de’Angelis, N.; Sforza, S.; de’Angelis, G.L.; et al. Gluten peptides drive healthy and celiac monocytes toward an M2-like polarization. J. Nutr. Biochem. 2018, 54, 11–17. [Google Scholar] [CrossRef]
- Barilli, A.; Rotoli, B.M.; Visigalli, R.; Dall’Asta, V. Gliadin activates arginase pathway in RAW264.7 cells and in human monocytes. Biochim. Et Biophys. Acta 2014, 1842, 1364–1371. [Google Scholar] [CrossRef] [Green Version]
- Barilli, A.; Rotoli, B.M.; Visigalli, R.; Ingoglia, F.; Cirlini, M.; Prandi, B.; Dall’Asta, V. Gliadin-mediated production of polyamines by RAW264.7 macrophages modulates intestinal epithelial permeability in vitro. Biochim. Et Biophys. Acta 2015, 1852, 1779–1786. [Google Scholar] [CrossRef] [Green Version]
- Rakhimova, M.; Esslinger, B.; Schulze-Krebs, A.; Hahn, E.G.; Schuppan, D.; Dieterich, W. In vitro differentiation of human monocytes into dendritic cells by peptic-tryptic digest of gliadin is independent of genetic predisposition and the presence of celiac disease. J. Clin. Immunol. 2009, 29, 29–37. [Google Scholar] [CrossRef]
- Serena, G.; Huynh, D.; Lima, R.S.; Vise, L.M.; Freire, R.; Ingano, L.; Leonard, M.M.; Senger, S.; Fasano, A. Intestinal Epithelium Modulates Macrophage Response to Gliadin in Celiac Disease. Front. Nutr. 2019, 6, 167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, K.E.; Sapone, A.; Fasano, A.; Vogel, S.N. Gliadin stimulation of murine macrophage inflammatory gene expression and intestinal permeability are MyD88-dependent: Role of the innate immune response in Celiac disease. J. Immunol. 2006, 176, 2512–2521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abenavoli, L.; Dastoli, S.; Bennardo, L.; Boccuto, L.; Passante, M.; Silvestri, M.; Proietti, I.; Potenza, C.; Luzza, F.; Nistico, S.P. The Skin in Celiac Disease Patients: The Other Side of the Coin. Medicina 2019, 55, 578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ross, E.A.; Devitt, A.; Johnson, J.R. Macrophages: The Good, the Bad, and the Gluttony. Front. Immunol. 2021, 12, 708186. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lu, Q.; Gao, W.; Yu, S. Recent advancement on development of drug-induced macrophage polarization in control of human diseases. Life Sci. 2021, 284, 119914. [Google Scholar] [CrossRef]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef]
- Yunna, C.; Mengru, H.; Lei, W.; Weidong, C. Macrophage M1/M2 polarization. Eur. J. Pharmacol. 2020, 877, 173090. [Google Scholar] [CrossRef]
- Tateyama, M.; Fujihara, K.; Misu, T.; Itoyama, Y. CCR7+ myeloid dendritic cells together with CCR7+ T cells and CCR7+ macrophages invade CCL19+ nonnecrotic muscle fibers in inclusion body myositis. J. Neurol. Sci. 2009, 279, 47–52. [Google Scholar] [CrossRef]
- Kong, W.N.; Zhao, S.E.; Duan, X.L.; Yang, Z.; Qian, Z.M.; Chang, Y.Z. Decreased DMT1 and increased ferroportin 1 expression is the mechanisms of reduced iron retention in macrophages by erythropoietin in rats. J. Cell. Biochem. 2008, 104, 629–641. [Google Scholar] [CrossRef]
- Feng, Q.; Xu, M.; Yu, Y.Y.; Hou, Y.; Mi, X.; Sun, Y.X.; Ma, S.; Zuo, X.Y.; Shao, L.L.; Hou, M.; et al. High-dose dexamethasone or all-trans-retinoic acid restores the balance of macrophages towards M2 in immune thrombocytopenia. J. Thromb. Haemost. JTH 2017, 15, 1845–1858. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.J.; Gu, Y.; Wang, C.Z.; Jin, Y.; Wen, X.M.; Ma, J.C.; Tang, L.J.; Mao, Z.W.; Qian, J.; Lin, J. The M2 macrophage marker CD206: A novel prognostic indicator for acute myeloid leukemia. Oncoimmunology 2020, 9, 1683347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashimoto, R.; Kakigi, R.; Miyamoto, Y.; Nakamura, K.; Itoh, S.; Daida, H.; Okada, T.; Katoh, Y. JAK-STAT-dependent regulation of scavenger receptors in LPS-activated murine macrophages. Eur. J. Pharmacol. 2020, 871, 172940. [Google Scholar] [CrossRef] [PubMed]
- Brunn, A.; Mihelcic, M.; Carstov, M.; Hummel, L.; Geier, F.; Schmidt, A.; Saupe, L.; Utermohlen, O.; Deckert, M. IL-10, IL-4, and STAT6 promote an M2 milieu required for termination of P0(106-125)-induced murine experimental autoimmune neuritis. Am. J. Pathol. 2014, 184, 2627–2640. [Google Scholar] [CrossRef] [PubMed]
- Recalcati, S.; Cairo, G. Macrophages and Iron: A Special Relationship. Biomedicines 2021, 9, 1585. [Google Scholar] [CrossRef] [PubMed]
- Recalcati, S.; Locati, M.; Marini, A.; Santambrogio, P.; Zaninotto, F.; De Pizzol, M.; Zammataro, L.; Girelli, D.; Cairo, G. Differential regulation of iron homeostasis during human macrophage polarized activation. Eur. J. Immunol. 2010, 40, 824–835. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, E.; Ganz, T. Hepcidin-Ferroportin Interaction Controls Systemic Iron Homeostasis. Int. J. Mol. Sci. 2021, 22, 6493. [Google Scholar] [CrossRef] [PubMed]
- Atri, C.; Guerfali, F.Z.; Laouini, D. Role of Human Macrophage Polarization in Inflammation during Infectious Diseases. Int. J. Mol. Sci. 2018, 19, 1801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turcotte, C.; Blanchet, M.R.; Laviolette, M.; Flamand, N. The CB2 receptor and its role as a regulator of inflammation. Cell. Mol. Life Sci. CMLS 2016, 73, 4449–4470. [Google Scholar] [CrossRef] [Green Version]
- Argenziano, M.; Tortora, C.; Bellini, G.; Di Paola, A.; Punzo, F.; Rossi, F. Correction: Argenziano, M., et al. The Endocannabinoid System in Pediatric Inflammatory and Immune Diseases. Int. J. Mol. Sci. 2019, 20, 5875. Int. J. Mol. Sci. 2020, 21, 2757. [Google Scholar] [CrossRef] [Green Version]
- Hryhorowicz, S.; Kaczmarek-Rys, M.; Zielinska, A.; Scott, R.J.; Slomski, R.; Plawski, A. Endocannabinoid System as a Promising Therapeutic Target in Inflammatory Bowel Disease-A Systematic Review. Front. Immunol. 2021, 12, 790803. [Google Scholar] [CrossRef]
- Rossi, F.; Bellini, G.; Alisi, A.; Alterio, A.; Maione, S.; Perrone, L.; Locatelli, F.; Miraglia del Giudice, E.; Nobili, V. Cannabinoid receptor type 2 functional variant influences liver damage in children with non-alcoholic fatty liver disease. PLoS ONE 2012, 7, e42259. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Bernardo, M.E.; Bellini, G.; Luongo, L.; Conforti, A.; Manzo, I.; Guida, F.; Cristino, L.; Imperatore, R.; Petrosino, S.; et al. The cannabinoid receptor type 2 as mediator of mesenchymal stromal cell immunosuppressive properties. PLoS ONE 2013, 8, e80022. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Tortora, C.; Palumbo, G.; Punzo, F.; Argenziano, M.; Casale, M.; Di Paola, A.; Locatelli, F.; Perrotta, S. CB2 Receptor Stimulation and Dexamethasone Restore the Anti-Inflammatory and Immune-Regulatory Properties of Mesenchymal Stromal Cells of Children with Immune Thrombocytopenia. Int. J. Mol. Sci. 2019, 20, 1049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maurer, M.; Gresnigt, M.S.; Last, A.; Wollny, T.; Berlinghof, F.; Pospich, R.; Cseresnyes, Z.; Medyukhina, A.; Graf, K.; Groger, M.; et al. A three-dimensional immunocompetent intestine-on-chip model as in vitro platform for functional and microbial interaction studies. Biomaterials 2019, 220, 119396. [Google Scholar] [CrossRef]
- Husby, S.; Koletzko, S.; Korponay-Szabo, I.; Kurppa, K.; Mearin, M.L.; Ribes-Koninckx, C.; Shamir, R.; Troncone, R.; Auricchio, R.; Castillejo, G.; et al. European Society Paediatric Gastroenterology, Hepatology and Nutrition Guidelines for Diagnosing Coeliac Disease 2020. J. Pediatric Gastroenterol. Nutr. 2020, 70, 141–156. [Google Scholar] [CrossRef] [Green Version]
- Strisciuglio, C.; Duijvestein, M.; Verhaar, A.P.; Vos, A.C.; van den Brink, G.R.; Hommes, D.W.; Wildenberg, M.E. Impaired autophagy leads to abnormal dendritic cell-epithelial cell interactions. J. Crohn’s Colitis 2013, 7, 534–541. [Google Scholar] [CrossRef] [Green Version]
- Varga, E.; Pap, R.; Janosa, G.; Sipos, K.; Pandur, E. IL-6 Regulates Hepcidin Expression Via the BMP/SMAD Pathway by Altering BMP6, TMPRSS6 and TfR2 Expressions at Normal and Inflammatory Conditions in BV2 Microglia. Neurochem. Res. 2021, 46, 1224–1238. [Google Scholar] [CrossRef]
- Li, L.; Luo, Q.; Shang, B.; Yang, X.; Zhang, Y.; Pan, Q.; Wu, N.; Tang, W.; Du, D.; Sun, X.; et al. Selective activation of cannabinoid receptor-2 reduces white matter injury via PERK signaling in a rat model of traumatic brain injury. Exp. Neurol. 2022, 347, 113899. [Google Scholar] [CrossRef]
- Rzeczycki, P.; Rasner, C.; Lammlin, L.; Junginger, L.; Goldman, S.; Bergman, R.; Redding, S.; Knights, A.J.; Elliott, M.; Maerz, T. Cannabinoid receptor type 2 is upregulated in synovium following joint injury and mediates anti-inflammatory effects in synovial fibroblasts and macrophages. Osteoarthr. Cartil. 2021, 29, 1720–1731. [Google Scholar] [CrossRef]
- Han, C.; Sheng, Y.; Wang, J.; Zhou, X.; Li, W.; Zhang, C.; Guo, L.; Yang, Y. NOX4 promotes mucosal barrier injury in inflammatory bowel disease by mediating macrophages M1 polarization through ROS. Int. Immunopharmacol. 2022, 104, 108361. [Google Scholar] [CrossRef]
- Li, Y.; Tian, X.; Li, S.; Chang, L.; Sun, P.; Lu, Y.; Yu, X.; Chen, S.; Wu, Z.; Xu, Z.; et al. Total polysaccharides of adlay bran (Coix lachryma-jobi L.) improve TNF-alpha induced epithelial barrier dysfunction in Caco-2 cells via inhibition of the inflammatory response. Food Funct. 2019, 10, 2906–2913. [Google Scholar] [CrossRef] [PubMed]
- Pires, C.L.; Praca, C.; Martins, P.A.T.; Batista de Carvalho, A.L.M.; Ferreira, L.; Marques, M.P.M.; Moreno, M.J. Re-Use of Caco-2 Monolayers in Permeability Assays-Validation Regarding Cell Monolayer Integrity. Pharmaceutics 2021, 13, 1563. [Google Scholar] [CrossRef] [PubMed]
- Di Sabatino, A.; Ciccocioppo, R.; Cupelli, F.; Cinque, B.; Millimaggi, D.; Clarkson, M.M.; Paulli, M.; Cifone, M.G.; Corazza, G.R. Epithelium derived interleukin 15 regulates intraepithelial lymphocyte Th1 cytokine production, cytotoxicity, and survival in coeliac disease. Gut 2006, 55, 469–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maiuri, L.; Ciacci, C.; Auricchio, S.; Brown, V.; Quaratino, S.; Londei, M. Interleukin 15 mediates epithelial changes in celiac disease. Gastroenterology 2000, 119, 996–1006. [Google Scholar] [CrossRef] [PubMed]
- Mayassi, T.; Jabri, B. Human intraepithelial lymphocytes. Mucosal Immunol. 2018, 11, 1281–1289. [Google Scholar] [CrossRef] [PubMed]







| Clinical Characteristics | CTR | CD |
|---|---|---|
| Median age, years (mean ± SD) | 12 ± 5 | 10 ± 4 |
| Sex (Female/Male) | 5/5 | 6/4 |
| Sideremia (µg/dL) | 87.1 ± 33.9 | 78.4 ± 25.9 |
| Ferritin (ng/mL) | 31.63 ± 20 | 19.5 ± 10 |
| Transferrin (mg/dL) | 256.5 ± 14 | 292.5 ± 34.5 |
| C-Reactive Protein (mg/L) | 0.08 ± 0.18 | 0.08 ± 0.11 |
| Hemoglobin (g/dL) | 13.3 ± 0.99 | 12.9 ± 0.89 |
| Mean Corpuscular Volume fl | 79.7 ± 3.6 | 77.2 ± 6.6 |
| Transferrin Saturation Index (%) | 30.5 ± 10 | 18.4 ± 6.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tortora, C.; Di Paola, A.; Argenziano, M.; Creoli, M.; Marrapodi, M.M.; Cenni, S.; Tolone, C.; Rossi, F.; Strisciuglio, C. Effects of CB2 Receptor Modulation on Macrophage Polarization in Pediatric Celiac Disease. Biomedicines 2022, 10, 874. https://doi.org/10.3390/biomedicines10040874
Tortora C, Di Paola A, Argenziano M, Creoli M, Marrapodi MM, Cenni S, Tolone C, Rossi F, Strisciuglio C. Effects of CB2 Receptor Modulation on Macrophage Polarization in Pediatric Celiac Disease. Biomedicines. 2022; 10(4):874. https://doi.org/10.3390/biomedicines10040874
Chicago/Turabian StyleTortora, Chiara, Alessandra Di Paola, Maura Argenziano, Mara Creoli, Maria Maddalena Marrapodi, Sabrina Cenni, Carlo Tolone, Francesca Rossi, and Caterina Strisciuglio. 2022. "Effects of CB2 Receptor Modulation on Macrophage Polarization in Pediatric Celiac Disease" Biomedicines 10, no. 4: 874. https://doi.org/10.3390/biomedicines10040874