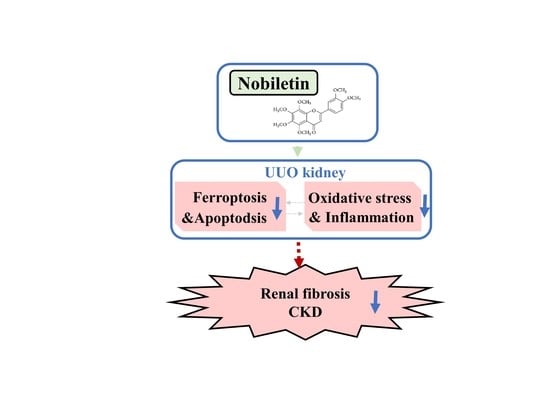
Nobiletin Alleviates Ferroptosis-Associated Renal Injury, Inflammation, and Fibrosis in a Unilateral Ureteral Obstruction Mouse Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Care, Nob Treatment, and UUO Surgical Administration
2.2. Histopathological Detection
2.3. Western Blotting Analysis
2.4. Immunohistological Staining
2.5. Fluorescent Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling (TUNEL) Staining
2.6. Real-Time Quantitative Polymerase Chain Reaction
2.7. Statistical Analysis
3. Results
3.1. Nob Treatment Ameliorates Pathological Changes and Renal Fibrosis in the Kidneys of UUO Mice
3.2. Administration of Nob Attenuates Oxidative Stress Injury, Ferroptosis, and Apoptotic Cell Death in the UUO Kidney
3.3. Administration of Nob Prevents Renal Apoptotic Cell Death in UUO Mice
3.4. Nob Treatment Mitigates Inflammatory Cells Infiltration in the Kidneys of UUO Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef] [Green Version]
- Carney, E.F. The impact of chronic kidney disease on global health. Nat. Rev. Nephrol. 2020, 16, 251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeisberg, M.; Neilson, E.G. Mechanisms of tubulointerstitial fibrosis. J. Am. Soc. Nephrol. 2010, 21, 1819–1834. [Google Scholar] [CrossRef] [Green Version]
- Andrade-Oliveira, V.; Foresto-Neto, O.; Watanabe, I.K.M.; Zatz, R.; Camara, N.O.S. Inflammation in Renal Diseases: New and Old Players. Front. Pharmacol. 2019, 10, 1192. [Google Scholar] [CrossRef]
- Branton, M.H.; Kopp, J.B. TGF-beta and fibrosis. Microbes Infect. 1999, 1, 1349–1365. [Google Scholar] [CrossRef]
- Dendooven, A.; Ishola, D.A., Jr.; Nguyen, T.Q.; Van der Giezen, D.M.; Kok, R.J.; Goldschmeding, R.; Joles, J.A. Oxidative stress in obstructive nephropathy. Int. J. Exp. Pathol. 2011, 92, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Mukhin, N.A.; Kozlovskaia, L.V.; Bobkova, I.N.; Rameev, V.V.; Chebotareva, N.B.; Plieva, O.K.; Shcherbak, A.V.; Varshavskii, V.A.; Golitsina, E.P. The key role of tubulointerstitium remodeling in progression of chronic renal diseases. Arch. Pathol. 2004, 66, 16–22. [Google Scholar]
- Nogueira, A.; Pires, M.J.; Oliveira, P.A. Pathophysiological Mechanisms of Renal Fibrosis: A Review of Animal Models and Therapeutic Strategies. In Vivo 2017, 31, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Meng, X.M.; Nikolic-Paterson, D.J.; Lan, H.Y. TGF-beta: The master regulator of fibrosis. Nat. Rev. Nephrol. 2016, 12, 325–338. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Wang, Y.; Sun, L. The Cross-Link between Ferroptosis and Kidney Diseases. Oxid. Med. Cell Longev. 2021, 2021, 6654887. [Google Scholar] [CrossRef]
- Belavgeni, A.; Meyer, C.; Stumpf, J.; Hugo, C.; Linkermann, A. Ferroptosis and Necroptosis in the Kidney. Cell Chem. Biol. 2020, 27, 448–462. [Google Scholar] [CrossRef] [PubMed]
- Borawski, B.; Malyszko, J. Iron, ferroptosis, and new insights for prevention in acute kidney injury. Adv. Med. Sci. 2020, 65, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Quan, F.; Cao, Q.; Lin, Y.; Yue, C.; Bi, R.; Cui, X.; Yang, H.; Yang, Y.; Birnbaumer, L.; et al. Quercetin alleviates acute kidney injury by inhibiting ferroptosis. J. Adv. Res. 2021, 28, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Carney, E.F. Ferroptotic stress promotes the AKI to CKD transition. Nat. Rev. Nephrol. 2021, 17, 633. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Li, L.; Shi, W.; Liu, H.; Yang, J.; Yuan, X.; Wu, L. The Multifunctional Effects of Nobiletin and Its Metabolites In Vivo and In Vitro. Evid. Based Complement Alternat. Med. 2016, 2016, 2918796. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Cheng, C.S.; Li, Q.; Chen, J.X.; Lv, L.L.; Xu, J.Y.; Zhang, K.Y.; Zheng, L. The Application of Citrus folium in Breast Cancer and the Mechanism of Its Main Component Nobiletin: A Systematic Review. Evid. Based Complement Alternat. Med. 2021, 2021, 2847466. [Google Scholar] [CrossRef]
- Braidy, N.; Behzad, S.; Habtemariam, S.; Ahmed, T.; Daglia, M.; Nabavi, S.M.; Sobarzo-Sanchez, E.; Nabavi, S.F. Neuroprotective Effects of Citrus Fruit-Derived Flavonoids, Nobiletin and Tangeretin in Alzheimer’s and Parkinson’s Disease. CNS Neurol. Disord. Drug Targets 2017, 16, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Gao, Z.; Zeng, L.; Liang, Z.; Zheng, D.; Wu, X. Nobiletin ameliorates cardiac impairment and alleviates cardiac remodeling after acute myocardial infarction in rats via JNK regulation. Pharmacol. Res. Perspect. 2021, 9, e00728. [Google Scholar] [CrossRef]
- Li, S.; Li, X.; Chen, F.; Liu, M.; Ning, L.; Yan, Y.; Shang, Z.; Huang, S.; Tu, C. Nobiletin mitigates hepatocytes death, liver inflammation, and fibrosis in a murine model of NASH through modulating hepatic oxidative stress and mitochondrial dysfunction. J. Nutr. Biochem. 2021, 100, 108888. [Google Scholar] [CrossRef]
- Martinez-Klimova, E.; Aparicio-Trejo, O.E.; Tapia, E.; Pedraza-Chaverri, J. Unilateral Ureteral Obstruction as a Model to Investigate Fibrosis-Attenuating Treatments. Biomolecules 2019, 9, 141. [Google Scholar] [CrossRef] [Green Version]
- Chiang, C.K.; Hsu, S.P.; Wu, C.T.; Huang, J.W.; Cheng, H.T.; Chang, Y.W.; Hung, K.Y.; Wu, K.D.; Liu, S.H. Endoplasmic reticulum stress implicated in the development of renal fibrosis. Mol. Med. 2011, 17, 1295–1305. [Google Scholar] [CrossRef] [PubMed]
- Higashi, K.; Oda, T.; Kushiyama, T.; Hyodo, T.; Yamada, M.; Suzuki, S.; Sakurai, Y.; Miura, S.; Kumagai, H. Additive antifibrotic effects of pioglitazone and candesartan on experimental renal fibrosis in mice. Nephrology 2010, 15, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.A.; Chen, C.M.; Guan, S.S.; Chiang, C.K.; Wu, C.T.; Liu, S.H. The antifibrotic and anti-inflammatory effects of icariin on the kidney in a unilateral ureteral obstruction mouse model. Phytomedicine 2019, 59, 152917. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.T.; Sheu, M.L.; Tsai, K.S.; Chiang, C.K.; Liu, S.H. Salubrinal, an eIF2alpha dephosphorylation inhibitor, enhances cisplatin-induced oxidative stress and nephrotoxicity in a mouse model. Free. Radic. Biol. Med. 2011, 51, 671–680. [Google Scholar] [CrossRef]
- Liu, S.H.; Wu, C.T.; Huang, K.H.; Wang, C.C.; Guan, S.S.; Chen, L.P.; Chiang, C.K. C/EBP homologous protein (CHOP) deficiency ameliorates renal fibrosis in unilateral ureteral obstructive kidney disease. Oncotarget 2016, 7, 21900–21912. [Google Scholar] [CrossRef] [Green Version]
- Chevalier, R.L.; Forbes, M.S.; Thornhill, B.A. Ureteral obstruction as a model of renal interstitial fibrosis and obstructive nephropathy. Kidney Int. 2009, 75, 1145–1152. [Google Scholar] [CrossRef] [Green Version]
- Allison, S.J. Fibrosis: Targeting EMT to reverse renal fibrosis. Nat. Rev. Nephrol. 2015, 11, 565. [Google Scholar] [CrossRef]
- Meran, S.; Steadman, R. Fibroblasts and myofibroblasts in renal fibrosis. Int. J. Clin. Exp. Pathol. 2011, 92, 158–167. [Google Scholar] [CrossRef]
- Hills, C.E.; Squires, P.E. TGF-beta1-induced epithelial-to-mesenchymal transition and therapeutic intervention in diabetic nephropathy. Am. J. Nephrol. 2010, 31, 68–74. [Google Scholar] [CrossRef] [Green Version]
- Docherty, N.G.; Calvo, I.F.; Quinlan, M.R.; Perez-Barriocanal, F.; McGuire, B.B.; Fitzpatrick, J.M.; Watson, R.W. Increased E-cadherin expression in the ligated kidney following unilateral ureteric obstruction. Kidney Int. 2009, 75, 205–213. [Google Scholar] [CrossRef] [Green Version]
- Kawada, N.; Moriyama, T.; Ando, A.; Fukunaga, M.; Miyata, T.; Kurokawa, K.; Imai, E.; Hori, M. Increased oxidative stress in mouse kidneys with unilateral ureteral obstruction. Kidney Int. 1999, 56, 1004–1013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Y.; Yan, Y.; Niu, F.; Wang, Y.; Chen, X.; Su, G.; Liu, Y.; Zhao, X.; Qian, L.; Liu, P.; et al. Ferroptosis: A cell death connecting oxidative stress, inflammation and cardiovascular diseases. Cell Death Discov. 2021, 7, 193. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Zhang, H.; Yang, S.K.; Wu, X.; He, D.; Cao, K.; Zhang, W. Emerging Role of Ferroptosis in Acute Kidney Injury. Oxid. Med. Cell Longev. 2019, 2019, 8010614. [Google Scholar] [CrossRef] [PubMed]
- Cebula, M.; Schmidt, E.E.; Arner, E.S. TrxR1 as a potent regulator of the Nrf2-Keap1 response system. Antioxid. Redox Signal. 2015, 23, 823–853. [Google Scholar] [CrossRef]
- Qiao, X.; Wang, L.; Wang, Y.; Su, X.; Qi, Y.; Fan, Y.; Peng, Z. Intermedin inhibits unilateral ureteral obstruction-induced oxidative stress via NADPH oxidase Nox4 and cAMP-dependent mechanisms. Ren. Fail. 2017, 39, 652–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Cao, F.; Yin, H.L.; Huang, Z.J.; Lin, Z.T.; Mao, N.; Sun, B.; Wang, G. Ferroptosis: Past, present and future. Cell Death Dis. 2020, 11, 88. [Google Scholar] [CrossRef]
- Lin, W.; Wang, C.; Liu, G.; Bi, C.; Wang, X.; Zhou, Q.; Jin, H. SLC7A11/xCT in cancer: Biological functions and therapeutic implications. Am. J. Cancer Res. 2020, 10, 3106–3126. [Google Scholar] [PubMed]
- Feng, H.; Schorpp, K.; Jin, J.; Yozwiak, C.E.; Hoffstrom, B.G.; Decker, A.M.; Rajbhandari, P.; Stokes, M.E.; Bender, H.G.; Csuka, J.M.; et al. Transferrin Receptor Is a Specific Ferroptosis Marker. Cell Rep. 2020, 30, 3411–3423.e3417. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.; Zhuang, Y.Y.; Wang, W.W.; Zhu, H.P.; Zhang, Y.J.; Zheng, S.L.; Yang, Y.R.; Chen, B.C.; Xia, P.; Zhang, Y. Role of Sirolimus in renal tubular apoptosis in response to unilateral ureteral obstruction. Int. J. Med. Sci. 2018, 15, 1433–1442. [Google Scholar] [CrossRef] [Green Version]
- Black, L.M.; Lever, J.M.; Agarwal, A. Renal Inflammation and Fibrosis: A Double-edged Sword. J. Histochem. Cytochem. 2019, 67, 663–681. [Google Scholar] [CrossRef] [Green Version]
- Boivin, G.; Faget, J.; Ancey, P.B.; Gkasti, A.; Mussard, J.; Engblom, C.; Pfirschke, C.; Contat, C.; Pascual, J.; Vazquez, J.; et al. Durable and controlled depletion of neutrophils in mice. Nat. Commun. 2020, 11, 2762. [Google Scholar] [CrossRef] [PubMed]
- Dos Anjos Cassado, A. F4/80 as a Major Macrophage Marker: The Case of the Peritoneum and Spleen. Results Probl. Cell Differ. 2017, 62, 161–179. [Google Scholar] [CrossRef] [PubMed]
- Eddy, A.A.; Lopez-Guisa, J.M.; Okamura, D.M.; Yamaguchi, I. Investigating mechanisms of chronic kidney disease in mouse models. Pediatr. Nephrol. 2012, 27, 1233–1247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.F. Ulinastatin inhibits renal tubular epithelial apoptosis and interstitial fibrosis in rats with unilateral ureteral obstruction. Mol. Med. Rep. 2017, 16, 8916–8922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, Y.; Zhang, F.; Wu, J.; Shao, C.; Gao, Y. Urinary candidate biomarker discovery in a rat unilateral ureteral obstruction model. Sci. Rep. 2015, 5, 9314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Zhao, R.; Wang, X.; Liu, F.; Zhao, J.; Yao, Q.; Zhi, W.; He, Z.; Niu, X. Nobiletin-Ameliorated Lipopolysaccharide-Induced Inflammation in Acute Lung Injury by Suppression of NF-kappaB Pathway In Vivo and Vitro. Inflammation 2018, 41, 996–1007. [Google Scholar] [CrossRef]
- Zhang, N.; Yang, Z.; Xiang, S.Z.; Jin, Y.G.; Wei, W.Y.; Bian, Z.Y.; Deng, W.; Tang, Q.Z. Nobiletin attenuates cardiac dysfunction, oxidative stress, and inflammatory in streptozotocin: Induced diabetic cardiomyopathy. Mol. Cell. Biochem. 2016, 417, 87–96. [Google Scholar] [CrossRef]
- Liu, B.; Deng, Q.; Zhang, L.; Zhu, W. Nobiletin alleviates ischemia/reperfusion injury in the kidney by activating PI3K/AKT pathway. Mol. Med. Rep. 2020, 22, 4655–4662. [Google Scholar] [CrossRef]
- Xu, M.; Wang, R.; Fan, H.; Ni, Z. Nobiletin ameliorates streptozotocin-cadmium-induced diabetic nephropathy via NF-kappaB signalling pathway in rats. Arch. Physiol. Biochem. 2021, 1–9. [Google Scholar] [CrossRef]
- Wang, S.W.; Lan, T.; Sheng, H.; Zheng, F.; Lei, M.K.; Wang, L.X.; Chen, H.F.; Xu, C.Y.; Zhang, F. Nobiletin Alleviates Non-alcoholic Steatohepatitis in MCD-Induced Mice by Regulating Macrophage Polarization. Front. Physiol. 2021, 12, 687744. [Google Scholar] [CrossRef]
- Guvenc, M.; Cellat, M.; Gokcek, I.; Ozkan, H.; Arkali, G.; Yakan, A.; Yurdagul Ozsoy, S.; Aksakal, M. Nobiletin attenuates acetaminophen-induced hepatorenal toxicity in rats. J. Biol. Mol. Toxicol. 2020, 34, e22427. [Google Scholar] [CrossRef]
- Ucero, A.C.; Benito-Martin, A.; Izquierdo, M.C.; Sanchez-Nino, M.D.; Sanz, A.B.; Ramos, A.M.; Berzal, S.; Ruiz-Ortega, M.; Egido, J.; Ortiz, A. Unilateral ureteral obstruction: Beyond obstruction. Int. Urol. Nephrol. 2014, 46, 765–776. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Y.; Xu, Y. Pyroptosis in Kidney Disease. J. Mol. Biol. 2021, 434, 167290. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Chen, X.; Ru, F.; Gan, Y.; Li, B.; Xia, W.; Dai, G.; He, Y.; Chen, Z. Liproxstatin-1 attenuates unilateral ureteral obstruction-induced renal fibrosis by inhibiting renal tubular epithelial cells ferroptosis. Cell Death Dis. 2021, 12, 843. [Google Scholar] [CrossRef]
- Kajarabille, N.; Latunde-Dada, G.O. Programmed Cell-Death by Ferroptosis: Antioxidants as Mitigators. Int. J. Mol. Sci. 2019, 20, 4968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, Y.Y.; Yan, J.Y.; Xia, X.; Liang, J.Q.; Li, F.; Xie, T.F.; Luo, W.S.; Feng, J.F. Effect and mechanism of total flavonoids of Lichi Semen on CCl_4-induced liver fibrosis in rats, and prediction of Q-marker. Zhongguo Zhong Yao Za Zhi 2020, 45, 5722–5731. [Google Scholar] [CrossRef] [PubMed]
- Bunbupha, S.; Apaijit, K.; Maneesai, P.; Prasarttong, P.; Pakdeechote, P. Nobiletin ameliorates high-fat diet-induced vascular and renal changes by reducing inflammation with modulating AdipoR1 and TGF-beta1 expression in rats. Life Sci. 2020, 260, 118398. [Google Scholar] [CrossRef]
- Da, C.; Liu, Y.; Zhan, Y.; Liu, K.; Wang, R. Nobiletin inhibits epithelial-mesenchymal transition of human non-small cell lung cancer cells by antagonizing the TGF-beta1/Smad3 signaling pathway. Oncol. Rep. 2016, 35, 2767–2774. [Google Scholar] [CrossRef] [Green Version]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lo, Y.-H.; Yang, S.-F.; Cheng, C.-C.; Hsu, K.-C.; Chen, Y.-S.; Chen, Y.-Y.; Wang, C.-W.; Guan, S.-S.; Wu, C.-T. Nobiletin Alleviates Ferroptosis-Associated Renal Injury, Inflammation, and Fibrosis in a Unilateral Ureteral Obstruction Mouse Model. Biomedicines 2022, 10, 595. https://doi.org/10.3390/biomedicines10030595
Lo Y-H, Yang S-F, Cheng C-C, Hsu K-C, Chen Y-S, Chen Y-Y, Wang C-W, Guan S-S, Wu C-T. Nobiletin Alleviates Ferroptosis-Associated Renal Injury, Inflammation, and Fibrosis in a Unilateral Ureteral Obstruction Mouse Model. Biomedicines. 2022; 10(3):595. https://doi.org/10.3390/biomedicines10030595
Chicago/Turabian StyleLo, Yi-Hsin, Shun-Fa Yang, Ching-Chang Cheng, Kuo-Chiang Hsu, Yu-Syuan Chen, Yu-Ya Chen, Chun-Wei Wang, Siao-Syun Guan, and Cheng-Tien Wu. 2022. "Nobiletin Alleviates Ferroptosis-Associated Renal Injury, Inflammation, and Fibrosis in a Unilateral Ureteral Obstruction Mouse Model" Biomedicines 10, no. 3: 595. https://doi.org/10.3390/biomedicines10030595