Arsenic Trioxide Triggers Apoptosis of Metastatic Oral Squamous Cells Carcinoma with Concomitant Downregulation of GLI1 in Hedgehog Signaling
Abstract
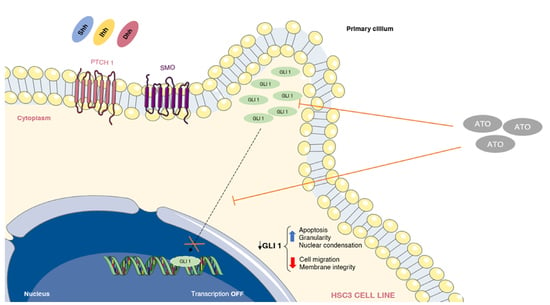
:1. Introduction
2. Materials and Methods
2.1. Cell Culturing and Characterization
2.2. Arsenic Trioxide Assessment and Treatment Protocol
2.3. Membrane Integrity Assessment Assay Using Trypan Blue
2.4. Cell Cycle and Internucleosomal DNA Fragmentation
2.5. Cell Death, Viability, and Morphology Evaluations
2.6. Western Blot
2.7. Immunofluorescence
2.8. Gene Expression of HH Pathway Components
2.9. Statistical Analysis
3. Results
3.1. ATO Exerts Cytotoxic Effects in a Metastatic Lineage of OSCC
3.2. ATO Reduces Membrane Integrity of HSC3 Cells
3.3. ATO Promotes Cell Death by Apoptosis through Significantly Increased Nuclear Fragmentation
3.4. ATO Promotes Cell Shrinkage, Increases Granularity and Nuclear Condensation in Metastatic OSCC
3.5. Reduction of GLI Protein and mRNA Expression after ATO Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- D’Souza, S.; Addepalli, V. Preventive Measures in Oral Cancer: An Overview. Biomed. Pharmacother. 2018, 107, 72–80. [Google Scholar] [CrossRef]
- Gobbo, M.; Bullo, F.; Perinetti, G.; Gatto, A.; Ottaviani, G.; Biasotto, M.; Tirelli, G. Diagnostic and therapeutic features associated with modification of quality-of-life’s outcomes between one and six months after major surgery for head and neck cancer. Braz. J. Otorhinolaryngol. 2016, 82, 548–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamat, M.; Rai, B.D.; Puranik, R.S.; Datar, U.V. A comprehensive review of surgical margin in oral squamous cell carcinoma highlighting the significance of tumor-free surgical margins. J. Cancer Res. Ther. 2019, 15, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Sigafoos, A.N.; Paradise, B.D.; Fernandez-Zapico, M.E. Hedgehog/GLI Signaling Pathway: Transduction, Regulation, and Implications for Disease. Cancers 2021, 13, 3410. [Google Scholar] [CrossRef] [PubMed]
- Dusek, C.O.; Hadden, M.K. Targeting the GLI family of transcription factors for the development of anti-cancer drugs. Expert Opin. Drug Discov. 2021, 16, 289–302. [Google Scholar] [CrossRef] [PubMed]
- Takabatake, K.; Shimo, T.; Murakami, J.; Anqi, C.; Kawai, H.; Yoshida, S.; Wathone Oo, M.; Haruka, O.; Sukegawa, S.; Tsujigiwa, H.; et al. The Role of Sonic Hedgehog Signaling in the Tumor Microenvironment of Oral Squamous Cell Carcinoma. Int. J. Mol. Sci. 2019, 20, 5779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doheny, D.; Manore, S.G.; Wong, G.L.; Lo, H.W. Hedgehog Signaling and Truncated GLI1 in Cancer. Cells 2020, 9, 2114. [Google Scholar] [CrossRef] [PubMed]
- Hui, M.; Cazet, A.; Nair, R.; Watkins, D.N.; O’Toole, S.A.; Swarbrick, A. The Hedgehog signalling pathway in breast development, carcinogenesis and cancer therapy. Breast Cancer Res. 2013, 15, 203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deep, G.; Panigrahi, G.K. Hypoxia-Induced Signaling Promotes Prostate Cancer Progression: Exosomes Role as Messenger of Hypoxic Response in Tumor Microenvironment. Crit. Rev. Oncog. 2015, 20, 419–434. [Google Scholar] [CrossRef] [Green Version]
- Mastrangelo, E.; Milani, M. Role and inhibition of GLI1 protein in cancer. Lung Cancer 2018, 9, 35–43. [Google Scholar] [CrossRef]
- Avery, J.T.; Zhang, R.; Boohaker, R.J. GLI1: A Therapeutic Target for Cancer. Front. Oncol. 2021, 25, 673154. [Google Scholar] [CrossRef] [PubMed]
- Bacelar Sacramento de Araújo, T.; de Oliveira Siquara da Rocha, L.; Torres Andion Vidal, M.; Cerqueira Coelho, P.L.; Galvão Dos Reis, M.; Solano de Freitas Souza, B.; Botelho Pereira Soares, M.; Almeida Pereira, T.; Della Coletta, R.; Pereira Bezerra, D.; et al. GANT61 Reduces Hedgehog Molecule (GLI1) Expression and Promotes Apoptosis in Metastatic Oral Squamous Cell Carcinoma Cells. Int. J. Mol. Sci. 2020, 21, 6076. [Google Scholar] [CrossRef] [PubMed]
- Leung, L.L.; Lam, S.K.; Li, Y.Y.; Ho, J.C. Tumour growth-suppressive effect of arsenic trioxide in squamous cell lung carcinoma. Oncol. Lett. 2017, 14, 3748–3754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Wang, L.; Yin, C.; An, B.; Hao, Y.; Wei, T.; Li, L.; Song, G. Arsenic trioxide inhibits breast cancer cell growth via microRNA-328/hERG pathway in MCF-7 cells. Mol. Med. Rep. 2015, 12, 1233–1238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, S.; Nagano, S.; Nagao, H.; Ishidou, Y.; Yokouchi, M.; Abematsu, M.; Yamamoto, T.; Komiya, S.; Setoguchi, T. Arsenic trioxide prevents osteosarcoma growth by inhibition of GLI transcription via DNA damage accumulation. PLoS ONE 2013, 8, e69466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Wang, R.; Fan, L.; Liang, W.; Liang, K.; Xu, Y.; Peng, G.; Ye, Q. Arsenic trioxide is an immune adjuvant in liver cancer treatment. Mol. Immunol. 2017, 81, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.J.; Yin, J.Z.; Huang, H.; Li, B.; Yang, M.H. Arsenic trioxide inhibits the growth of cancer stem cells derived from small cell lung cancer by downregulating stem cell-maintenance factors and inducing apoptosis via the Hedgehog signaling blockade. Transl. Lung Cancer Res. 2020, 9, 1379–1396. [Google Scholar] [CrossRef] [PubMed]
- Mäkelä, M.; Salo, T.; Larjava, H. MMP-9 from TNF alpha-stimulated keratinocytes binds to cell membranes and type I collagen: A cause for extended matrix degradation in inflammation? Biochem. Biophys. Res. Commun. 1998, 253, 325–335. [Google Scholar] [CrossRef]
- Sobral, L.M.; Bufalino, A.; Lopes, M.A.; Graner, E.; Salo, T.; Coletta, R.D. Myofibroblasts in the stroma of oral cancer promote tumorigenesis via secretion of activin A. Oral Oncol. 2011, 47, 840–846. [Google Scholar] [CrossRef] [Green Version]
- Eble, J.A.; Niland, S. The extracellular matrix in tumor progression and metastasis. Clin. Exp. Metastasis 2019, 36, 171–198. [Google Scholar] [CrossRef] [PubMed]
- Najafi, M.; Farhood, B.; Mortezaee, K. Extracellular matrix (ECM) stiffness and degradation as cancer drivers. J. Cell. Biochem. 2019, 120, 2782–2790. [Google Scholar] [CrossRef] [PubMed]
- Dourado, M.R.; Korvala, J.; Åström, P.; De Oliveira, C.E.; Cervigne, N.K.; Mofatto, L.S.; Campanella Bastos, D.; Pereira Messetti, A.C.; Graner, E.; Paes Leme, A.F.; et al. Extracellular vesicles derived from cancer-associated fibroblasts induce the migration and invasion of oral squamous cell carcinoma. J. Extracell. Vesicles 2019, 8, 1578525. [Google Scholar] [CrossRef] [Green Version]
- Nakaoka, T.; Ota, A.; Ono, T.; Karnan, S.; Konishi, H.; Furuhashi, A.; Ohmura, Y.; Yamada, Y.; Hosokawa, Y.; Kazaoka, Y. Combined arsenic trioxide-cisplatin treatment enhances apoptosis in oral squamous cell carcinoma cells. Cell. Oncol. 2014, 37, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Wang, G.; Wu, J.; Zuo, Y.; Zhang, J.; Jin, X. Skp2 Expression Is Inhibited by Arsenic Trioxide through the Upregulation of miRNA-330-5p in Pancreatic Cancer Cells. Mol. Ther. Oncolytics 2019, 12, 214–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Y.; Cao, T.; Huang, H.; Lian, C.; Yang, Y.; Wang, Z.; Ma, J.; Xia, J. Arsenic trioxide inhibits cell growth and motility via up-regulation of let-7a in breast cancer cells. Cell Cycle 2017, 16, 2396–2403. [Google Scholar] [CrossRef] [Green Version]
- Kotowski, U.; Heiduschka, G.; Brunner, M.; Erovic, B.M.; Martinek, H.; Thurnher, D. Arsenic trioxide enhances the cytotoxic effect of cisplatin in head and neck squamous cell carcinoma cell lines. Oncol. Lett. 2012, 3, 1326–1330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medda, N.; De, S.K.; Maiti, S. Different mechanisms of arsenic related signaling in cellular proliferation, apoptosis and neo-plastic transformation. Ecotoxicol. Environ. Saf. 2021, 208, 111752. [Google Scholar] [CrossRef] [PubMed]
- Linder, B.; Wehle, A.; Hehlgans, S.; Bonn, F.; Dikic, I.; Rödel, F.; Seifert, V.; Kögel, D. Arsenic Trioxide and (-)-Gossypol Synergistically Target Glioma Stem-Like Cells via Inhibition of Hedgehog and Notch Signaling. Cancers 2019, 11, 350. [Google Scholar] [CrossRef] [Green Version]
- Cai, X.; Yu, K.; Zhang, L.; Li, Y.; Li, Q.; Yang, Z.; Shen, T.; Duan, L.; Xiong, W.; Wang, W. Synergistic inhibition of colon carcinoma cell growth by Hedgehog-Gli1 inhibitor arsenic trioxide and phosphoinositide 3-kinase inhibitor LY294002. OncoTargets Ther. 2015, 8, 877–883. [Google Scholar]
- Boehme, K.A.; Zaborski, J.J.; Riester, R.; Schweiss, S.K.; Hopp, U.; Traub, F.; Kluba, T.; Handgretinger, R.; Schleicher, S.B. Targeting hedgehog signalling by arsenic trioxide reduces cell growth and induces apoptosis in rhabdomyosarcoma. Int. J. Oncol. 2016, 48, 801–812. [Google Scholar] [CrossRef] [Green Version]
- Wu, B.; Tan, M.; Cai, W.; Wang, B.; He, P.; Zhang, X. Arsenic trioxide induces autophagic cell death in osteosarcoma cells via the ROS-TFEB signaling pathway. Biochem. Biophys. Res. Commun. 2018, 496, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Sanchez, M.; Menzies, F.M.; Chang, Y.Y.; Simecek, N.; Neufeld, T.P.; Rubinsztein, D.C. The Hedgehog signalling pathway regulates autophagy. Nat. Commun. 2012, 3, 1200. [Google Scholar] [CrossRef] [PubMed]







| Arsenic Trioxide | 5-FU | |
|---|---|---|
| Tumor cells | ||
| CAL 27 | >126.4 | 30.8 |
| HSC 3 | 37.4 | 16.75 |
| SCC 4 | >126.4 | 0.07 |
| Cancer-associated fibroblasts | ||
| CAF 1 | 0.28 | >192 |
| CAF 2 | >126.4 | >192 |
| Non-tumor cells | ||
| HGK | >126.4 | >192 |
| NOF | >126.4 | 39.59 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nogueira, R.L.R.; de Araújo, T.B.S.; Valverde, L.F.; Silva, V.A.O.; Cavalcante, B.R.R.; Rossi, E.A.; Allahdadi, K.J.; dos Reis, M.G.; Pereira, T.A.; Coletta, R.D.; et al. Arsenic Trioxide Triggers Apoptosis of Metastatic Oral Squamous Cells Carcinoma with Concomitant Downregulation of GLI1 in Hedgehog Signaling. Biomedicines 2022, 10, 3293. https://doi.org/10.3390/biomedicines10123293
Nogueira RLR, de Araújo TBS, Valverde LF, Silva VAO, Cavalcante BRR, Rossi EA, Allahdadi KJ, dos Reis MG, Pereira TA, Coletta RD, et al. Arsenic Trioxide Triggers Apoptosis of Metastatic Oral Squamous Cells Carcinoma with Concomitant Downregulation of GLI1 in Hedgehog Signaling. Biomedicines. 2022; 10(12):3293. https://doi.org/10.3390/biomedicines10123293
Chicago/Turabian StyleNogueira, Raphael Luís Rocha, Taís Bacelar Sacramento de Araújo, Ludmila Faro Valverde, Viviane Aline Oliveira Silva, Bruno Raphael Ribeiro Cavalcante, Erik Aranha Rossi, Kyan James Allahdadi, Mitermayer Galvão dos Reis, Thiago Almeida Pereira, Ricardo D. Coletta, and et al. 2022. "Arsenic Trioxide Triggers Apoptosis of Metastatic Oral Squamous Cells Carcinoma with Concomitant Downregulation of GLI1 in Hedgehog Signaling" Biomedicines 10, no. 12: 3293. https://doi.org/10.3390/biomedicines10123293