How Does Changing Environment Influence Plant Seed Movements as Populations of Dispersal Vectors Decline?
Abstract
:1. Introduction
2. Results
3. Discussion
3.1. Geographical Distribution of Studies on Seed Dispersal in a Changing Environment
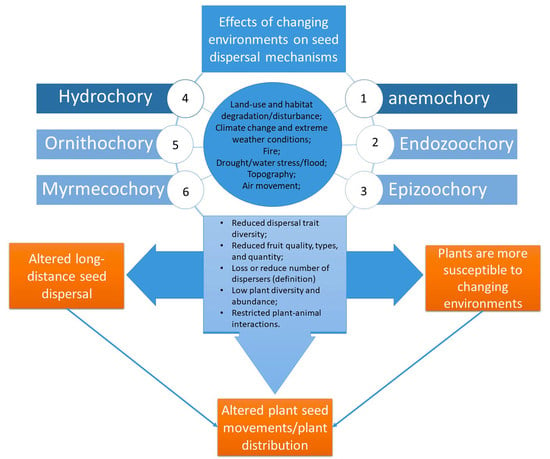
3.2. How Does Changing Environment Affect Seed Dispersal?
3.3. Will the Changing Environment Reduce or Enhance Plants’ Dispersal Abilities?
3.4. Do Fruit Traits Control Long-Distance Seed Dispersal in a Changing Environment?
3.5. Future Perspectives
4. Methodology
4.1. Literature Search and Data Sources
4.2. Article Screening and Appraisal
4.3. Data Extraction, Categorization, and Analysis
4.4. Scope and Limitations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pecl, G.T.; Araújo, M.B.; Bell, J.D.; Blanchard, J.; Bonebrake, T.C.; Chen, I.-C.; Clark, T.D.; Colwell, R.K.; Danielsen, F.; Evengård, B.; et al. Biodiversity redistribution under climate change: Impacts on ecosystems and human well-being. Science 2017, 355, 6332. [Google Scholar] [CrossRef] [PubMed]
- Lenoir, J.; Gégout, J.C.; Marquet, P.A.; de Ruffray, P.; Brisse, H. A significant upward shift in plant species optimum elevation during the 20th century. Science 2008, 320, 1768–1771. [Google Scholar] [CrossRef] [PubMed]
- Lenoir, J.; Bertrand, R.; Comte, L.; Bourgeaud, L.; Hattab, T.; Murienne, J.; Grenouillet, G. Species better track climate warming in the oceans than on land. Nat. Ecol. Evol. 2020, 4, 1044–1059. [Google Scholar] [CrossRef] [PubMed]
- Nathan, R.; Muller-Landau, H.C. Spatial patterns of seed dispersal, their determinants and consequences for recruitment. Trends Ecol. Evol. 2000, 15, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Treep, J.; de Jager, M.; Bartumeus, F.; Soons, M.B. Seed dispersal as a search strategy: Dynamic and fragmented landscapes select for multi-scale movement strategies in plants. Mov. Ecol. 2021, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Donoso, I.; Fricke, E.C.; Hervías-Parejo, S.; Rogers, H.S.; Traveset, A. Drivers of ecological and evolutionary disruptions in the Seed Dispersal Process: Research trends and Biases. Front. Ecol. Evol. 2022, 10, 794481. [Google Scholar] [CrossRef]
- San-José, M.; Arroyo-Rodríguez, V.; Jordano, P.; Meave, J.A.; Martínez-Ramos, M. The scale of landscape effect on seed dispersal depends on both response variables and landscape predictor. Landsc. Ecol. 2019, 34, 1069–1080. [Google Scholar] [CrossRef]
- Traveset, A.; Rodríguez-Pérez, J. Seed dispersal. Encycl. Ecol. 2008, 3188–3194. [Google Scholar] [CrossRef]
- Rojas, T.N.; Zampini, I.C.; Isla, M.I.; Blendinger, P.G. Fleshy fruit traits and seed dispersers: Which traits define syndromes? Ann. Bot. 2021, 129, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Maciel, E.A.; Martins, V.F.; de Paula, M.D.; Huth, A.; Guilherme, F.A.G.; Fischer, R.; Giles, A.; Barbosa, R.I.; Cavassan, O.; Martins, F.R. Defaunation and changes in climate and fire frequency have synergistic effects on aboveground biomass loss in the Brazilian Savanna. Ecol. Model. 2021, 454, 109628. [Google Scholar] [CrossRef]
- Smith, J.R.; Bagchi, R.; Ellens, J.; Kettle, C.J.; Burslem, D.F.; Maycock, C.R.; Khoo, E.; Ghazoul, J. Predicting dispersal of auto-gyrating fruit in tropical trees: A case study from the Dipterocarpaceae. Ecol. Evol. 2015, 5, 1794–1801. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.-B.; Shen-Tu, X.-L.; Dong, M. Intraspecific variation of Samara dispersal traits in the endangered tropical tree Hopea hainanensis (Dipterocarpaceae). Front. Plant Sci. 2020, 11, 599764. [Google Scholar] [CrossRef] [PubMed]
- Valdesolo, T.; Del Vecchio, S.; Buffa, G. Patterns of seed dispersal in coastal dune plant communities. Sustainability 2022, 14, 10983. [Google Scholar] [CrossRef]
- Li, J.; Charles, L.S.; Yang, Z.; Du, G.; Fu, S.J.F.i.P.S. Differential mechanisms drive species loss under artificial shade and fertilization in the alpine meadow of the Tibetan Plateau. Front. Plant Sci. 2022, 13, 63. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.S.; Cantrell, R.S.; Cosner, C.; Hartig, F.; Hastings, A.; Rogers, H.S.; Schupp, E.W.; Shea, K.; Teller, B.J.; Yu, X.; et al. Rapid changes in seed dispersal traits may modify plant responses to Global Change. AoB PLANTS 2019, 11, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lei, B.; Cui, J.; Newman, C.; Buesching, C.D.; Xie, Z.; Macdonald, D.W.; Zhou, Y. Seed dispersers shape the pulp nutrients of fleshy-fruited plants. Proc. R. Soc. B Biol. Sci. 2021, 288, 20210817. [Google Scholar] [CrossRef]
- Janson, C.H. Adaptation of fruit morphology to dispersal agents in a Neotropical forest. Science 1983, 219, 187–189. [Google Scholar] [CrossRef]
- Yu, S.; Katz, O.; Fang, W.; Li, D.; Sang, W.; Liu, C. Shift of fleshy fruited species along elevation: Temperature, canopy coverage, phylogeny and origin. Sci. Rep. 2017, 7, 40417. [Google Scholar] [CrossRef] [Green Version]
- Contreras-Calderón, J.; Calderón-Jaimes, L.; Guerra-Hernández, E.; García-Villanova, B. Antioxidant capacity, phenolic content and vitamin C in pulp, Peel and seed from 24 exotic fruits from Colombia. Food Res. Int. 2011, 44, 2047–2053. [Google Scholar] [CrossRef]
- Howe, H.F.; Estabrook, G.F. On intraspecific competition for avian dispersers in tropical trees. Am. Nat. 1977, 111, 817–832. [Google Scholar] [CrossRef]
- Gómez, M.D.; Vera-Sirera, F.; Pérez-Amador, M.A. Molecular programme of senescence in dry and fleshy fruits. J. Exp. Bot. 2013, 65, 4515–4526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Godwin, J.; Raviv, B.; Grafi, G. Dead pericarps of dry fruits function as long-term storage for active hydrolytic enzymes and other substances that affect germination and microbial growth. Plants 2017, 6, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammed, S.; Bhattacharya, S.; Gesing, M.A.; Klupsch, K.; Theißen, G.; Mummenhoff, K.; Müller, C. Morphologically and physiologically diverse fruits of two lepidium species differ in allocation of glucosinolates into immature and mature seed and pericarp. PLoS ONE 2020, 15, 0227528. [Google Scholar] [CrossRef] [PubMed]
- Damschen, E.I.; Baker, D.V.; Bohrer, G.; Nathan, R.; Orrock, J.L.; Turner, J.R.; Brudvig, L.A.; Haddad, N.M.; Levey, D.J.; Tewksbury, J.J. How fragmentation and corridors affect wind dynamics and seed dispersal in open habitats. Proc. Natl. Acad. Sci. USA 2014, 111, 3484–3489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renton, M.; Shackelford, N.; Standish, R.J. Habitat restoration will help some functional plant types persist under climate change in fragmented landscapes. Glob. Change Biol. 2012, 18, 2057–2070. [Google Scholar] [CrossRef]
- Rogers, H.S.; Cavazos, B.R.; Gawel, A.M.; Karnish, A.; Ray, C.A.; Rose, E.; Thierry, H.; Fricke, E.C. Frugivore gut passage increases seed germination: An updated meta-analysis. bioRxiv 2021. [Google Scholar] [CrossRef]
- Willson, M.F.; Whelan, C.J. Variation in postdispersal survival of vertebrate-dispersed seeds: Effects of density, habitat, location, season, and species. Oikos 1990, 57, 191. [Google Scholar] [CrossRef]
- Pan, C.; Yang, K.; Erhunmwunsee, F.; Li, Y.-X.; Liu, M.; Pan, S.; Yang, D.; Lu, G.; Ma, D.; Tian, J.J.F.C. Inhibitory effect of cinnamaldehyde on Fusarium solani and its application in postharvest preservation of sweet potato. Food Chem. 2023, 408, 135213. [Google Scholar] [CrossRef]
- Liu, J.; Slik, F.; Coomes, D.A.; Corlett, R.T.; Wang, Y.; Wilson, M.; Hu, G.; Ding, P.; Yu, M. The distribution of plants and seed dispersers in response to habitat fragmentation in an artificial island archipelago. J. Biogeogr. 2019, 46, 1152–1162. [Google Scholar] [CrossRef]
- Brodie, J.F.; Helmy, O.E.; Brockelman, W.Y.; Maron, J.L. Bushmeat poaching reduces the seed dispersal and population growth rate of a mammal-dispersed tree. Ecol. Appl. 2009, 19, 854–863. [Google Scholar] [CrossRef]
- Donoso, I.; Schleuning, M.; García, D.; Fründ, J. Defaunation effects on plant recruitment depend on size matching and size trade-offs in seed-dispersal networks. Proc. R. Soc. B Biol. Sci. 2017, 284, 20162664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Redford, K.H. The empty forest. BioScience 1992, 42, 412–422. [Google Scholar] [CrossRef]
- Hamaoui-Laguel, L.; Vautard, R.; Liu, L.; Solmon, F.; Viovy, N.; Khvorostyanov, D.; Essl, F.; Chuine, I.; Colette, A.; Semenov, M.A.; et al. Effects of climate change and seed dispersal on airborne ragweed pollen loads in Europe. Nat. Clim. Change 2015, 5, 766–771. [Google Scholar] [CrossRef]
- Koo, K.A.; Uk Park, S. The effect of interplays among climate change, land-use change, and dispersal capacity on plant redistribution. Ecol. Indic. 2022, 142, 109192. [Google Scholar] [CrossRef]
- Li, N.; Tang, N.; Ren, Y.; Wang, Z. Effects of forest ropeway construction on bird diversity and its seed dispersal mutualism for endangered Taxus chinensis, Southeast China. Glob. Ecol. Conserv. 2022, 38, e02227. [Google Scholar] [CrossRef]
- Nowak, L.; Schleuning, M.; Bender, I.M.; Böhning-Gaese, K.; Dehling, D.M.; Fritz, S.A.; Kissling, W.D.; Mueller, T.; Neuschulz, E.L.; Pigot, A.L.; et al. Avian seed dispersal may be insufficient for plants to track future temperature change on Tropical Mountains. Glob. Ecol. Biogeogr. 2022, 31, 848–860. [Google Scholar] [CrossRef]
- Johnson, A.L.; Borowy, D.; Swan, C.M. Land use history and seed dispersal drive divergent plant community assembly patterns in urban vacant lots. J. Appl. Ecol. 2017, 55, 451–460. [Google Scholar] [CrossRef] [Green Version]
- Effects of fragmentation on grassland plant diversity depend on the habitat specialization of species. Biol. Conserv. 2022, 275, 109773. [CrossRef]
- Neuenkamp, L.; Lewis, R.J.; Koorem, K.; Zobel, K.; Zobel, M. Changes in dispersal and light capturing traits explain post-abandonment community change in semi-natural grasslands (ed Z. Botta-Dukát). J. Veg. Sci. 2016, 27, 1222–1232. [Google Scholar] [CrossRef]
- Yang, K.; Geng, Q.; Luo, Y.; Xie, R.; Sun, T.; Wang, Z.; Qin, L.; Zhao, W.; Liu, M.; Li, Y.J.E.M. Dysfunction of FadA-cAMP signalling decreases Aspergillus flavus resistance to antimicrobial natural preservative Perillaldehyde and AFB1 biosynthesis. Environ. Microbiol. 2022, 24, 1590–1607. [Google Scholar] [CrossRef]
- Stanley, A.; Arceo-Gómez, G. Urbanization increases seed dispersal interaction diversity but decreases dispersal success in Toxicodendron radicans. Glob. Ecol. Conserv. 2020, 22, e01019. [Google Scholar] [CrossRef]
- North, A.; Cornell, S.; Ovaskainen, O. Evolutionary responses of dispersal distance to landscape structure and habitat loss. Evolution 2011, 65, 1739–1751. [Google Scholar] [CrossRef] [PubMed]
- Dener, E.; Ovadia, O.; Shemesh, H.; Altman, A.; Chen, S.-C.; Giladi, I. Direct and indirect effects of fragmentation on seed dispersal traits in a fragmented agricultural landscape. Agric. Ecosyst. Environ. 2021, 309, 107273. [Google Scholar] [CrossRef]
- Travis, J.M.; Smith, H.S.; Ranwala, S.M. Towards a mechanistic understanding of dispersal evolution in plants: Conservation implications. Divers. Distrib. 2010, 16, 690–702. [Google Scholar] [CrossRef]
- Garcia, D.; Zamora, R.; Amico, G. Birds as suppliers of seed dispersal in temperate ecosystems: Conservation guidelines from real-world landscapes. Conserv. Biol. 2010, 24, 1070–1079. [Google Scholar] [CrossRef]
- LaRue, E.A.; Holland, J.D.; Emery, N.C. Environmental predictors of dispersal traits across a species’ geographic range. Ecology 2018, 99, 1857–1865. [Google Scholar] [CrossRef]
- Cramer, J.M.; Mesquita, R.C.G.; Bruce Williamson, G. Forest fragmentation differentially affects seed dispersal of large and small-seeded tropical trees. Biol. Conserv. 2007, 137, 415–423. [Google Scholar] [CrossRef]
- Moran, C.; Catterall, C.P.; Kanowski, J. Reduced dispersal of native plant species as a consequence of the reduced abundance of frugivore species in fragmented rainforest. Biol. Conserv. 2009, 142, 541–552. [Google Scholar] [CrossRef] [Green Version]
- Markl, J.S.; Schleuning, M.; Forget, P.M.; Jordano, P.; Lambert, J.E.; Traveset, A.; Wright, S.J.; Böhning-Gaese, K. Meta-analysis of the effects of human disturbance on seed dispersal by animals. Conserv. Biol. 2012, 26, 1072–1081. [Google Scholar] [CrossRef] [Green Version]
- Herrera, J.M.; Garcia. Effects of forest fragmentation on seed dispersal and seedling establishment in ornithochorous trees. Conserv. Biol. 2010, 24, 1089–1098. [Google Scholar] [CrossRef]
- Lehouck, V.; Spanhove, T.; Demeter, S.; Groot, N.E.; Lens, L. Complementary seed dispersal by three avian frugivores in a fragmented Afromontane forest. J. Veg. Sci. 2009, 20, 1110–1120. [Google Scholar] [CrossRef]
- Rey, P.J.; Alcántara, J.M. Effects of habitat alteration on the effectiveness of plant-avian seed dispersal mutualisms: Consequences for plant regeneration. Perspect. Plant Ecol. Evol. Syst. 2014, 16, 21–31. [Google Scholar] [CrossRef]
- Guariguata, M.R.; Adame, J.J.; Finegan, B. Seed removal and fate in two selectively logged lowland forests with constrasting protection levels. Conserv. Biol. 2000, 14, 1046–1054. [Google Scholar] [CrossRef]
- Mokany, K.; Prasad, S.; Westcott, D.A. Loss of frugivore seed dispersal services under climate change. Nat. Commun. 2014, 5, 4971. [Google Scholar] [CrossRef]
- Imbach, P.A.; Locatelli, B.; Molina, L.G.; Ciais, P.; Leadley, P.W. Climate change and plant dispersal along corridors in fragmented landscapes of Mesoamerica. Ecol. Evol. 2013, 3, 2917–2932. [Google Scholar] [CrossRef]
- Fontúrbel, F.E.; Lara, A.; Lobos, D.; Little, C. The cascade impacts of climate change could threaten key ecological interactions. Ecosphere 2018, 9, 2485. [Google Scholar] [CrossRef] [Green Version]
- Soons, M.B.; Groot, G.A.; Cuesta Ramirez, M.T.; Fraaije, R.G.; Verhoeven, J.T.; Jager, M. Directed dispersal by an abiotic vector: Wetland plants disperse their seeds selectively to suitable sites along the hydrological gradient via water. Funct. Ecol. 2017, 31, 499–508. [Google Scholar] [CrossRef]
- González-Varo, J.P.; López-Bao, J.V.; Guitián, J. Seed dispersers help plants to escape global warming. Oikos 2017, 126, 1600–1606. [Google Scholar] [CrossRef] [Green Version]
- Karsai, I.; Stanley, A.; Gomez, G.A. Population models reveal synergistic fitness effects of climate change and urbanization on Poison ivy (Toxicodendron radicans) via disruption of seed dispersal interactions. Urban Ecosyst. 2022, 25, 1503–1514. [Google Scholar] [CrossRef]
- Mokany, K.; Prasad, S.; Westcott, D.A. Impacts of climate change and management responses in tropical forests depend on complex frugivore-mediated seed dispersal. Glob. Ecol. Biogeogr. 2015, 24, 685–694. [Google Scholar] [CrossRef]
- Fricke, E.C.; Ordonez, A.; Rogers, H.S.; Svenning, J.-C. The effects of defaunation on plants’ capacity to track climate change. Science 2022, 375, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Sales, L.; Culot, L.; Pires, M.M. Climate niche mismatch and the collapse of primate seed dispersal services in the Amazon. Biol. Conserv. 2020, 247, 108628. [Google Scholar] [CrossRef]
- Pagel, J.; Treurnicht, M.; Bond, W.J.; Kraaij, T.; Nottebrock, H.; Schutte-Vlok, A.L.; Tonnabel, J.; Esler, K.J.; Schurr, F.M. Mismatches between demographic niches and geographic distributions are strongest in poorly dispersed and highly persistent plant species. Proc. Natl. Acad. Sci. 2020, 117, 3663–3669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabarelli, M.; Vicente, A.; Barbosa, D.C.A. Variation of seed dispersal spectrum of woody plants across a rainfall gradient in north-eastern Brazil. J. Arid Environ. 2003, 53, 197–210. [Google Scholar] [CrossRef]
- Zhao, Y.; Cao, H.; Xu, W.; Chen, G.; Lian, J.; Du, Y.; Ma, K. Contributions of precipitation and temperature to the large scale geographic distribution of fleshy-fruited plant species: Growth form matters. Sci. Rep. 2018, 8, 17017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarneel, J.M. Effects of experimental snowmelt and rain on dispersal of six plant species. Ecohydrology 2016, 9, 1464–1470. [Google Scholar] [CrossRef] [Green Version]
- Almeida-Neto, M.; Campassi, F.; Galetti, M.; Jordano, P.; Oliveira-Filho, A. Vertebrate dispersal syndromes along the Atlantic Forest: Broad-scale patterns and macroecological correlates. Glob. Ecol. Biogeogr. 2008, 17, 503–513. [Google Scholar] [CrossRef]
- Travis, J.M.; Delgado, M.; Bocedi, G.; Baguette, M.; Bartoń, K.; Bonte, D.; Boulangeat, I.; Hodgson, J.A.; Kubisch, A.; Penteriani, V.; et al. Dispersal and species’ responses to climate change. Oikos 2013, 122, 1532–1540. [Google Scholar] [CrossRef] [Green Version]
- Kuparinen, A.; Katul, G.; Nathan, R.; Schurr, F.M. Increases in air temperature can promote wind-driven dispersal and spread of plants. Proc. R. Soc. B Biol. Sci. 2009, 276, 3081–3087. [Google Scholar] [CrossRef] [Green Version]
- Thomas, C.F.; Brain, P.; Jepson, P.C. Aerial activity of linyphiid spiders: Modelling dispersal distances from meteorology and behaviour. J. Appl. Ecol. 2003, 40, 912–927. [Google Scholar] [CrossRef]
- Bullock, J.M.; White, S.M.; Prudhomme, C.; Tansey, C.; Perea, R.; Hooftman, D.A. Modelling spread of British wind-dispersed plants under future wind speeds in a changing climate. J. Ecol. 2011, 100, 104–115. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Wang, J.; Li, D.; Liu, S.; Yin, J.; Lai, Z.; Yang, G.J.C.C.L. A Hg (II)-specific probe for imaging application in living systems and quantitative analysis in environmental/food samples. Chin. Chem. Lett. 2021, 32, 1527–1531. [Google Scholar] [CrossRef]
- Thuiller, W.; Albert, C.; Araújo, M.B.; Berry, P.M.; Cabeza, M.; Guisan, A.; Hickler, T.; Midgley, G.F.; Paterson, J.; Schurr, F.M.; et al. Predicting global change impacts on plant species’ distributions: Future challenges. Perspect. Plant Ecol. Evol. Syst. 2008, 9, 137–152. [Google Scholar] [CrossRef]
- Bläß, C.; Ronnenberg, K.; Tackenberg, O.; Hensen, I.; Wesche, K. The relative importance of different seed dispersal modes in dry Mongolian Rangelands. J. Arid Environ. 2010, 74, 991–997. [Google Scholar] [CrossRef]
- Yusefi, G.H.; Safi, K.; Tarroso, P.; Brito, J.C. The impacts of extreme climate change on mammals differ among functional groups at regional scale: The case of Iranian terrestrial mammals. Divers. Distrib. 2021, 27, 1634–1647. [Google Scholar] [CrossRef]
- Cunze, S.; Heydel, F.; Tackenberg, O. Are plant species able to keep pace with the rapidly changing climate? PLoS ONE 2013, 8, 0067909. [Google Scholar] [CrossRef] [PubMed]
- Beyer, R.M.; Manica, A. Historical and projected future range sizes of the world’s mammals, birds, and Amphibians. Nat. Commun. 2020, 11, 5633. [Google Scholar] [CrossRef]
- Machado, I.C.S.; Barros, L.M.; Sampaio, E.V.S.B. Phenology of Caatinga species at Serra Talhada, PE, Northeastern Brazil. Biotropica 1997, 29, 57–68. [Google Scholar] [CrossRef]
- González-Varo, J.P.; Rumeu, B.; Albrecht, J.; Arroyo, J.M.; Bueno, R.S.; Burgos, T.; da Silva, L.P.; Escribano-Ávila, G.; Farwig, N.; García, D.; et al. Limited potential for bird migration to disperse plants to cooler latitudes. Nature 2021, 595, 75–79. [Google Scholar] [CrossRef]
- Liang, C.; Liu, J.; Pan, B.; Wang, N.; Yang, J.; Yang, G.; Feng, G. Precipitation is the dominant driver for bird species richness, phylogenetic and functional structure in university campuses in Northern China. Avian Res. 2020, 11, 26. [Google Scholar] [CrossRef]
- López Calderón, C.; Balbontín Arenas, J.; Hobson, K.A.; Møller, A.P. Age-dependent carry-over effects in a long-distance migratory bird. Sci. Rep. 2019, 9, 12032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cotton, P.A. Avian migration phenology and global climate change. Proc. Natl. Acad. Sci. 2003, 100, 12219–12222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knudsen, E.; Lindén, A.; Both, C.; Jonzén, N.; Pulido, F.; Saino, N.; Sutherland, W.J.; Bach, L.A.; Coppack, T.; Ergon, T.; et al. Challenging claims in the study of Migratory Birds and climate change. Biol. Rev. 2011, 86, 928–946. [Google Scholar] [CrossRef] [Green Version]
- Rosamond, K.M.; Goded, S.; Soultan, A.; Kaplan, R.H.; Glass, A.; Kim, D.H.; Arcilla, N. Not singing in the rain: Linking migratory songbird declines with increasing precipitation and brood parasitism vulnerability. Front. Ecol. Evol. 2020, 8, 536769. [Google Scholar] [CrossRef]
- Akresh, M.E.; King, D.I.; Marra, P.P. Rainfall and habitat interact to affect the condition of a wintering migratory songbird in the Bahamas. Ecol. Evol. 2019, 9, 8042–8061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blendinger, P.G. Functional equivalence in seed dispersal effectiveness of Podocarpus parlatorei in Andean fruit-eating bird assemblages. Front. Ecol. Evol. 2017, 5, 57. [Google Scholar] [CrossRef] [Green Version]
- Correa, D.F.; Stevenson, P.R.; Umaña, M.N.; Coelho, L.; de Lima Filho, D.; de Salomão, R.P.; Amaral, I.L.; Wittmann, F.; Matos, F.D.; Castilho, C.V.; et al. Geographic patterns of tree dispersal modes in Amazonia and their ecological correlates. Glob. Ecol. Biogeogr. 2022, 32, 49–69. [Google Scholar] [CrossRef]
- Nilsson, C.; Brown, R.L.; Jansson, R.; Merritt, D.M. The role of hydrochory in structuring riparian and wetland vegetation. Biol. Rev. 2010, 85, 837–858. [Google Scholar] [CrossRef]
- Vogt, K.; Rasran, L.; Jensen, K. Water-borne seed transport and seed deposition during flooding in a small river-valley in Northern Germany. Flora Morphol. Distrib. Funct. Ecol. Plants 2004, 199, 377–388. [Google Scholar] [CrossRef]
- Kubitzki, K.; Ziburski, A. Seed dispersal in flood plain forests of Amazonia. Biotropica 1994, 26, 30. [Google Scholar] [CrossRef]
- Ma, Y.-R.; Chen, S.-H.; Chen, F.-Q.; Chen, G.-H.; Xie, Z.-Q.; Liu, Y.-Y. Effects of flooding on seed viability and nutrient composition in three riparian shrubs and implications for restoration. J. Freshw. Ecol. 2018, 33, 449–460. [Google Scholar] [CrossRef] [Green Version]
- Holguín, J.E.; Crepy, M.; Striker, G.G.; Mollard, F.P.O. Dormancy breakage and germination are tightly controlled by hypoxic submergence water on Echinochloa crusgalli seeds from an accession resistant to anaerobic germination. Seed Sci. Res. 2020, 30, 262–267. [Google Scholar] [CrossRef]
- Stuble, K.L.; Patterson, C.M.; Rodriguez-Cabal, M.A.; Ribbons, R.R.; Dunn, R.R.; Sanders, N.J. Ant-mediated seed dispersal in a warmed world. PeerJ 2014, 2, 286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parr, C.L.; Andersen, A.N.; Chastagnol, C.; Duffaud, C. Savanna fires increase rates and distances of seed dispersal by ants. Oecologia 2007, 151, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Parr, C.L.; Bishop, T.R. The response of ants to climate change. Glob. Change Biol. 2022, 28, 3188–3205. [Google Scholar] [CrossRef]
- Kwon, T.-S.; Li, F.; Kim, S.-S.; Chun, J.H.; Park, Y.-S. Modelling vulnerability and range shifts in ant communities responding to future global warming in temperate forests. PLoS ONE 2016, 11, 0159795. [Google Scholar] [CrossRef] [Green Version]
- Rossetto, M.; Kooyman, R.; Yap, J.-Y.S.; Laffan, S.W. From ratites to rats: The size of fleshy fruits shapes species’ distributions and Continental Rainforest Assembly. Proc. R. Soc. B Biol. Sci. 2015, 282, 20151998. [Google Scholar] [CrossRef] [Green Version]
- Dennis, A.J.; Westcott, D.A. Reducing complexity when studying seed dispersal at community scales: A functional classification of vertebrate seed dispersers in tropical forests. Oecologia 2006, 149, 620–634. [Google Scholar] [CrossRef]
- Rodríguez, A.; Alquézar, B.; Peña, L. Fruit aromas in mature fleshy fruits as signals of readiness for predation and seed dispersal. New Phytologist 2012, 197, 36–48. [Google Scholar] [CrossRef]
- Cazetta, E.; Schaefer, H.M.; Galetti, M. Why are fruits colorful? the relative importance of achromatic and chromatic contrasts for detection by birds. Evol. Ecol. 2007, 23, 233–244. [Google Scholar] [CrossRef]
- Ramírez, N.; Barrios, Y.; Briceño, H. Correlations between morphological fruit types, fruit and seed colors, and functional groups. BiotaNeotropica 2021, 21, e20211238. [Google Scholar] [CrossRef]
- Vander Wall, S.B. The evolutionary ecology of Nut Dispersal. Bot. Rev. 2001, 67, 74–117. [Google Scholar] [CrossRef]
- Moran, E.V.; Clark, J.S. Between-site differences in the scale of dispersal and gene flow in Red Oak. PLoS ONE 2012, 7, 0036492. [Google Scholar] [CrossRef] [PubMed]
- Pesendorfer, M.B.; Sillett, T.S.; Koenig, W.D.; Morrison, S.A. Scatter-hoarding corvids as seed dispersers for Oaks and pines: A review of a widely distributed mutualism and its utility to habitat restoration. Condor 2016, 118, 215–237. [Google Scholar] [CrossRef]
- Wan, X.; Yan, C.; Wang, Z.; Zhang, Z. Sustained population decline of rodents is linked to accelerated climate warming and human disturbance. BMC Ecol. Evol. 2022, 22, 102. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Tan, D.; Baskin, J.M.; Baskin, C.C. Fruit and seed heteromorphism in the Cold Desert Annual ephemeral Diptychocarpus Strictus (Brassicaceae) and possible adaptive significance. Ann. Bot. 2010, 105, 999–1014. [Google Scholar] [CrossRef]
- Sheldon, J.C.; Burrows, F.M. The dispersal effectiveness of the achene-pappus units of selected Compositae in steady winds with convection. New Phytol. 1973, 72, 665–675. [Google Scholar] [CrossRef]
- Matteodo, M.; Wipf, S.; Stöckli, V.; Rixen, C.; Vittoz, P. Elevation gradient of successful plant traits for colonizing alpine summits under climate change. Environ. Res. Lett. 2013, 8, 024043. [Google Scholar] [CrossRef]
- McEvoy, P.B.; Cox, C.S. Wind dispersal distances in dimorphic achenes of ragwort, Senecio Jacobaea. Ecology 1987, 68, 2006–2015. [Google Scholar] [CrossRef]
- Beckman, N.G.; Aslan, C.E.; Rogers, H.S.; Kogan, O.; Bronstein, J.L.; Bullock, J.M.; Hartig, F.; HilleRisLambers, J.; Zhou, Y.; Zurell, D.; et al. Advancing an interdisciplinary framework to study seed dispersal ecology. AoB PLANTS 2019, 12, 48. [Google Scholar] [CrossRef] [Green Version]
- Kerr, J.T. Racing against change: Understanding dispersal and persistence to improve species’ conservation prospects. Proc. R. Soc. B Biol. Sci. 2020, 287, 20202061. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, J.O. Ecophysiological Effects of Groundwater Drawdown on Phreatophytes: Research Trends during the Last Three Decades. Land 2022, 11, 2061. [Google Scholar] [CrossRef]





| Dispersal Mode | Relative Count (%) | Fruit Type | Example Species |
|---|---|---|---|
| Anemochory | 17.21 | Achenes, capsule, winged seeds/samara | Erigeron bonariensis, Pinus halepensis, Swietenia humilis |
| Autochory/Ballochory | 3.24 | Dehiscent pod | Pentaclethra macroloba |
| Endozoochory | 33.33 | Capsule, drupe, fleshy, nuts, | Tristerix corymbosus, Ficus carica, Duckeodendron cestroides |
| Epizoochory | 6.45 | Achenes, drupe, dry dehiscent, fleshy, winged seeds/samara | Choerospondias axillaris, Carapa procera, Heterosperma pinnatum |
| Hydrochory/ichthyochory | 9.68 | Buoyant seeds, drupe | Lolium perenne, Trifolium repens, Iris pseudocorus |
| Myrmecochory | 7.53 | Achenes, capsule, fleshy | Eucalyptus tetrodonta, Hepatica nobilis, Trillium undulatum |
| Ornithochory | 22.58 | Capsule, drupe, fleshy, nuts, | Prunus avium, Quercus serrata, Olea europaea |
| Fruit/Seed Types | Examples | Adaptive Traits in a Changing Environment | |
|---|---|---|---|
| Morpho-Structural Traits | Biochemical and Molecular Traits | ||
| Fleshy | Berry, drupe, hesperidium, pepo, pome | Soft pericarp, some have tough rind for edibility and long-distance seed dispersal; size and color | Nutrient and water content of mature fleshy fruit pulp for dietary requirements of frugivorous seed dispersers [16,17,18]; sugar, fat, and protein, antioxidants, minerals, and vitamins [19,20]; pigments and volatile compounds, cell wall modifications (softening) [21] |
| Dehiscent dry fruits | Capsule, silique, follicle, legume | Dry with single or multiple pod walls that facilitate fruit opening for seed dispersal | Programmed cell death (PCD) of pericarps; dead pericarps for long-term storage of nucleases, proteases, and chitinases [22] |
| Indehiscent dry fruits | Caryopsis, nut (acorn), samara, achene | Hard, dry, and dead pericarps for embryo protection and long-distance dispersal | Concentration of glucosinolates (GSLs) in seeds and pericarps that could explain dispersal strategies and dormancy of indehiscent fruits [23]. |
| Search Keywords | ScienceDirect | PubMed | Google Scholar | Direct Search | Total |
|---|---|---|---|---|---|
| “seed dispersal” AND “climate change” | 1675 | 135 | 1700 | 21 | 3531 |
| “spore dispersal” OR “propagule dispersal” AND “changing climate” | 783 | 30 | 1110 | 10 | 1933 |
| “plant spread” AND “climate” | 280 | 931 | 3940 | 15 | 5151 |
| Extraction Criteria | Information Considered and Justification |
|---|---|
| Between 1990 and 2022, to obtain enough studies about seed dispersal in a changing environment. |
| Global, to map the geographical distribution of studies and the trends of in studies about the reviewed topic. |
| Keywords in the title, to provide insights into the research trends in seed dispersal-changing environment studies during the last three decades. |
| The plant species mentioned as the experimental plant species in the paper, to validate the dispersal modes of the species depending on their fruit types or plant traits. |
| Fruit types of the experimental plant species, to validate whether the dispersal modes/vectors match with fruit types. |
| Any mentioned mechanism/agent of seed dispersal, to determine which among the dispersal modes and vectors are well-studied. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernandez, J.O.; Naeem, M.; Zaman, W. How Does Changing Environment Influence Plant Seed Movements as Populations of Dispersal Vectors Decline? Plants 2023, 12, 1462. https://doi.org/10.3390/plants12071462
Hernandez JO, Naeem M, Zaman W. How Does Changing Environment Influence Plant Seed Movements as Populations of Dispersal Vectors Decline? Plants. 2023; 12(7):1462. https://doi.org/10.3390/plants12071462
Chicago/Turabian StyleHernandez, Jonathan O., Muhammad Naeem, and Wajid Zaman. 2023. "How Does Changing Environment Influence Plant Seed Movements as Populations of Dispersal Vectors Decline?" Plants 12, no. 7: 1462. https://doi.org/10.3390/plants12071462