Plant Functional Dispersion, Vulnerability and Originality Increase Arthropod Functions from a Protected Mountain Mediterranean Area in Spring
Abstract
:1. Introduction
2. Results
2.1. Plant Community Composition
2.2. Plant Functional Diversity in the Multidimensional Space
2.3. Response of the Plant Taxonomic Diversity to Habitat and Month
2.4. Response of Plant Functional Diversity to Habitat and Month
2.5. Arthropods Functional Community
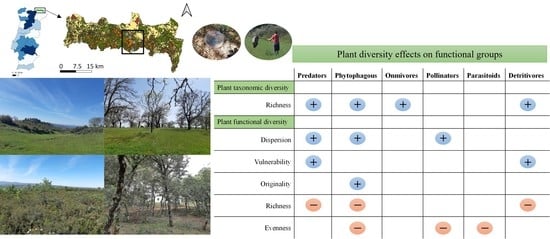
2.6. Response of Functional Groups (Abundance) to Functional and Taxonomic Diversity of Plants
3. Discussion
3.1. Plant Community
3.2. Bottom-Up Relationships between Plants and Arthropods
- The trait selection constitutes a critical and not easy step in the trait-based ecology [6] and here, because it was the first attempt to establish arthropod–plant interactions in the region, the selection of plant traits and their categorization was performed at a coarse level and might be inaccurate. For example, theoretical amounts of N and P were used [31,32,33,34,35], but the composition in the growing conditions of the study region must vary (at least) to some extent. Moreover, the human spectra visual perception was used to record the flower color, but the perception of different arthropod species performs at variable UV reflectance [29]. Important plant traits for arthropods such as the resource quality or quantity, resource types (e.g., stems, roots, sap, nectar pollen), olfactory and gustatory signals, chemical attractant or repellents, leaf structure traits (e.g., cuticula thickness, lignin content, leaf area, etc.) and other structural properties of plants (e.g., grown form, density and orientation of branches and leaves, surface features, etc.) or of vegetation (e.g., density or litter quantity) were not considered [1]. Despite the potential trait inappropriateness, Gagic et al. [25] found that “most multi-trait functional diversity indices were weakly affected by trait choice, and while excluding traits worsen explanatory power in some cases, it rarely increased it”.
- Different arthropod taxonomic groups, even of the same functional group, may show different responses to the plant functional diversity. For example, the predators Chrysopidae and Syrphidae increased with the total amount of available resources [36], but Carabidae also responded to the diversity of these resources [37]. In addition, grasshopper herbivory depended on the plant toughness and insect mandibular strength [28], Carabidae varied in body size and shape with the vegetation structure [38] and Ebeling et al. [22] found that the plant species richness was associated with shifts in many taxa, but not all. Thus, a study about the response of arthropod taxa (and not only functional groups) to the plants might provide further insights.
- This study only addressed spring months, and arthropod dynamics most probably show seasonal variations.
- Arthropods were captured using pitfall traps and sweeping nets but different sample methods may capture different arthropods, e.g., [39].
- We focused on the effects of local factors (i.e., habitats and plants within the habitats), but it is known that the effects of the surrounding landscape structure (i.e., the degree of simplification or heterogeneity/complexity) can dominate the functional community composition and even buffer local effects [40].
4. Materials and Methods
4.1. Study Sites
4.2. Plant Sampling
4.3. Arthropod Sampling
4.4. Plant Traits Selection
- Resource: the resource type at observation (only leaves, leaves and flowers, leaves and fruits.
- Flower attractiveness:
- Flower color: brown, pink, purple, red, white, yellow and inconspicuous for not-apparent flowers (e.g., Poaceae, Fagaceae, Chenopodium spp. or Plantago spp.).
- Flower area: this was considered as the bloomed area, i.e., the unique flower, the inflorescence or clusters of nearby small solitary flowers and inflorescences forming bloomed clusters. Three categorical levels were established: small = approx. <0.05 cm; medium = approx. 0.05 to 2 cm; large = approx. >2 cm.
- Flower accessibility:
- Flower shape: the flower shape was ranked according to the degree of openness of the corolla in three levels (for each plant species)—total openness (rosaceous and rotate corollas), medium openness (cruciferous, ligulate, hypocrateriform, campanulate and infundibuliform corollas) and low openness (papilionaceus, bilabiate, tubular, personate, orchidaceae and urceolate corollas) (see Aguiar [46] for corolla descriptions and Flora Iberica [45] for species corolla information). This trait was considered as an indication of flower accessibility to arthropods.
- Nutritional quality:
- The leaf texture (herbaceous, fleshy, and semi-sclerophyllous/sclerophyllous).
- The leaf nitrogen (N) and phosphorous (P) composition (mg g−1). Mean values—for the species when possible, or for the plant family—from the literature were used [31,32,33,34,35]. For species/families with no information, “Not Available” (NA) was introduced in the data matrix. The values were ranked as follows: for N—low (<19 mg g−1), medium (>19 and <23 mg g−1), high (>23 mg g−1); for P—low (<1.1 mg g−1), medium (>1.1 and <1.7 mg g−1), high (>1.7 mg g−1) (Table S4). This ranking was arbitrary because there is no previous information about the leaf amount of N and P on the general biodiversity of arthropods. In addition, found interactions related with this trait in this study must be taken as preliminary because plant composition can show a high degree of variability depending on the growing conditions, and there is no information about the composition of these plants in the study area.
4.5. Plant Functional and Taxonomic Diversity
- Functional dispersion: the biomass weighted deviation of species traits values from the center of the functional space filled by the assemblage, i.e., the biomass-weighted mean distance to the biomass-weighted mean trait values of the assemblage. Changes in the functional dispersion reflect changes in the abundance-weighted deviation of species trait values from the center of the functional space filled by the community [49].
- Functional richness: the volume of multidimensional space occupied by all species in a community within the functional space. The importance of this index relies on the fact that while species (taxonomic) richness is assumed to peak for intermediate disturbance levels, functional richness, through trait reduction, is expected to decrease under high disturbance levels when species decrease [49].
- Functional divergence (the proportion of total abundance supported by species with the most extreme trait values within a community) and functional evenness (the regularity of the distribution and relative abundance of species in the functional space for a given community): the importance of both traits is based on the fact that after a disturbance, the species abundance is expected to be modified, with species having a combination of traits that are lost and others that remains stable before local extinctions. Thus, reductions in the functional divergence and evenness will reveal disturbance impacts earlier than functional richness [49].
- Functional specialization: the mean distance of a species from the rest of the species pool in functional space. This indicates generalist species (i.e., species close to the center of the functional space) or specialist species (i.e., having extreme trait combinations) [49].
- Functional originality: the weighted mean distance to the nearest species from the global species pool. Changes in functional originality quantify how changes in species abundances modify the functional redundancy between species [49].
- Functional redundancy reflects the average number of species per FE.
- Functional vulnerability that reflects the proportion of FE with only one species.
4.6. Data Analyses
4.6.1. Response of the Plant Taxonomic and Functional Diversity to Habitat and Month
4.6.2. Response of Arthropod Functional Groups to Plants
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gardarin, A.; Plantegenest, M.; Bischoff, A.; Valantin-Morison, M. Understanding plant–arthropod interactions in multitrophic communities to improve conservation biological control: Useful traits and metrics. J. Pest Sci. 2018, 91, 943–955. [Google Scholar] [CrossRef]
- Wäckers, F.L. Suitability of (extra-) floral nectar, pollen, and honeydew as insect food sources. In Plant-Provided Food for Car-nivorous Insects: A Protective Mutualism and Its Applications; Wäckers, F.L., van Rijn, P.C.J., Bruin, J., Eds.; Cambridge University Press: Cambridge, UK, 2005; pp. 17–74. [Google Scholar]
- Noriega, J.A.; Hortal, J.; Azcárate, F.M.; Berg, M.P.; Bonada, N.; Briones, M.J.; Del Toro, I.; Goulson, D.; Ibanez, S.; Landis, D.A.; et al. Research trends in ecosystem services provided by insects. Basic Appl. Ecol. 2018, 26, 8–23. [Google Scholar] [CrossRef]
- Chowdhury, S.; Jennions, M.D.; Zalucki, M.P.; Maron, M.; Watson, J.E.; Fuller, R.A. Protected areas and the future of insect conservation. Trends Ecol. Evol. 2022, 38, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Stork, N.E. How Many Species of Insects and Other Terrestrial Arthropods Are There on Earth? Annu. Rev. Èntomol. 2018, 63, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.K.L.; Guénard, B.; Lewis, O. Trait-based ecology of terrestrial arthropods. Biol. Rev. 2018, 94, 999–1022. [Google Scholar] [CrossRef] [PubMed]
- Haddad, N.M.; Crutsinger, G.M.; Gross, K.; Haarstad, J.; Knops, J.M.; Tilman, D. Plant species loss decreases arthropod diversity and shifts trophic structure. Ecol. Lett. 2009, 12, 1029–1039. [Google Scholar] [CrossRef] [PubMed]
- Haddad, N.M.; Crutsinger, G.M.; Gross, K.; Haarstad, J.; Tilman, D. Plant diversity and the stability of foodwebs. Ecol. Lett. 2010, 14, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Isbell, F.; Gonzalez, A.; Loreau, M.; Cowles, F.I.J.; Díaz, S.; Hector, A.; Mace, G.M.; Wardle, D.A.; O’Connor, M.I.; Duffy, J.E.; et al. Linking the influence and dependence of people on biodiversity across scales. Nature 2017, 546, 65–72. [Google Scholar] [CrossRef]
- Barnes, A.D.; Scherber, C.; Brose, U.; Borer, E.T.; Ebeling, A.; Gauzens, B.; Giling, D.P.; Hines, J.; Isbell, F.; Ristok, C.; et al. Biodiversity enhances the multitrophic control of arthropod herbivory. Sci. Adv. 2020, 6, eabb6603. [Google Scholar] [CrossRef]
- Violle, C.; Navas, M.-L.; Vile, D.; Kazakou, E.; Fortunel, C.; Hummel, I.; Garnier, E. Let the concept of trait be functional! Oikos 2007, 116, 882–892. [Google Scholar] [CrossRef]
- Wood, S.A.; Karp, D.S.; DeClerck, F.; Kremen, C.; Naeem, S.; Palm, C.A. Functional traits in agriculture: Agrobiodiversity and ecosystem services. Trends Ecol. Evol. 2015, 30, 531–539. [Google Scholar] [CrossRef]
- Vukomanovic, J.; Randall, J. Research trends in U.S. national parks, the world’s “living laboratories”. Conserv. Sci. Pract. 2021, 3, e414. [Google Scholar] [CrossRef]
- COS 2018, Carta de Uso e Ocupação do Solo Para 2018. Available online: https://www.dgterritorio.gov.pt/Carta-de-Uso-e-Ocupacao-do-Solo-para-2018?language=en (accessed on 22 October 2022).
- Barros, P.; Moreira, P.; Ferreira, S. Contribution to the knowledge of the Odonata fauna of Northern Portugal. Bol. SEA 2010, 46, 533–539. [Google Scholar]
- Maes, D.; Verovnik, R.; Wiemers, M.; Brosens, D.; Beshkov, S.; Bonelli, S.; Buszko, J.; Cantú-Salazar, L.; Cassar, L.-F.; Collins, S.; et al. Integrating national Red Lists for prioritising conservation actions for European butterflies. J. Insect Conserv. 2019, 23, 301–330. [Google Scholar] [CrossRef]
- Miranda-Arabolaza, M.J.; Barranco, P. Os ortópteros da bacia do rio Sabor (Trás-os-Montes e Alto Douro, Portugal (Insecta, Orthoptera). Bol. SEA 2005, 37, 173–200. [Google Scholar]
- Santos, A.L.; Santos, S.A.P.; Almeida, J.; Bento, A. Parasitoides autóctones associados a Dryocosmus kuriphilus Yasumatsu: Principais espécies e taxas de parasitismo. Rev. De Ciências Agrar. 2018, 41, 141–144. [Google Scholar] [CrossRef]
- Cadotte, M.W.; Carscadden, K.; Mirotchnick, N. Beyond species: Functional diversity and the maintenance of ecological processes and services. J. Appl. Ecol. 2011, 48, 1079–1087. [Google Scholar] [CrossRef]
- Borer, E.T.; Seabloom, E.W.; Tilman, D. Plant diversity controls arthropod biomass and temporal stability. Ecol. Lett. 2012, 15, 1457–1464. [Google Scholar] [CrossRef]
- Graça, M.B.; Morais, J.W.; Franklin, E.; Pequeno, P.A.C.L.; Souza, J.L.P.; Bueno, A.S. Combining Taxonomic and Functional Approaches to Unravel the Spatial Distribution of an Amazonian Butterfly Community. Environ. Èntomol. 2015, 45, 301–309. [Google Scholar] [CrossRef]
- Ebeling, A.; Hines, J.; Hertzog, L.R.; Lange, M.; Meyer, S.T.; Simons, N.K.; Weisser, W.W. Plant diversity effects on arthropods and arthropod-dependent ecosystem functions in a biodiversity experiment. Basic Appl. Ecol. 2018, 26, 50–63. [Google Scholar] [CrossRef]
- Storkey, J.; Brooks, D.; Haughton, A.; Hawes, C.; Smith, B.M.; Holland, J.M. Using functional traits to quantify the value of plant communities to invertebrate ecosystem service providers in arable landscapes. J. Ecol. 2012, 101, 38–46. [Google Scholar] [CrossRef]
- Frenette-Dussault, C.; Shipley, B.; Hingrat, Y. Linking plant and insect traits to understand multitrophic community structure in arid steppes. Funct. Ecol. 2013, 27, 786–792. [Google Scholar] [CrossRef]
- Gagic, V.; Bartomeus, I.; Jonsson, T.; Taylor, A.; Winqvist, C.; Fischer, C.; Slade, E.M.; Steffan-Dewenter, I.; Emmerson, M.; Potts, S.G.; et al. Functional identity and diversity of animals predict ecosystem functioning better than species-based indices. Proc. R. Soc. B Boil. Sci. 2015, 282, 20142620. [Google Scholar] [CrossRef] [PubMed]
- Albacete, S.; Mac Nally, R.; Carles-Tolrá, M.; Domènech, M.; Vives, E.; Espadaler, X.; Pujadé-Villar, J.; Serra, A.; Maceda-Veiga, A. Stream distance and vegetation structure are among the major factors affecting various groups of arthropods in non-riparian chestnut forests. For. Ecol. Manag. 2020, 460, 117860. [Google Scholar] [CrossRef]
- Ibanez, S. Optimizing size thresholds in a plant–pollinator interaction web: Towards a mechanistic understanding of ecological networks. Oecologia 2012, 170, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Stang, M.; Klinkhamer, P.G.L.; van der Meijden, E. Size constraints and flower abundance determine the number of interactions in a plant-flower visitor web. Oikos 2006, 112, 111–121. [Google Scholar] [CrossRef]
- Fornoff, F.; Klein, A.; Hartig, F.; Benadi, G.; Venjakob, C.; Schaefer, H.M.; Ebeling, A. Functional flower traits and their diversity drive pollinator visitation. Oikos 2017, 126, 1020–1030. [Google Scholar] [CrossRef]
- Scherber, C.; Eisenhauer, N.; Weisser, W.W.; Schmid, B.; Voigt, W.; Fischer, M.; Schulze, E.-D.; Roscher, C.; Weigelt, A.; Allan, E.; et al. Bottom-up effects of plant diversity on multitrophic interactions in a biodiversity experiment. Nature 2010, 468, 553–556. [Google Scholar] [CrossRef]
- Margaris, N.S.; Adamandiadou, S.; Siafaca, L.; Diamantopoulos, J. Nitrogen and phosphorus content in plant species of Mediterranean ecosystems in Greece. Plant Ecol. 1984, 55, 29–35. [Google Scholar] [CrossRef]
- Badre, B.; Nobelis, P.; Trémolières, M. Quantitative study and modelling of the litter decomposition in a European alluvial forest. Is there an influence of overstorey tree species on the decomposition of ivy litter (Hedera helix L.)? Acta Oecologica 1998, 19, 491–500. [Google Scholar] [CrossRef]
- Tian, D.; Yan, Z.; Ma, S.; Ding, Y.; Luo, Y.; Chen, Y.; Du, E.; Han, W.; Kovacs, E.D.; Shen, H.; et al. Family-level leaf nitrogen and phosphorus stoichiometry of global terrestrial plants. Sci. China Life Sci. 2019, 62, 1047–1057. [Google Scholar] [CrossRef]
- Mohammadzadeh, S.; Pirzad, A. Biochemical responses of mycorrhizal-inoculated Lamiaceae (Lavender, Rosemary and Thyme) plants to drought: A field study. Soil Sci. Plant Nutr. 2020, 67, 41–49. [Google Scholar] [CrossRef]
- Navarro, T.; Hidalgo-Triana, N. Variations in Leaf Traits Modulate Plant Vegetative and Reproductive Phenological Sequencing Across Arid Mediterranean Shrublands. Front. Plant Sci. 2021, 12, 117860. [Google Scholar] [CrossRef] [PubMed]
- Pfiffner, L.; Wyss, E. Use of sown wildflower strips to enhance natural enemies of agricultural pests. In Ecological Engineering for Pest Management: Advances in Habitat Manipulation for Arthropods; CABI Publishing: Wallingford, UK, 2004; pp. 165–186. [Google Scholar]
- Harwood, J.D.; Phillips, S.W.; Lello, J.; Sunderland, K.D.; Glen, D.M.; Bruford, M.W.; Harper, G.L.; Symondson, W.O. Invertebrate biodiversity affects predator fitness and hence potential to control pests in crops. Biol. Control. 2009, 51, 499–506. [Google Scholar] [CrossRef]
- Ng, K.; Barton, P.S.; Blanchard, W.; Evans, M.J.; Lindenmayer, D.B.; Macfadyen, S.; McIntyre, S.; Driscoll, D.A. Disentangling the effects of farmland use, habitat edges, and vegetation structure on ground beetle morphological traits. Oecologia 2018, 188, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Stašiov, S.; Čiliak, M.; Wiezik, M.; Svitok, M.; Wieziková, A.; Diviaková, A. Pitfall trap design affects the capture efficiency of harvestmen (Opiliones) and millipedes (Diplopoda). Ecol. Evol. 2021, 11, 9864–9875. [Google Scholar] [CrossRef]
- Gámez-Virués, S.; Perovic, D.J.; Gossner, M.M.; Börschig, C.; Blüthgen, N.; De Jong, H.; Simons, N.K.; Klein, A.M.; Krauss, J.; Maier, G.; et al. Landscape simplification filters species traits and drives biotic homogenization. Nat. Commun. 2015, 6, 8568. [Google Scholar] [CrossRef] [PubMed]
- Küchler, A.W.; Mueller-Dombois, D.; Ellenberg, H. Aims and Methods of Vegetation Ecology. Geogr. Rev. 1976, 66, 114. [Google Scholar] [CrossRef]
- Pérez-Harguindeguy, N.; Díaz, S.; Garnier, E.; Lavorel, S.; Poorter, H.; Jaureguiberry, P.; Bret-Harte, M.S.; Cornwell, W.K.; Craine, J.M.; Gurvich, D.E.; et al. Corrigendum to: New handbook for standardised measurement of plant functional traits worldwide. Aust. J. Bot. 2016, 64, 715–716. [Google Scholar] [CrossRef]
- Carboni, M.; Acosta, A.T.; Ricotta, C. Are differences in functional diversity among plant communities on Mediterranean coastal dunes driven by their phylogenetic history? J. Veg. Sci. 2013, 24, 932–941. [Google Scholar] [CrossRef]
- Mahdavi, P.; Bergmeier, E. Plant functional traits and diversity in sand dune ecosystems across different biogeographic regions. Acta Oecologica 2016, 74, 37–45. [Google Scholar] [CrossRef]
- Castroviejo, S. (coord. gen.). Flora Iberica 1-8, 10-15, 17-18, 21; Real Jardín Botánico, CSIC: Madrid, Spain, 1986–2012. [Google Scholar]
- Aguiar, C. Estrutura E Biologia das Plantas; Imprensa Nacional Casa da Moeda: Lisboa, Portugal, 2020; p. 343. [Google Scholar]
- Oksanen, J.; Simpson, G.; Blanchet, F.; Kindt, R.; Legendre, P.; Minchin, P.; O’Hara, R.; Solymos, P.; Stevens, M.; Szoecs, E.; et al. Vegan: Community Ecology Package. R Package Version 2.6-2 April 2022. 2022. Available online: https://CRAN.R-project.org/package=vegan (accessed on 22 October 2022).
- Magneville, C.; Loiseau, N.; Albouy, C.; Casajus, N.; Claverie, T.; Escalas, A.; Leprieur, F.; Maire, E.; Mouillot, D.; Villéger, S. mFD: An R package to compute and illustrate the multiple facets of functional diversity. Ecography 2021, 2022, e05904. [Google Scholar] [CrossRef]
- Mouillot, D.; Graham, N.A.; Villéger, S.; Mason, N.W.; Bellwood, D.R. A functional approach reveals community responses to disturbances. Trends Ecol. Evol. 2013, 28, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S., 4th ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Fox, J.; Weisberg, S. An {R} Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2019; Available online: https://socialsciences.mcmaster.ca/jfox/Books/Companion/ (accessed on 22 October 2022).
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef] [PubMed]
- Hartig, F. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models. R Package Version 0.4.6. 2022. Available online: https://CRAN.R-project.org/package=DHARMa (accessed on 22 October 2022).
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J.; et al. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Mollie, E.B.; Kristensen, K.; Koen, J.; Magnusson, A.; Casper, W.B.; Nielsen, A.; Hans, J.S.; Mächler, M.; Benjamin, M.B. glmmTMB Balances Speed and Flexibility Among Packages for Zero-inflated Generalized Linear Mixed Modeling. R J. 2017, 9, 378–400. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Multimodel Inference: Understanding AIC and BIC in Model Selection. Sociol. Methods Res. 2004, 33, 261–304. [Google Scholar] [CrossRef]






| Predators | Phytophagous | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| β | SE | Z | p | β | SE | Z | p | ||
| (Intercept) | 3.102 | 0.121 | 25.602 | <0.001 | (Intercept) | 1.100 | 0.178 | 6.194 | <0.001 |
| Functional dispersion | 0.214 | 0.100 | 2.142 | 0.032 | Functional dispersion | 0.507 | 0.149 | 3.409 | 0.001 |
| Functional richness | −0.536 | 0.116 | −4.625 | <0.001 | Functional evenness | −0.235 | 0.102 | −2.306 | 0.021 |
| Functional vulnerability | 0.197 | 0.062 | 3.156 | 0.002 | Functional richness | −0.987 | 0.185 | −5.334 | <0.001 |
| Taxonomic richness | 0.230 | 0.070 | 3.308 | 0.001 | Functional originality | 0.227 | 0.106 | 2.141 | 0.032 |
| June (vs. May) | −0.382 | 0.139 | −2.744 | 0.006 | Functional vulnerability | 0.130 | 0.103 | 1.266 | 0.205 |
| Vegetation (vs. Ground) | −1.248 | 0.102 | −12.200 | <0.001 | Taxonomic richness | 0.416 | 0.109 | 3.802 | <0.001 |
| June (vs. May) | 0.379 | 0.228 | 1.662 | 0.097 | |||||
| Vegetation (vs. Ground) | 1.214 | 0.155 | 7.824 | <0.001 | |||||
| Pollinators | Omnivores | ||||||||
| β | SE | Z | p | β | SE | Z | p | ||
| (Intercept) | −0.377 | 0.215 | −1.752 | 0.080 | (Intercept) | 2.892 | 0.216 | 13.359 | <0.001 |
| Functional dispersion | 0.196 | 0.116 | 1.696 | 0.090 | Functional evenness | 0.162 | 0.104 | 1.550 | 0.121 |
| Functional evenness | −0.369 | 0.120 | −3.066 | 0.002 | Functional richness | −0.052 | 0.095 | −0.552 | 0.581 |
| June (vs. May) | 1.872 | 0.234 | 8.016 | <0.001 | Functional divergence | 0.095 | 0.106 | 0.896 | 0.370 |
| Vegetation (vs. Ground) | −0.687 | 0.209 | −3.295 | 0.001 | Functional originality | 0.176 | 0.107 | 1.639 | 0.101 |
| Taxonomic richness | 0.285 | 0.123 | 2.324 | 0.020 | |||||
| Vegetation (vs. Ground) | −3.085 | 0.185 | −16.704 | <0.001 | |||||
| Parasitoids | Detritivores | ||||||||
| β | SE | Z | p | β | SE | Z | p | ||
| (Intercept) | −0.007 | 0.238 | −0.031 | 0.975 | (Intercept) | 0.009 | 0.326 | 0.028 | 0.977 |
| Functional dispersion | 0.177 | 0.172 | 1.029 | 0.304 | Functional richness | −1.438 | 0.432 | −3.328 | 0.001 |
| Functional evenness | −0.187 | 0.112 | −1.680 | 0.093 | Functional vulnerability | 0.650 | 0.255 | 2.547 | 0.011 |
| Functional richness | −0.112 | 0.203 | −0.554 | 0.580 | Taxonomic richness | 0.486 | 0.190 | 2.550 | 0.011 |
| Functional originality | 0.115 | 0.092 | 1.251 | 0.211 | June (vs. May) | −2.305 | 0.516 | −4.470 | <0.001 |
| Taxonomic richness | 0.071 | 0.100 | 0.712 | 0.477 | |||||
| June (vs. May) | 1.530 | 0.297 | 5.153 | <0.001 | |||||
| Vegetation (vs. Ground) | −0.619 | 0.175 | −3.534 | <0.001 | |||||
| Ecosystem Function for Arthropods | Functional Trait | Variable Category | Classification | Trait Selection Source | Level Classification Source |
|---|---|---|---|---|---|
| Resource | Resource type | Nominal | 1 = Vegetative (leaves); 2 = Flower (leaves and flowers); 3 = Fruit (leaves and fruits) | Field observation | Field observation |
| Attractiveness | Flower color | Nominal | 1 = brown; 2 = inconspicuous; 3 = pink; 4 = purple; 5 = red; 6 = white; 7 = yellow | Field observation | Field observation |
| Flower area | Ordinal | small = approx. < 0.05 cm; medium = approx. 0.05 to 4 cm; large = approx. > 4 cm | Adapted from Fornoff et al., [29] | Classified according to potential arthropod meaningfulness | |
| Accessibility | Corolla shape | Ordinal | 1 = total openness; 2 = medium openness; 3 = low openness | Adapted from Fornoff et al., [29]. Flowers typologies from Aguiar [46] | Classified according to potential arthropod meaningfulness |
| Nutritional quality | Leaf texture | Ordinal | 1 = herbaceous; 2 = fleshy; 3 = Semi-sclerophyllous or sclerophyllous | Leaves typologies by consistency from Aguiar [46] | Literature |
| P | Ordinal | Literature | Literature | ||
| N | Ordinal | Literature | Literature | ||
| Architecture | Plant height at observation | Ordinal | 1 = 0 to 5 cm; 2 = 5 to 30 cm; 3 = 30 to 100 cm; 4 = > 100 cm | Adapted from Mahdavi and Bergmeier [44] | Observation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calheiros-Nogueira, B.; Aguiar, C.; Villa, M. Plant Functional Dispersion, Vulnerability and Originality Increase Arthropod Functions from a Protected Mountain Mediterranean Area in Spring. Plants 2023, 12, 889. https://doi.org/10.3390/plants12040889
Calheiros-Nogueira B, Aguiar C, Villa M. Plant Functional Dispersion, Vulnerability and Originality Increase Arthropod Functions from a Protected Mountain Mediterranean Area in Spring. Plants. 2023; 12(4):889. https://doi.org/10.3390/plants12040889
Chicago/Turabian StyleCalheiros-Nogueira, Bruno, Carlos Aguiar, and María Villa. 2023. "Plant Functional Dispersion, Vulnerability and Originality Increase Arthropod Functions from a Protected Mountain Mediterranean Area in Spring" Plants 12, no. 4: 889. https://doi.org/10.3390/plants12040889