4.1. Evaluation of Physiological Status
In the case of studies with rare and endangered plants, especially with respect to legally protected species, special care is necessary to avoid any disturbances and harm to the native populations. In natural conditions, chlorophyll
a fluorescence-derived parameters have been shown to be reliable indicators of the physiological state of rare plant populations [
6,
17,
18,
19,
20]. However, to understand a possible degree of generalizability of such results, it is necessary to point out that (i) the results cannot be easily compared between different genotypes and sites with different sets of environmental conditions, (ii) the growth and physiological indices do not always correlate tightly, and (iii) changes in various chlorophyll
a fluorescence parameters may have different physiological meaning. These important restrictions need to be discussed in some detail in the context of the present study.
The first restriction has been previously thoroughly discussed in a specialized literature [
30]. Most importantly, it must be considered that fluorescence data can be used only for the comparison of the impact of certain environmental factor(s) on the photochemistry of photosynthesis, but any absolute values have no physiological meaning. Therefore, genetically different plant populations can be evaluated only with respect to the impact of some environmental factors, rather than a direct comparison of their fluorescence parameter values. The only partial exception is for F
v/F
m, representing a maximum quantum yield of photochemistry of photosystem II, which is around 0.8 for plants in conditions near a physiological optimum, and diminishes in unfavorable conditions most likely related to the photoinhibition of photosynthesis through direct damage to D1 proteins [
31]. The chlorophyll fluorescence ratio F
v/F
m in
S. esthonica plants at the Pope site was below 0.8, indicating photoinhibition of photosynthesis. In turn, in the Apšuciems site, this ratio was slightly above 0.8 (except for in August 2011 in the generative plants). This was probably due to increased precipitation in Pope, because as rainfall increases, photosystem II activity-related indicators decrease (
Figure 6). The sharp decrease in F
v/F
m in Pope in August 2011 could be due to increased precipitation. A similar negative relationship between diminished F
v/F
m and increased summary precipitation in the previous months has been found for a the coastal-specific endangered species
Eryngium maritimum [
8]. However,
E. maritimum is a xerophytic drought-tolerant species well adapted to the hot Mediterranean conditions, and these plants have a relatively low vitality in Northern Europe, while
S. esthonica seems to be well adapted to high substrate moisture—even requiring it for optimal growth.
While all chlorophyll fluorescence-derived parameters obtained through fast induction kinetics are interlinked, each of them shows effects at specific points in the electron transport chain [
30]. F
v/F
0 is mostly affected by photochemical reactions at the donor side of photosystem II, including water-splitting activity [
30], and changes in this parameter have been shown to be an important determinant of biomass accumulation of
Dracocephalum moldavica plants at various fertilization regimes [
32]. However, F
v/F
0 did not show a direct relationship with growth at different mineral regimes in
S. esthonica (
Figure 8E) or biomass accumulation in plants at different soil moisture regimes (
Figure 11). Instead, both the Performance Index and RC/ABS showed better resolution for biomass accumulation, separating treatments with good vs poor growth (
Figure 8B,D and
Figure 11B,D). Consequently, fluorescence emission on the absorption basis together with energy fluxes related to a reduction in end electron acceptors of photosystem I [
30] are important indicators of biomass accumulation in
S. esthonica.
It is suggested that changes in the chlorophyll concentration in plants reflects the impact of relatively long-term environmental changes [
33]. However, even a relatively drastic decrease in leaf chlorophyll content usually has only minor negative effects on the photochemical activity of photosystem II [
34,
35]. In the present study, at the end of the vegetation season, chlorophyll concentration tended to decrease, which can be explained by the aging of leaves [
35] The chlorophyll concentration in flowering individuals was larger than that in the leaves of vegetative individuals in the Pope and Estonian populations, however, in the Apšuciems population, the opposite relationship was observed. A study of chlorophyll in leaves of various rose species and varieties found that in most cases, the amount of chlorophyll decreases during flowering and increases during the fruit-ripening stage [
36]. A study of corn hybrids found that after flowering, chlorophyll concentration and F
v/F
m was reduced in most cases as a result of aging [
37]. In the present study, measurements in July correspond to the flowering phase, while the August measurements correspond to the seed-maturation phase. In addition, it was observed that, somehow, the Pope plants flowered longer, and some plants were still flowering in August. The variation in the flowering dynamic was also observed between different seasons. In general, chlorophyll concentration increased slightly between flowering and fruit ripening, however, in 2011, this increase was less pronounced in the Apšuciems population. The leaf chlorophyll concentration of vegetative individuals began to decrease as early as July (except in 2011 in the Apšuciems population) (
Figure 1). In senescing leaves, chlorophyll concentration decreases, since metabolites diffuse to reaction centers, and the chlorophyll synthesis rate decreases [
35]. Consequently, the present study shows that the amount of chlorophyll depends on both environmental and ontogenesis factors.
It is evident that photosynthesis-related parameters, chlorophyll concentration, and chlorophyll a fluorescence-derived parameters do not always perfectly correlate with the growth performance of plants. Therefore, special care needs to be taken for the complex evaluation of all available indices in order to predict the physiological performance of model plants.
4.2. Effect of Mineral Nutrition
The presence of several
Saussurea species in calcareous soils indicates a good adaptability to high bicarbonate conditions. These soils are identified by the presence of a high amount of CaCO
3 and a relatively high pH reaction, and HCO
3– concentrations in calcareous soils increases with the rise in soil moisture [
38]. The growth of carbonate-susceptible species in calcareous soils is significantly reduced together with negative effects on mineral nutrition and photosynthesis at the level of enhancing photoinhibition [
39]. With respect to mineral nutrition, Fe deficiency is a common symptom in calcareous soils, resulting in leaf chlorosis [
40]. However, calcicole species can control mineral nutrient uptake through local soil acidification and the release of chelating compounds [
38]. Two subspecies of
S. alpina in the Western Alps were found to be natively growing in soils with different pH values: 6.00–7.31 for
S. alpina subsp.
alpina and 7.93–8.65 for
S. alpina subsp.
depressa [
41]. In the present study, the soil pH range found for
S. esthonica (pH 6.2–7.3) was closer to that of
S. alpina subsp.
alpina (
Table 2). In the soil containing the highest concentration of Ca (in the Apšuciems site), the highest concentration of Fe was also present (
Table 2), but
S. esthonica plants did not show any signs of photoinhibition of photosynthesis, as F
v/F
m values tended to be higher than in the site with a lower soil Ca level (
Figure 5).
It was initially hypothesized that
S. esthonica plants can adapt to a relatively wide range of soil mineral nutrient concentrations. Indeed, the concentration of plant-available mineral elements in the natural soils of
S. esthonica was highly variable between the sites, with especially high variability for Ca, Mg, S, Fe, and Mn (
Table 2). Several times higher concentrations of Fe, S, and Mn in the native soil of the Apšuciems site could be associated with continuously expressed higher performance of Apšuciems plants with respect to the photochemistry of photosynthesis (
Figure 4 and
Figure 5). The functional basis of this effect is related to the fact that all these elements are important for light-dependent reactions of photosynthesis: Fe is a cofactor in photosynthesis complexes and participates in many components of the photosynthetic electron transport chain, Mn is a functional component of a water-splitting complex of photosystem II, and Fe–S clusters are integral structural components of photosystem I reaction centers, cytochrome b6f, and ferredoxin [
42].
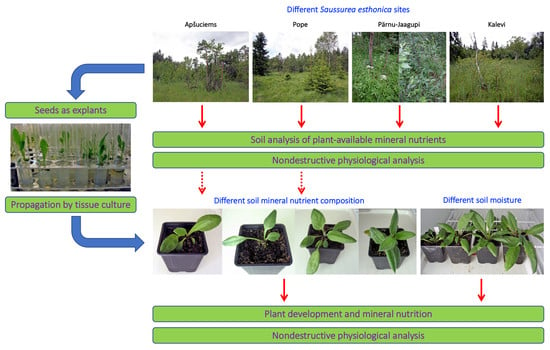
Therefore, we decided to establish four substrates with different mineral nutrient concentrations for a study in controlled conditions, based on the composition that is optimal for a majority of crop plants (control), soil in Apšuciems site (“Apšuciems”), soil in Pope site with lower N, Mg, S, Fe, Mn, and B than in “Apšuciems” soil (“Pope”), and soil based on the previous soil but with increased N and S concentrations (“Pope+”). Surprisingly, plants in “Apšuciems” soil had very low growth (
Table 5) and suppressed development (
Figure 7A), and chlorophyll fluorescence-related parameters significantly decreased in these plants at the last phase of cultivation (
Figure 8). However, the chlorophyll content in the leaves of plants in “Apšuciems” soil was stable and high throughout the cultivation period (
Figure 8A). Consequently, some other factors in the native site of
S. esthonica at Apšuciems were responsible for the increased photochemical performance of photosynthesis, both physicochemical and biological. It is possible that this discrepancy was related to differences in photosynthetically active radiation intensity between natural and controlled conditions, which did not allow for positive effects in low-light conditions, as many plant responses to single factors differ to those in presence of additional factors [
43]. However, an increase in N and S concentrations in “Pope+” substrate in comparison to those in “Pope” substrate significantly improved shoot growth (
Table 3) and increased leaf chlorophyll content, but not chlorophyll fluorescence indices of
S. esthonica plants.
4.3. Effect of Soil Moisture
In a vegetation study performed in Estonia,
S. esthonica plants were located in the part of the calcareous fen with the shallow depth to water level, characterized by high electrical conductivity, but multivariate analysis did not show any significant association between the presence of the individuals and any environmental variables [
44]. However, it was suggested that
S. esthonica can benefit from increasing shade due to the presence of shrubs and sparse trees [
44].
Cladium mariscus, another typical but extremely rare species of calcareous fens, did not show any immediate effect of habitat water level changes on the growth of generative shoots [
45]. Seasonal measurements of F
v/F
m indicated good adaptability of the species to high substrate water level, while being sensitive to a decrease in the water level below 5 cm [
45].
Soil moisture has a substantial influence on soil chemistry, leading to differences in mineral nutrient availability and uptake in plants [
38,
46]. However, the response is usually genotype-specific, as significant differences were found in mineral nutrient responses to increasing soil moisture in different species including
Veronica spicata and
Phleum phleoides [
38]. Thus, shoot Ca, K, P, and Mg concentration increased only in
Veronica spicata plants with increasing soil moisture levels, with no significant changes in
Phleum phleoides. However, only the concentrations of Mg and S gradually increased with increasing soil moisture in leaves of
S. esthonica plants (
Table 7).
S. esthonica is closely related, both morphologically and genetically, to other species of the genus including
S. alpina and
Saussurea discolor [
12,
13]. However, the results obtained in the present study are difficult to compare with those obtained with taxonomically related
Saussurea species due to the rather unique ecological niche of
S. esthonica.
Saussurea salsa, a species from salt marshes, showed morphological changes in leaves as a part of the adaptation strategy of plants from low to deep flooding, involving an increase in specific leaf area, together with a plasticity of photochemical reactions of photosynthesis [
47]. This was similar to the results of the present study, showing that waterlodged conditions were well tolerated up to 6 weeks, as indicated by stable trends both in the amount of leaf chlorophyll and indices of photochemistry of photosynthesis, followed by a decrease in the later stages of cultivation (
Figure 11). The optimal growth of
S. esthonica plants was evident in waterlodged conditions, decreasing with a decrease in soil moisture (
Table 5,
Figure 10), but physiological performance, as indicated by chlorophyll levels and the chlorophyll fluorescence parameters Performance Index and RC/ABS, was better for plants at moderate- (100%) and high- (150%) moisture conditions (
Figure 11). However, the maximum quantum efficiency of photosystem II (F
v/F
m), an indicator of photoinhibition of photosynthesis, showed that only at 150% moisture plants showed no signs of metabolic disturbance. Therefore,
S. esthonica plants can be characterized as a moisture-demanding species, with extremely high tolerance to soil waterlodged at the level of growth and photosynthesis. Other relatively waterlodged-tolerant species, such as
Trifolium fragiferum, usually show chlorosis and other signs of metabolic disturbance even after several weeks in waterlodged conditions [
48]. In
S. esthonica, certain visual signs of metabolic disturbances due to waterlodging appeared in the form of purple spots after 4 weeks and in the form of chlorotic lesions only after 12 weeks.
4.4. Future Perspectives
Calcareous fens have been recognized as local hotspots of biological diversity and refugia for rare and endangered plant species [
49]. A set of specific environmental conditions is responsible for the development of unique vegetation in calcareous fen habitats, but also determines its high vulnerability. In particular, alkaline fens are highly dependent on the inflow of calcareous groundwater resulting in a stable near-surface water level [
50]. Seasonal fluctuations in the water level occur mainly due to the differences in precipitation. The composition of plant species in calcareous fens depends on the amplitude of seasonal water level fluctuations, and changes less than 20–25 cm are suggested to be optimal for calcicole vascular plant species [
44]. Results of the present study suggest that
S. esthonica is strongly dependent on high substrate moisture. Therefore, the preservation of the natural water regime in the natural habitats of
S. esthonica is critical to the conservation of the species.
Genetic differentiation of
S. esthonica plants between the two Latvian sites, Apšuciems and Pope, was only 3–5%, pointing to their common provenance [
51]. However, there was a 10–13% differentiation between the two Latvian populations and those from Estonia (Pärnu-Jaagupi and Kalevi), but genetic polymorphism was lower in the Estonian populations. Therefore, in future studies, it will be necessary to compare the response of
S. esthonica plants from Latvian and Estonian populations to changes in environmental factors under controlled conditions, in order to find out whether genetic differences are related to differences in the ability of plants to adapt to a changing environment.