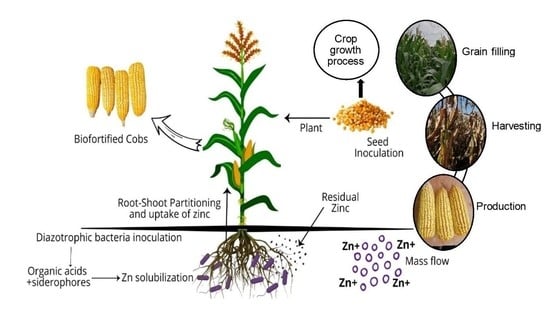
Diazotrophic Bacteria Is an Alternative Strategy for Increasing Grain Biofortification, Yield and Zinc Use Efficiency of Maize
Abstract
:1. Introduction
2. Results
2.1. Plant Height, Dry Matter and Grain Yield
2.2. Zinc Concentration in Leaf, Shoot, and Grains
2.3. Zinc Shoot and Grain Accumulation, Partitioning, and Intake in Maize
2.4. Zinc Efficiencies
2.5. Pearson’s Correlation among Evaluated Attributes of Maize
3. Discussion
4. Materials and Methods
4.1. Experimental Site and Climate Description
4.2. Soil Analysis
4.3. Experimental Design and Treatments
4.4. Plant Materials
4.5. Evaluations and Analysis
4.5.1. Growth and Yield Attributes
4.5.2. Nutritional Analysis
4.5.3. Zinc Partitioning Index, Intake, and Use Efficiencies
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xue, Y.F.; Yue, S.C.; Liu, D.Y.; Zhang, W.; Chen, X.P.; Zou, C.Q. Dynamic zinc accumulation and contributions of pre-and/or post-silking zinc uptake to grain zinc of maize as affected by nitrogen supply. Front. Plant Sci. 2019, 10, 1203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayyar, S.; Appavoo, S.; Basker, M.; Pandiyarajan, P.; Kavimani, R. Effect of Zinc and microbial inoculation on soil enzyme activities for maize (Zea mays L.) in black soil. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 1804–1814. [Google Scholar] [CrossRef]
- Conab Companhia Nacional de Abastecimento. Grains Report 11/2020. Brasília: Conab. Available online: https://www.conab.gov.br/info-agro/safras (accessed on 15 March 2022). (In Portuguese)
- United States Department of Agriculture. National Agricultural Statistics Service. Crop Production 2020. Available online: http://www.nass.usda.gov/Publications/index.php (accessed on 25 March 2022).
- Obaid, H.; Shrestha, R.K.; Liu, D.; Elsayed, N.S.; Ni, J.; Ni, C. Biofortification of Maize with Zinc and Its Effect on Human Health. J. Soil Sci. Plant Nutr. 2022, 7, 1–13. [Google Scholar] [CrossRef]
- Cakmak, I.; McLaughlin, M.J.; White, P. Zinc for better crop production and human health. Plant Soil 2017, 411, 1–4. [Google Scholar] [CrossRef]
- Masood, F.; Ahmad, S.; Malik, A. Role of Rhizobacterial Bacilli in Zinc Solubilization. In Microbial Biofertilizers and Micronutrient Availability; Khan, S.T., Malik, A., Eds.; Springer: Cham, Switzerland, 2022; pp. 361–377. [Google Scholar] [CrossRef]
- Suganya, A.; Saravanan, A.; Manivannan, N. Role of zinc nutrition for increasing zinc availability, uptake, yield, and quality of maize (Zea mays L.) grains: An overview. Commun. Soil Sci. Plant Anal. 2020, 51, 2001–2021. [Google Scholar]
- Dhaliwal, S.S.; Sharma, V.; Shukla, A.K.; Verma, V.; Kaur, M.; Shivay, Y.S.; Nisar, S.; Gaber, A.; Brestic, M.; Barek, V.; et al. Biofortification—A Frontier Novel Approach to Enrich Micronutrients in Field Crops to Encounter the Nutritional Security. Molecules 2022, 27, 1340. [Google Scholar] [CrossRef]
- Jalal, A.; Galindo, F.S.; Boleta, E.H.M.; da Silva Oliveira, C.E.; dos Reis, A.R.; Nogueira, T.A.R.; Moretti Neto, M.J.; Mortinho, E.S.; Fernandes, G.C.; Teixeira Filho, M.C.M. Common Bean Yield and Zinc Use Efficiency in Association with Diazotrophic Bacteria Co-Inoculations. Agronomy 2021, 11, 959. [Google Scholar] [CrossRef]
- Ahsin, M.; Hussain, S.; Rengel, Z.; Amir, M. Zinc status and its requirement by rural adults consuming wheat from control or zinc-treated fields. Environ. Geochem. Health 2020, 42, 1877–1892. [Google Scholar] [CrossRef]
- Hakim, S.; Naqqash, T.; Nawaz, M.S.; Laraib, I.; Siddique, M.J.; Zia, R.; Mirza, M.S.; Imran, A. Rhizosphere engineering with plant growth-promoting microorganisms for agriculture and ecological sustainability. Front. Sustain. Food Syst. 2021, 5, 16. [Google Scholar] [CrossRef]
- Bashir, S.; Basit, A.; Abbas, R.N.; Naeem, S.; Bashir, S.; Ahmed, N.; Ahmed, M.S.; Ilyas, M.Z.; Aslam, Z.; Alotaibi, S.S.; et al. Combined application of zinc-lysine chelate and zinc-solubilizing bacteria improves yield and grain biofortification of maize (Zea mays L.). PLoS ONE 2021, 16, e0254647. [Google Scholar] [CrossRef]
- Mumtaz, M.Z.; Ahmad, M.; Jamil, M.; Hussain, T. Zinc solubilizing Bacillus spp. potential candidates for biofortification in maize. Microbiol. Res. 2017, 202, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.; Farooq, M.; Naveed, M.; Nawaz, A.; Shahzad, B. Seed priming of Zn with endophytic bacteria improves the productivity and grain biofortification of bread wheat. Eur. J. Agron. 2018, 94, 98–107. [Google Scholar] [CrossRef]
- Upadhayay, V.K.; Singh, A.V.; Khan, A. Cross Talk between Zinc-Solubilizing Bacteria and Plants: A Short Tale of Bacterial-Assisted Zinc Biofortification. Front. Soil Sci. 2022, 1, 788170. [Google Scholar] [CrossRef]
- Galindo, F.S.; Bellotte, J.L.; Santini, J.M.; Buzetti, S.; Rosa, P.A.; Jalal, A.; Teixeira Filho, M.C.M. Zinc use efficiency of maize-wheat cropping after inoculation with Azospirillum brasilense. Nutr. Cycl. Agroecosyst. 2021, 120, 205–221. [Google Scholar] [CrossRef]
- Chandra, A.K.; Kumar, A.; Bharati, A.; Joshi, R.; Agrawal, A.; Kumar, S. Microbial-assisted and genomic-assisted breeding: A two-way approach for the improvement of nutritional quality traits in agricultural crops. 3 Biotech 2020, 10, 2. [Google Scholar] [CrossRef]
- Noulas, C.; Tziouvalekas, M.; Karyotis, T. Zinc in soils, water and food crops. J. Trace Elem. Med. Biol. 2018, 49, 252–260. [Google Scholar] [CrossRef]
- Jalal, A.; Shah, S.; Teixeira Filho, M.C.M.; Khan, A.; Shah, T.; Hussain, Z.; Younis, M.; Ilyas, M. Yield and phenological indices of wheat as affected by exogenous fertilization of Zinc and Iron. Braz. J. Agric. Sci./Rev. Bras. Cienc. Agrar. 2020, 15, e7730. [Google Scholar] [CrossRef] [Green Version]
- Marag, P.S.; Suman, A. Growth stage and tissue specific colonization of endophytic bacteria having plant growth promoting traits in hybrid and composite maize (Zea mays L.). Microbiol. Res. 2018, 214, 101–113. [Google Scholar] [CrossRef]
- Jalal, A.; Oliveira, C.; Freitas, L.; Galindo, F.; Lima, B.; Boleta, E.; Silva, E.C.; Nascimento, V.; Nogueira, T.A.R.; Buzetti, S.; et al. Agronomic biofortification and productivity of wheat with soil zinc and diazotrophic bacteria under tropical savannah. Crop Pasture Sci. 2022, 74, A–N. [Google Scholar] [CrossRef]
- Malavolta, E.; Vitti, G.C.; Oliveira, S.A. Evaluation of the Nutritional Status of Plants: Principles and Applications, 2nd ed.; Potafos: Piracicaba, Brazil, 1997; p. 319. (In Portuguese) [Google Scholar]
- Rehman, A.; Farooq, M.; Naveed, M.; Ozturk, L.; Nawaz, A. Pseudomonas-aided zinc application improves the productivity and biofortification of bread wheat. Crop Pasture Sci. 2018, 69, 659–672. [Google Scholar] [CrossRef]
- Mitter, B.; Pfaffenbichler, N.; Flavell, R.; Compant, S.; Antonielli, L.; Petric, A.; Berninger, T.; Naveed, M.; Sheibani-Tezerji, R.; von Maltzahn, G.; et al. A new approach to modify plant microbiomes and traits by introducing beneficial bacteria at flowering into progeny seeds. Front. Microbiol. 2017, 8, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, D.; Prasanna, R. Potential of microbes in the biofortification of Zn and Fe in dietary food grains. A review. Agron. Sustain. Dev. 2020, 40, 15. [Google Scholar] [CrossRef]
- Jalal, A.; Azeem, K.; Teixeira Filho, M.C.M.; Khan, A. Enhancing soil properties and maize yield through organic and inorganic nitrogen and diazotrophic bacteria. In Sustainable Crop Production; IntechOpen: London, UK, 2020; Volume 20, pp. 165–178. [Google Scholar]
- Pereira, N.C.M.; Galindo, F.S.; Gazola, R.P.D.; Dupas, E.; Rosa, P.A.L.; Mortinho, E.S. Corn yield and phosphorus use efficiency response to phosphorus rates associated with plant growth promoting bacteria. Front. Environ. Sci. 2020, 8, 40. [Google Scholar] [CrossRef]
- Abadi, V.A.J.M.; Sepehri, M.; Khatabi, B.; Rezaei, M. Alleviation of zinc deficiency in wheat inoculated with root endophytic fungus Piriformospora indica and rhizobacterium Pseudomonas putida. Rhizosphere 2021, 17, 100311. [Google Scholar] [CrossRef]
- Khan, A.; Singh, J.; Upadhayay, V.K.; Singh, A.V.; Shah, S. Microbial biofortification: A green technology through plant growth promoting microorganisms. In Sustainable Green Technologies for Environmental Management; Springer: Singapore, 2019; pp. 255–269. [Google Scholar]
- Jalal, A.; Shah, S.; Teixeira Filho, M.C.M.; Khan, A.; Shah, T.; Ilyas, M.; Rosa, P.A.L. Agro-biofortification of zinc and iron in wheat grains. Gesunde Pflanz. 2020, 72, 227–236. [Google Scholar] [CrossRef]
- Kaur, T.; Rana, K.L.; Kour, D.; Sheikh, I.; Yadav, N.; Kumar, V.; Yadav, A.N.; Dhaliwal, H.S.; Saxena, A.K. Microbe-mediated biofortification for micronutrients: Present status and future challenges. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–17. [Google Scholar] [CrossRef]
- Singh, D.; Rajawat, M.V.S.; Kaushik, R.; Prasanna, R.; Saxena, A.K. Beneficial role of endophytes in biofortification of Zn in wheat genotypes varying in nutrient use efficiency grown in soils sufficient and deficient in Zn. Plant Soil 2017, 416, 107–116. [Google Scholar] [CrossRef]
- Shakeel, M.; Rais, A.; Hassan, M.N.; Hafeez, F.Y. Root associated Bacillus sp. improves growth, yield and zinc translocation for basmati rice (Oryza sativa) varieties. Front. Microbiol. 2015, 6, 1286. [Google Scholar] [CrossRef]
- Shivay, Y.S.; Prasad, R.; Rahal, A. Relative efficiency of zinc oxide and zinc sulphate-enriched urea for spring wheat. Nutr. Cycl. Agroecosyst. 2008, 82, 259–264. [Google Scholar] [CrossRef]
- Galindo, F.S.; Buzetti, S.; Rodrigues, W.L.; Boleta, E.H.M.; Silva, V.M.; Tavanti, R.F.R.; Fernandes, G.C.; Biagini, A.L.C.; Rosa, P.A.L.; Teixeira Filho, M.C.M. Inoculation of Azospirillum brasilense associated with silicon as a liming source to improve nitrogen fertilization in wheat. Crops Sci. 2020, 10, 6160. [Google Scholar] [CrossRef]
- Schütz, L.; Gattinger, A.; Meier, M.; Müller, A.; Boller, T.; Mader, P.; Mathimaran, N. Improving Crop Yield and Nutrient Use Efficiency via Biofertilization—A Global Meta-Analysis. Front. Plant Sci. 2018, 8, 2204. [Google Scholar] [CrossRef] [Green Version]
- Soil Survey Staff. Keys to Soil Taxonomy, 12th ed.; USDA, Natural Resources Conservation Service: Washington, DC, USA, 2014.
- Santos, H.G.; Jacomina, P.K.T.; Anjos, L.H.C.; Oliveira, V.A.; Lumbreras, J.F.; Coelho, M.R.; Almeida, J.A.; Araujo Filho, J.C.; Oliveira, J.B.; Cunha, T.J.F. Brazilian Soil Classification System, 5th ed.; Embrapa: Brasília, Brazil, 2018; Available online: https://www.embrapa.br/en/busca-de-publicacoes/-/publicacao/1094001/brazilian-soil-classification-system (accessed on 18 February 2022).
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; Gonçalves, J.D.M.; Sparovek, G. Köppen’s climate classification map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef]
- Raij, B.; van Andrade, J.C.; Cantarella, H.; Quaggio, J.A. Chemical Analysis for Fertility Evaluation of Tropical Soils; IAC: Campinas, Brazil, 2001; p. 285. (In Portuguese) [Google Scholar]
- Teixeira, P.C.; Donagemma, G.K.; Fontana, A.; Teixeira, W.G. Manual of Soil Analysis Methods; Centro Nacional de Pesquisa de Solos, Embrapa: Rio de Janeiro, Brazil, 2017. (In Portuguese) [Google Scholar]
- Rengel, Z.; Graham, R.D. Uptake of zinc from chelate-buffered nutrient solutions by wheat genotypes differing in zinc efficiency. J. Exp. Bot. 1996, 47, 217–226. [Google Scholar] [CrossRef]
- Lessa, J.H.D.L.; Araujo, A.M.; Ferreira, L.A.; da Silva, E.C., Jr.; De Oliveira, C.; Corguinha, A.P.B.; Martins, F.A.D.; De Carvalho, H.W.P.; Guilherme, L.R.G.; Lopes, G. Agronomic biofortification of rice (Oryza sativa L.) with selenium and its effect on element distributions in biofortified grains. Plant Soil 2019, 444, 331–342. [Google Scholar] [CrossRef]
- Statistica Research Department. Brazil. Per Capita Consumption of Selected Cereals in Brazil. 2022. Available online: https://www.statista.com/statistics/1121036/brazil-per-capita-consumption-cereals-type/ (accessed on 24 March 2022).
- Fageria, N.K.; Dos Santos, A.B.; Cobucci, T. Zinc Nutrition of Lowland Rice. Commun. Soil Sci. Plant Anal. 2011, 42, 1719–1727. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015; Available online: https://www.R-project.org/ (accessed on 9 February 2022).





| Treatments | Plant Height | First Cob Insertion | Shoot Dry Matter | 100 Grains Mass | Grain Yield | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| m | kg ha−1 | g | kg ha−1 | |||||||
| 2019–2020 | 2020–2021 | 2019–2020 | 2020–2021 | 2019–2020 | 2020–2021 | 2019–2020 | 2020–2021 | 2019–2020 | 2020–2021 | |
| Inoculations (I) | ||||||||||
| Without | 2.66 b | 2.67 b | 1.22 b | 1.25 b | 11,945 b | 11,832 | 30.6 b | 31.7 | 7379 | 7307 |
| A. brasilense | 2.78 a | 2.79 a | 1.29 ab | 1.31 a | 12,642 a | 12,654 | 33.5 a | 34.4 | 8109 | 8233 |
| B. subtilis | 2.67 ab | 2.72 ab | 1.27 ab | 1.31 a | 12,381 a | 12,381 | 32.7 ab | 35.4 | 8449 | 8555 |
| P. fluorescens | 2.72 ab | 2.77 a | 1.29 a | 1.33 a | 12,355 a | 12,243 | 31.8 ab | 33.5 | 7911 | 7952 |
| Residual Zinc (Zn) Doses (kg ha−1) | ||||||||||
| 0 | 2.67 b | 2.71 b | 1.25 a | 1.28 b | 12,102 b | 12,040 | 31.3 b | 32.7 | 7709 | 7806 |
| 8 | 2.72 a | 2.77 a | 1.28 a | 1.32 a | 12,559 a | 12,515 | 32.9 a | 34.8 | 8215 | 8218 |
| F-values | ||||||||||
| I | 0.004 ** | 0.00 ** | 0.03 * | 0.00 ** | 0.00 ** | 0.00 ** | 0.01 * | 0.00 ** | 0.00 ** | 0.00 ** |
| Zn | 0.01 ** | 0.00 ** | 0.11ns | 0.008 ** | 0.00 ** | 0.00 ** | 0.01 * | 0.00 ** | 0.00 ** | 0.00 ** |
| I × Zn | 0.63 ns | 0.19 ns | 0.99 ns | 0.86 ns | 0.36 ns | 0.04 * | 0.94 ns | 0.02 * | 0.03 * | 0.02 ** |
| CV (%) | 2.1 | 1.7 | 3.9 | 2.4 | 2.3 | 1.8 | 5.2 | 2.8 | 4.0 | 3.6 |
| Treatments | Leaf Zn Concentration | Shoot Zn Concentration | Grain Zn Concentration | |||
|---|---|---|---|---|---|---|
| mg kg−1 | ||||||
| 2019–2020 | 2020–2021 | 2019–2020 | 2020–2021 | 2019–2020 | 2020–2021 | |
| Inoculations (I) | ||||||
| Without (control) | 20.6 b | 21.7 | 29.1 b | 29.5 b | 28.2 b | 32.5 |
| A. brasilense | 23.5 ab | 27.4 | 35.8 ab | 35.7 a | 35.4 a | 41.0 |
| B. subtilis | 23.9 ab | 28.0 | 33.4 ab | 33.9 ab | 32.9 a | 36.9 |
| P. fluorescens | 25.5 a | 29.1 | 37.7 a | 38.5 a | 34.6 a | 38.5 |
| Residual Zinc (Zn) Doses (kg ha−1) | ||||||
| 0 | 21.7 b | 24.4 | 31.6 b | 31.9 b | 30.8 b | 34.1 |
| 8 | 25.0 a | 28.7 | 36.4 a | 36.9 a | 34.7 a | 40.3 |
| F-values | ||||||
| I | 0.03 * | 0.00 * | 0.01 * | 0.002 ** | 0.00 ** | 0.00 ** |
| Zn | 0.008 ** | 0.00 ** | 0.01 * | 0.002 ** | 0.00 ** | 0.00 ** |
| I × Zn | 0.43 ns | 0.04 * | 0.76 ns | 0.78 ns | 0.33 ns | 0.03 * |
| CV (%) | 13.5 | 6.2 | 14.2 | 12.0 | 8.6 | 6.7 |
| Treatments | Shoot Zn Accumulation | Grain Zn Accumulation | Zn Partitioning Index | Zn Intake (Brazil) | ||||
|---|---|---|---|---|---|---|---|---|
| g ha−1 | % | g person−1 day−1 | ||||||
| 2019–2020 | 2020–2021 | 2019–2020 | 2020–2021 | 2019–2020 | 2020–2021 | 2019–2020 | 2020–2021 | |
| Inoculations (I) | ||||||||
| Without I | 348.5 b | 349.3 b | 208.9 | 237.7 | 51.8 a | 47.5 a | 1.9 b | 2.2 |
| A. brasilense | 452.8 a | 453.4 a | 286.7 | 337.6 | 50.2 a | 46.6 a | 2.4 a | 2.7 |
| B. subtilis | 413.5 ab | 420.0 ab | 279.5 | 317.9 | 50.1 a | 47.8 a | 2.2 a | 2.5 |
| P. fluorescens | 466.3 a | 471.6 a | 273.9 | 306.2 | 51.8 a | 49.8 a | 2.3 a | 2.6 |
| Residual Zinc (Zn) Doses (kg ha−1) | ||||||||
| 0 | 383.4 b | 384.2 b | 238.2 | 266.9 | 50.5 a | 48.1 a | 2.1 b | 2.3 |
| 8 | 457.2 a | 463.0 a | 286.3 | 332.7 | 51.1 a | 47.8 a | 2.4 a | 2.7 |
| F-values | ||||||||
| I | 0.002 ** | 0.00 ** | 0.00 ** | 0.00 ** | 0.86 ns | 0.31 ns | 0.00 ** | 0.00 ** |
| Zn | 0.001 ** | 0.00 ** | 0.00 ** | 0.00 ** | 0.71 ns | 0.77 ns | 0.00 ** | 0.00 ** |
| I × Zn | 0.63 ns | 0.80 ns | 0.04 * | 0.00 ** | 0.99 ns | 0.78 ns | 0.33 ns | 0.02 ** |
| CV (%) | 13.7 | 12.5 | 8.9 | 7.3 | 8.6 | 6.8 | 8.6 | 6.6 |
| Treatments | ZnUE | APE | UE | AZnR | ||||
|---|---|---|---|---|---|---|---|---|
| kg kg−1 | % | |||||||
| 2019–2020 | 2020–2021 | 2019–2020 | 2020–2021 | 2019–2020 | 2020–2021 | 2019–2020 | 2020–2021 | |
| Inoculations (I) | ||||||||
| Without (control) | 164 c | 68 c | 19 a | 4.5 b | 297 b | 131 b | 12 b | 15 b |
| A. brasilense | 233 b | 178 b | 7 a | 3.8 b | 483 a | 353 a | 35 a | 47 a |
| B. subtilis | 317 a | 270 a | 10 a | 6.5 a | 509 a | 381 a | 32 a | 42 a |
| P. fluorescens | 190 c | 135 b | 7 a | 3.2 b | 379 b | 219 b | 33 a | 43 a |
| F-values | ||||||||
| I | 0.00 ** | 0.00 ** | 0.05 * | 0.003 ** | 0.00 ** | 0.00 ** | 0.008 ** | 0.00 ** |
| CV (%) | 8.3 | 13 | 54 | 20 | 10 | 17 | 29 | 21 |
| Properties | Units | Status | |
|---|---|---|---|
| 2019–2020 | 2020–2021 | ||
| pH (CaCl2) | ---- | 5.2 | 5.3 |
| Organic matter | mg dm−3 | 18 | 23 |
| P (resin) | mg dm−3 | 38 | 40 |
| K | mmolc dm−3 | 1.7 | 1.9 |
| Ca | mmolc dm−3 | 21 | 22 |
| Mg | mmolc dm−3 | 15 | 12 |
| B (hot water) | mg dm−3 | 0.14 | 0.39 |
| Cu (DTPA) * | mg dm−3 | 3.4 | 3.7 |
| Fe (DTPA) * | mg dm−3 | 25 | 28 |
| Mn (DTPA) * | mg dm−3 | 38.1 | 37.3 |
| S-SO4 | mg dm−3 | 4.0 | 22 |
| H + Al | mmolc dm−3 | 34 | 31 |
| CEC (pH7) * | mmolc dm−3 | 75.7 | 66.9 |
| V * | % | 50 | 54 |
| Zn content (DTPA) | |||
| Without Zn fertilization | mg dm−3 | 0.9 | 1.1 |
| Residual Zn fertilization | mg dm−3 | 2.2 | 3.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jalal, A.; Oliveira, C.E.d.S.; Fernandes, H.B.; Galindo, F.S.; Silva, E.C.d.; Fernandes, G.C.; Nogueira, T.A.R.; De Carvalho, P.H.G.; Balbino, V.R.; Lima, B.H.d.; et al. Diazotrophic Bacteria Is an Alternative Strategy for Increasing Grain Biofortification, Yield and Zinc Use Efficiency of Maize. Plants 2022, 11, 1125. https://doi.org/10.3390/plants11091125
Jalal A, Oliveira CEdS, Fernandes HB, Galindo FS, Silva ECd, Fernandes GC, Nogueira TAR, De Carvalho PHG, Balbino VR, Lima BHd, et al. Diazotrophic Bacteria Is an Alternative Strategy for Increasing Grain Biofortification, Yield and Zinc Use Efficiency of Maize. Plants. 2022; 11(9):1125. https://doi.org/10.3390/plants11091125
Chicago/Turabian StyleJalal, Arshad, Carlos Eduardo da Silva Oliveira, Henrique Benetasse Fernandes, Fernando Shintate Galindo, Edson Cabral da Silva, Guilherme Carlos Fernandes, Thiago Assis Rodrigues Nogueira, Pedro Henrique Gomes De Carvalho, Vinícius Rodrigues Balbino, Bruno Horschut de Lima, and et al. 2022. "Diazotrophic Bacteria Is an Alternative Strategy for Increasing Grain Biofortification, Yield and Zinc Use Efficiency of Maize" Plants 11, no. 9: 1125. https://doi.org/10.3390/plants11091125