1. Introduction
According to FAO [
1] more than 424 million hectares of topsoil (0–30 cm) and 833 million hectares of subsoil (30–100 cm) are salt-affected. Furthermore, approximately 20% of agricultural soils have excessive salt contents [
2]. Salinity as a stress factor decreases the osmotic potential of the soil, which severely affects water absorption by plants and, therefore, cell turgor, hindering plant growth and yield, with great losses to the agricultural industry [
3,
4,
5]. Due to weathering processes and the use of wastewater in agricultural irrigation, different concentrations and types of salts can be found in soils, rivers, lakes, and groundwater layers. Sodium chloride (NaCl), sodium sulfate (Na
2SO
4), and sodium carbonate monohydrate (NaHCO
3) are highly soluble salts and the most common in cultivated soils [
6].
The high accumulation of the Na
+, Cl
−, and SO
42− ions at the cellular level in plants induces nutritional and osmotic imbalances, as well as the overaccumulation of reactive oxygen species, which as a whole negatively affects cell metabolism and function. Indeed, cell walls can collapse and cause cell death as a consequence of excessive salt [
3]. On the other hand, plants under this type of stress can develop homeostatic control of water and ions, tolerance to Na
+, and synthesis of low-molecular-weight organic solutes in the cytoplasm (i.e., soluble sugars, organic acids, amino acids, and their methylated derivatives), all this to decrease the cellular osmotic potential and maintain the level of turgor [
7,
8].
Attempts to reclaim arable lands affected by salinity and maintain a productive agricultural system have proved to be expensive and thus unaffordable, with only temporary success, so the introduction of novel agricultural practices such as plant biostimulation may represent a creditable alternative to cope with salt stress [
9,
10], especially in horticultural crops such as tomato (
Solanum lycopersicum L.).
Tomato is the second most important vegetable produced worldwide, just after potato. There is a great diversity of tomato genotypes within this species, which can be cultivated in practically every country of the world, in fields, greenhouses and net houses. Given its versatility, tomato can be consumed either fresh or processed into many different products [
11]. In both cases, world production and consumption has exhibited a constant growth rate over the last three decades. Importantly, tomatoes represent a major source of bioactive compounds including lycopene, vitamin C, β-carotene, phenolic compounds, and tocopherol, as well as minerals. Many of these compounds are powerful antioxidants with anti-inflammatory properties that also have the ability to modulate the immune system [
12], and the concentrations of such compounds in fruit tissues can be increased under certain salt stress conditions [
13,
14]. Tomato has been classified as moderately sensitive to salinity and most cultivars currently available on the market may exhibit yield reduction when exposed to growth media with electrical conductivity values above 2.5 dS m
−1 [
15], but the application of certain biostimulants has proven to trigger efficient tolerance mechanisms to cope with salinity [
9,
16]. Importantly, tomato production worldwide is of high economic value. Worldwide production of fresh and processed tomatoes was 186.8 billion metric tons in 2020, with China being the world’s largest producer, followed by India, Turkey, the United States and Egypt, which together supply 70% of global tomato demand [
17]. Since 2015, Mexico has been the leading tomato exporter, reaching 3.7 billion pounds in 2020, which accounts for 90.7% of US total imports of tomatoes [
18]. In the last decade, the mean annual growth rate of tomato exports in Mexico has been 5.8% [
19], which can still grow with the use of novel technologies such asthe application of biostimulants.
Inorganic biostimulants such as beneficial elements have gained momentum in the development of measures to mitigate abiotic stress factors in agriculture. Titanium (Ti) is a beneficial element found in most rocks, sediments, and sands. It is the ninth most abundant element in the Earth’s crust and the second of the transition metals. Ti has a great affinity for oxygen, thanks to which it can be found naturally as titanium dioxide (TiO
2) in three forms: anatase (tetragonal form), rutile (tetragonal form), and brookite (orthorhombic form), as well as in the form of ilmenite (FeTiO
3). These forms of Ti in the soil are insoluble, and therefore not available to plants [
20,
21,
22].
Currently, anatase is one of the most used sources to extract Ti, and China has large deposits of this mineral, which have not been fully exploited. In 2012, the world production capacity of TiO
2 pigments increased to 6.5 million Mg. By 2016, the United States alone imported 247,000 Mg Ti, representing a 12% increase over the previous year; its main supplier countries were Canada (31%) and China (28%). The extracted Ti is used in alloys (aeronautics, aerospace, jewelry, and medicine), paper, paints, pigments, plastics, and porcelain, among other products. It is estimated that by the year 2025, the global demand for TiO
2 will increase by 4.1% per year, that is, 8.82 million Mg year
−1 [
22,
23].
Nowadays, another form of Ti exists in the environment, TiO
2 nanoparticles (TiO
2NPs). Global TiO
2NPs production is estimated at 88,000 Mg year
−1; they are used in cosmetics, and as sweeteners and flavor enhancers in foods. This type of material reaches crops mainly through wastewater and wastewater sludge, in addition to air, which causes high availability of this element for plants. However, the mechanisms of access, absorption, and transport of Ti in higher plants are still unknown. In Europe, it has been estimated that TiO
2NPs residues can reach up to 0.13 mg kg
−1 soil year
−1, and in soils irrigated with wastewater, they can reach up to 1200 mg kg
−1 year
−1 [
21,
24].
Several studies classify Ti as a beneficial element. In mung beans (
Vigna radiata L.), Ti improves root and stem length [
25]. In soybean (
Glycine max L.), Ti increases stem height and biomass [
26]. In various legumes such as faba bean (
Vicia faba L.), the application of Ti increases the concentrations of sugars, amino acids, chlorophyll, and proline [
27], and in cucumber (
Cucumis sativus L.), it increases the concentrations of P and K [
28]. The objective of this study was to evaluate the main effect of leaf-applied titanium (at concentrations of 0, 500, and 1000 mg Ti L
−1) and NaCl (at doses of 0, 50, and 100 mM in the nutrient solution), and their interactions, on some physiological, biochemical, and nutritional variables of tomato seedlings, in order to shed light on the role of Ti in tomato exposed to salt stress.
3. Discussion
In plants, the growth process is negatively affected by excess salinity in the growth medium. Excess NaCl in the substrate increases osmotic pressure and reduces water absorption, decreasing cell turgor and elongation. Inside the plant, the Na
+ cation and the Cl
− anion are concentrated in senescent leaves and through the flow of transpiration they are translocated to the aerial part, affecting the cells in leaves with greater photosynthetic activity and thus reducing the rate of photosynthesis, carbon uptake, and accumulation of fresh biomass [
29]. In our study, salinity significantly affected the variables of seedling height, stem diameter, and leaf area (
Table 1 and
Table 2). Similarly, the application of 50 mM NaCl decreased plant height and stem diameter in hybrid Saladette-type “Pony Express” tomato seedlings [
30], and 150 mg L
−1 NaCl affected the leaf area of Chonto-type tomato seedlings [
31]. This indicates that, at a higher NaCl concentration, lower seedling height, stem diameter, and leaf area are observed, that is, these variables are affected inversely proportional to the level of salinity tested [
32,
33,
34].
The biological effects of Ti on plant physiology and metabolism (i.e., synthesis of the Ti chelating α-hydroxy carboxylic acids
—citric and malic acids-; synthesis of ascorbic acid; modulation of Fe and Mg contents in plant tissues; chlorophyll biosynthesis; as well as enzymatic activity of nitrate reductase and of other proteins involved in the antioxidant system) are dose-dependent and therefore display hormesis [
35,
36]. Hormesis is a natural phenomenon characterized by favorable responses to low
-level exposures to a chemical compound or to adverse conditions [
37]. This phenomenon has a plethora of applications in different disciplines and can generally be utilized as a quantitative measure of biological plasticity through adaptive responses under stressful conditions [
38]. In the primary producer, nitrogen-fixing cyanobacteria
Anabaena PCC 7120, a nano-TiO
2 dose-dependent production of amino acids involved in N assimilation and intracellular N storage was observed, which possibly contributes to the increase in newly synthesized proteins needed for detoxification in response to the cellular stresses induced by nano-TiO
2 treatments in a hormetic manner [
39]. Though approaches to address the uptake, translocation, phytotoxicity, and hormetic effects of nano-TiO
2 have been undertaken [
40], in-depth studies on the hormetic effects of bulk Ti on plant biology are lacking. Therefore, our study may contribute with novel data to generate the information needed to estimate the hermetic responses of plants to Ti applications.
In this research, the beneficial effect of Ti on the growth variables evaluated was not observed at the saline doses tested. Rather, it is likely that the doses of Ti applied to the tomato seedlings caused an additional stress to that already induced by salinity, which triggered negative effects on the variables evaluated (
Table 1 and
Table 2). Similar effects with Ti were reported in rice (
Oryza sativa L.) and Narbon bean (
Vicia narbonensis), since the exposure of plants to doses between 500 and 2000 mg Ti L
−1 and 0.2%
nTiO
2 inhibited germinative and growth processes [
41,
42]. Potentially, Ti may induce toxicity in mitotic cell chromosomes [
43]. Furthermore, it could negatively affect the activity of enzymes related to nitrogen metabolism (nitrate reductase, glutamate dehydrogenase, glutamine synthase, and glutamic-pyruvic transaminase), an essential element (i.e., the major plant macronutrient) highly related to growth and development in plants [
44].
The accumulation of the Cl
− anion over time produces leaf necrosis, which decreases the photosynthetic capacity of the leaves and inhibits the absorption of nitrate. Similarly, excess Na
+ exerts an antagonistic effect on K
+, which regulates nitrate metabolism by acting as a companion ion and as an activator of the nitrate reductase enzyme, responsible for initiating the nitrate reduction process [
45,
46]. Similarly, by adding 50 mM NaCl to rough lemon (
Citrus jambhiri Lush.), the mean value of SPAD units decreased [
47]. Likewise, the addition of 20, 40, and 60 mM NaCl in common bean (
Phaseolus vulgaris L.) cv. Ica Cerinza decreased SPAD units 28 days after the treatments were applied [
48]. This negative effect of NaCl on SPAD units was evident in this study (
Table 2).
Decreases in chlorophyll contents in seedlings under saline stress can be attributed to an increase in the activity of the enzyme chlorophyllase, in addition to the degradation of chloroplasts [
49]. This was not observed in our study, since there were no significant differences in chlorophylls
a or
b among treatments (
Table 3). Coincidentally, red radish (
Raphanus sativus L.) seedlings at the V18 stage exposed to 70 mM NaCl did not decrease their chlorophyll concentrations, which was attributed to the sufficiency of N and Mg to cope with stress [
50]. This result is also in full agreement with our study since there were no significant differences in N with the addition of NaCl (
Table 7). Sodium chloride inhibits transpiration and stomatal conductance; it affects components of photosynthesis such as chlorophylls and carotenoids, and the quantum yield of PSII electron transport [
51,
52]. This effect was only observed in the total chlorophyll variable, since the treatment with 100 mM NaCl and 1000 mg Ti L
−1 reduced the concentration of total chlorophyll with respect to the treatment without salinity with 500 mg Ti L
−1 (
Table 3).
Sugars are organic solutes associated with salt tolerance in higher plants. At the cellular level, an osmotic balance is created between the tonoplast and the external environment, through the synthesis and accumulation of sugars in the cytoplasm and vacuole, which allow tolerance to salinity through ionic homeostasis and water absorption [
53,
54,
55,
56]. On the other hand, salinity improves the accumulation and transport of carbohydrates within the plant, as it promotes the expression of the
LeSUT1 gene, which encodes a sucrose transporter from source leaves, through the phloem, to other organs such as stems and fruits [
57,
58]. In our study, the highest accumulation of sugars was observed in the treatments without salinity in leaves and roots. However, in stems, the concentration of sugars was higher in the treatment with 50 mM NaCl compared to that observed in seedlings exposed to the treatment without NaCl (
Table 4). This finding coincides with a study carried out in tomato cv. INCA 17 treated with 50 mM NaCl, where increases in dry weight were observed, due to greater synthesis of sugars in the stems [
59].
In this research, the leaf-applied TiO
2 did not significantly affect the concentration of sugars in stems or roots, which is also in full agreement with what was observed in tomato fruits cv. ISI 68249 treated with 80, 240, 480, and 960 g Ti ha
−1 [
60]. Nevertheless, Ti did decrease the concentration of sugars in leaves (
Table 4).
When a crop is challenged with biotic or abiotic stress factors, there is an overaccumulation of free radicals at the cellular level that cause cascade reactions, potentially damaging biomolecules such as proteins, lipids, and nucleic acids, which can lead to the activation of apoptotic processes. During the process of defense against oxidative damage, plants activate both enzymatic and non-enzymatic antioxidant systems. In tomato, the main antioxidant metabolites are lycopene and ascorbic acid, which are responsible for capturing most reactive oxygen species [
61].
Table 5 and
Table 6 show the evaluated antioxidant activity in leaves, stems, and roots. Of the three evaluated organs, the NaCl factor caused the greatest antioxidant activity in leaves, while the NaCl × Ti interaction increased this activity in roots exposed to 50 mM NaCl at every level of Ti tested. Among the possible antioxidant enzymes activated by the tested treatments, we can find superoxide dismutase (SOD), catalase (CAT) and various peroxidases (GPX and APX) [
62,
63]. In seedlings exposed to stress, Ti can contribute to reducing the concentration of reactive oxygen species through the potential stimulation of these enzymes [
64].
In pepper, iron-depending enzymes as well as nitrate reductase were stimulated by Ti applications, thus activating the antioxidant system and protecting the plants against reactive oxygen species damage [
65]. In different strawberry (
Fragaria x
ananassa Duch.) cultivars, the application of Ti increased the concentrations of L-ascorbic acid and total anthocyanins, while significant enhancements of scavenging 2,2’-azinobis-(3-ethylbenzothiazoline-6 sulfonic acid) (ABTS) and 2-diphenyl-1-picrylhydrazyl (DPPH) were also observed, demonstrating an increased antioxidant activity triggered by Ti in this species [
66]. In henbane (
Hyoscyamus niger L.), both nano-sized TiO
2 (NT) and bulk TiO
2 (BT) increased SOD activity with increasing TiO
2 concentrations, whereas tropane alkaloid biosynthesis was also stimulated at low NT levels. In general, all tested antioxidant enzymes displayed higher activity in NT- treated plants, as compared to those of BT-treated ones [
67]. In maize (
Zea mays L.), Ti increased antioxidant enzyme activity and decreased malondialdehyde (MDA) accumulation, thus preventing lipid peroxidation and oxidative damage [
68].
Saline increases in the growth media (i.e., soil, substrates or nutrient solutions) prevent plants from absorbing enough water, due to an increase in the osmotic pressure at the root level. In turn, the increased osmotic pressure provokes stomatal closure, decreased transpiration rate, or reduced nutrient load in the xylem [
69]. Sodium chloride affects N absorption and metabolism, in addition to its transport and distribution to the aerial part, since the Cl
− anion has an antagonistic effect with both NO
3− and NH
4+ [
70,
71,
72]. However, this effect was not observed in our study, as N was not affected by the treatments with 50 and 100 mM NaCl. On the other hand, salinity enhanced the accumulation of P in leaves. This result is consistent with that observed in lavender (
Lavandula angustifolia Mill.) exposed to 25 and 50 mM NaCl, which increased P concentrations in leaf tissues as compared to the control not exposed to NaCl [
72]. Negative effects were observed in the leaf concentration of K, since salinity reduced it by 28 and 32%, with respect to the control. This is because the Na
+ cation competes for binding sites in the transport of the K
+ cation, in addition to the damage caused by Na
+ in the cell membrane, allowing K
+ leakage [
73,
74], as can be seen in
Table 7.
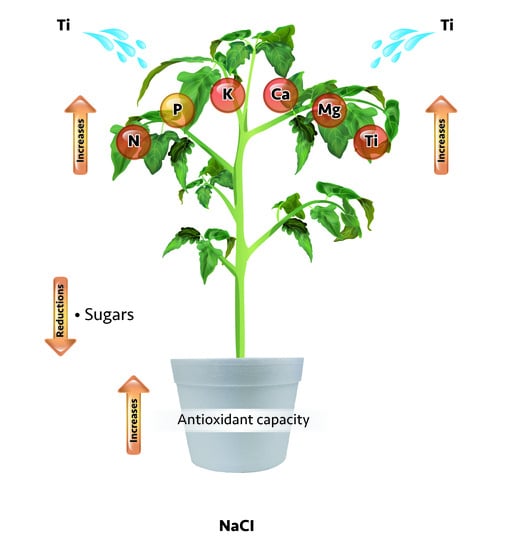
The NaCl factor tended to reduce the concentrations of K, Ca, and Mg, but increased those of P and Ti. The Ti factor increased the concentrations of N, P, K, Mg, and Ti in comparison with each respective control. In the case of Ca, Ti increased its concentration only with 500 mg Ti L
−1, in the absence of NaCl. The interaction of the study factors (NaCl × Ti) showed a higher mean of N when the seedlings were treated with 1000 mg Ti L
−1, regardless of the NaCl value tested. The P concentration was higher when applying 100 mM NaCl and 1000 mg Ti L
−1. The K concentration was higher with increasing Ti levels, in the absence of NaCl, which was also observed for Ca and Mg. The concentration of Ti was higher when applying 100 mM NaCl and 1000 mg Ti L
−1 (
Table 7). Similar effects have been reported in tomato cv. ISI 68249 treated with 960 g Ti ha
−1 [
60].
In some pioneering experiments with pepper, the concentration of some essential macro- and micronutrients were enhanced when plants were supplied with Ti [
75]. Interestingly, Ti effects were maximal under field conditions with traditional fertilization and minimal in optimal conditions under controlled environments in hydroponics [
75,
76]. Coincidently, when withdrawing N or P from the fertilizer applied to Ti-treated crops, no nutritional imbalances in the plants were observed [
77,
78]. Importantly, the concentration of Fe in Ti-treated pepper plants was higher than in controls, and the increase was higher for Fe as compared to the other nutrients analyzed [
79], which indicates that Fe may be a critical element implicated in the action mechanism of Ti in plants [
80]. In peach (
Prunus persica L.), Ti application showed significant increases in Fe, Cu and Zn concentrations in the peel, and Ca concentration in the peel and flesh [
81,
82], which can be explained by the beneficial effect of Ti on the processes of nutrient absorption, translocation and assimilation within the plant. In the M.26 EMLA apple (
Malus sp.) rootstock, foliar Ti applications enhanced plant dry matter and levels of P, Fe, Mn, and Zn in leaf tissues, with leaves being greener and having higher Fe
2+ and chlorophyll concentrations than those of control plants. These responses could be attributed to a higher concentration of Fe
2+ in leaves promoted by Ti applications, which enhanced chlorophyll synthesis and uptake of essential nutrients [
83]. In spinach (
Spinacia oleracea L.), nano-TiO
2 properly applied accelerated germination of naturally aged seeds and increased seedling vigor. Furthermore, nano-TiO
2 improved formation of chlorophyll and enhanced the enzymatic activity of Rubisco and the photosynthetic rate as a result of improved uptake and absorption of essential nutrients such as N and Mg [
84]. At typical Ti concentrations in the organism, Ti might exist as a labile pool of Ti(IV) within the cell, similar to Fe. Thus, Ti could mimic the functions of Fe in the cell. Various intracellular targets of Ti include phosphoproteins, DNA, ribonucleotide reductase, and ferritin [
85]. We are currently further investigating the effects of Ti on Fe concentrations in different tissues of tomato and other horticultural crops to answer this question.