Chemical Composition of Kickxia aegyptiaca Essential Oil and Its Potential Antioxidant and Antimicrobial Activities
Abstract
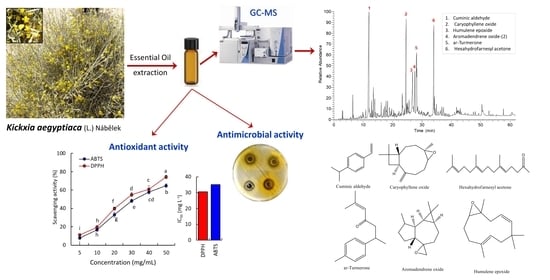
:1. Introduction
2. Results and Discussion
2.1. Chemical Characterization of K. aegyptiaca EO
2.2. Antioxidant Activity of K. aegyptiaca EO
2.3. Antibacterial Activity of K. aegyptiaca EO
3. Materials and Methods
3.1. Plant Materials
3.2. Extraction of EO and GC-MS Analysis
3.3. Antioxidant Activity of the EO
3.4. Antibacterial Activity of the EO
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Li, C.-J.; Anastas, P.T. Green Chemistry: Present and future. Chem. Soc. Rev. 2012, 41, 1413–1414. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Gawad, A.M. Chemical constituents, antioxidant and potential allelopathic effect of the essential oil from the aerial parts of Cullen plicata. Ind. Crop. Prod. 2016, 80, 36–41. [Google Scholar] [CrossRef]
- Elshamy, A.I.; Ammar, N.M.; Hassan, H.A.; Al-Rowaily, S.L.; Ragab, T.I.; El Gendy, A.E.-N.G.; Abd-ElGawad, A.M. Essential oil and its nanoemulsion of Araucaria heterophylla resin: Chemical characterization, anti-inflammatory, and antipyretic activities. Ind. Crop. Prod. 2020, 148, 112272. [Google Scholar] [CrossRef]
- Abd El-Gawad, A.M.A.; El-Amier, Y.A.; Bonanomi, G. Allelopathic activity and chemical composition of Rhynchosia minima (L.) DC. essential oil from Egypt. Chem. Biodivers. 2017, 15, e1700438. [Google Scholar] [CrossRef]
- Ghebrehiwet, M. Taxonomy, phylogeny and biogeography of Kickxia and Nanorrhinum (Scrophulariaceae). Nord. J. Bot. 2000, 20, 655–690. [Google Scholar] [CrossRef]
- Boulos, L. Flora of Egypt Checklist, Revised Annotated Edition; Al-Hadara Publishing: Cairo, Egypt, 2009; pp. 198–201. [Google Scholar]
- Kassem, F.F. Flavonoids of Kickxia aegyptiaca (Dum.) Nabelek. Alex. J. Pharm. Sci. 1992, 6, 62–65. [Google Scholar]
- Binish, Z.; Bibi, Y.; Zahara, K.; Nisa, S.; Manaf, A.; Qayyum, A.; Sher, A. Protective effect of Kickxia ramosissima (Wall.) Janchn extracts against pathogenic bacterial strains and free radicals. Arab. J. Sci. Eng. 2020, 46, 83–91. [Google Scholar] [CrossRef]
- Jan, S.; Khan, M.R. Antipyretic, analgesic and anti-inflammatory effects of Kickxia ramosissima. J. Ethnopharmacol. 2016, 182, 90–100. [Google Scholar] [CrossRef]
- Morteza-Semnani, K.; Saeedi, M.; Akbarzadeh, M. The essential oil composition of Kickxia spuria (L.) Dum. J. Essent. Oil Res. 2008, 20, 24–25. [Google Scholar] [CrossRef]
- Venditti, A.; Frezza, C.; Serafini, I.; Ciccola, A.; Sciubba, F.; Serafini, M.; Bianco, A. Iridoids of chemotaxonomy relevance, a new antirrhinoside ester and other constituents from Kickxia spuria subsp. integrifolia (Brot.) R.Fern. Chem. Biodivers. 2018, 15, e1700473. [Google Scholar] [CrossRef]
- Cheriet, T.; Mancini, I.; Seghiri, R.; Benayache, F.; Benayache, S. Chemical constituents and biological activities of the genus Linaria (Scrophulariaceae). Nat. Prod. Res. 2014, 29, 1589–1613. [Google Scholar] [CrossRef] [PubMed]
- Boulos, L. Flora of Egypt: Vol. 3 (Verbenaceae—Compositae); Al-Hadara Publishing: Cairo, Egypt, 2002. [Google Scholar]
- Ercil, D.; Sakar, M.K.; Del Olmo, E.; San Feliciano, A. Chemical constituents of Linaria aucheri. Turk. J. Chem. 2004, 28, 133–140. [Google Scholar]
- Farid, M.M.; Marzouk, M.M.; El-Shabrawy, M.; Salem, M.A.; Mounier, M.M.; Hussein, S.R. Isoscutellarein 8, 4′-dimethyl ether glycosides as cytotoxic agents and chemotaxonomic markers in Kickxia aegyptiaca. Biocatal. Agric. Biotechnol. 2019, 22, 101431. [Google Scholar] [CrossRef]
- Al-Karmalawy, A.A.; Farid, M.M.; Mostafa, A.; Ragheb, A.Y.; Mahmoud, S.H.; Shehata, M.; Shama, N.M.A.; GabAllah, M.; Mostafa-Hedeab, G.; Marzouk, M.M. Naturally available flavonoid aglycones as potential antiviral drug candidates against SARS-CoV-2. Molecules 2021, 26, 6559. [Google Scholar] [CrossRef]
- Moustafa, S.; Menshawi, B.; Wassel, G.; Mahmoud, K.; Mounier, M. Screening of some wild and cultivated Egyptian plants for their free radical scavenging activity. Int. J. PharmTech Res. 2014, 6, 1271–1278. [Google Scholar]
- El-Hela, A.A.; Abdel-Hady, N.M.; Dawoud, G.; Hamed, A.; Morsy, T.A. Phenolic content, antioxidant potential and Aedes aegyptii ecological friend larvicidal activity of some selected Egyptian plants. J. Egypt. Soc. Parasitol. 2013, 43, 215–234. [Google Scholar]
- Moustafa, S.M.; Menshawi, B.M.; Wassel, G.M.; Mahmoud, K.; Mounier, M.M. Screening of some plants in Egypt for their cytotoxicity against four human cancer cell lines. Int. J. PharmTech Res. 2014, 6, 1074–1084. [Google Scholar]
- Reda, E.H.; Shakour, Z.T.A.; El-Halawany, A.M.; El-Kashoury, E.-S.A.; Shams, K.A.; Mohamed, T.A.; Saleh, I.; Elshamy, A.I.; Atia, M.A.; El-Beih, A.A. Comparative study on the essential oils from five wild Egyptian Centaurea species: Effective extraction techniques, antimicrobial activity and in-silico analyses. Antibiotics 2021, 10, 252. [Google Scholar] [CrossRef]
- Judzentiene, A.; Budiene, J.; Butkiene, R.; Kupcinskiene, E.; Laffont-Schwob, I.; Masotti, V. Caryophyllene oxide-rich essential oils of lithuanian Artemisia campestris ssp. campestris and their toxicity. Nat. Prod. Commun. 2010, 5, 1981–1984. [Google Scholar] [CrossRef] [Green Version]
- Dougnon, G.; Ito, M. Essential oil from the leaves of Chromolaena odorata, and sesquiterpene caryophyllene oxide induce sedative activity in mice. Pharmaceuticals 2021, 14, 651. [Google Scholar] [CrossRef]
- Abd-ElGawad, A.M.; ElShamy, A.I.; Al-Rowaily, S.L.; El-Amier, Y.A. Habitat affects the chemical profile, allelopathy, and antioxidant properties of essential oils and phenolic enriched extracts of the invasive plant Heliotropium curassavicum. Plants 2019, 8, 482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abd-ElGawad, A.; El Gendy, A.; Assaeed, A.; Al-Rowaily, S.; Alharthi, A.; Mohamed, T.; Nassar, M.; Dewir, Y.; Elshamy, A. Phytotoxic effects of plant essential oils: A systematic review and structure-activity relationship based on chemometric analyses. Plants 2020, 10, 36. [Google Scholar] [CrossRef] [PubMed]
- Abd-ElGawad, A.M.; Bonanomi, G.; Al-Rashed, S.A.; Elshamy, A.I. Persicaria lapathifolia essential oil: Chemical constituents, antioxidant activity, and allelopathic effect on the weed Echinochloa colona. Plants 2021, 10, 1798. [Google Scholar] [CrossRef] [PubMed]
- Al-Rowaily, S.L.; Abd-ElGawad, A.M.; Assaeed, A.M.; Elgamal, A.M.; Gendy, A.E.-N.G.E.; Mohamed, T.A.; Dar, B.A.; Mohamed, T.K.; Elshamy, A.I. Essential oil of Calotropis procera: Comparative chemical profiles, antimicrobial activity, and allelopathic potential on weeds. Molecules 2020, 25, 5203. [Google Scholar] [CrossRef]
- Abd-ElGawad, A.M.; Elshamy, A.I.; El-Amier, Y.A.; El Gendy, A.E.-N.G.; Al-Barati, S.A.; Dar, B.A.; Al-Rowaily, S.L.; Assaeed, A. Chemical composition variations, allelopathic, and antioxidant activities of Symphyotrichum squamatum (Spreng.) Nesom essential oils growing in heterogeneous habitats. Arab. J. Chem. 2020, 13, 4237–4245. [Google Scholar] [CrossRef]
- Mehdizadeh, L.; Pirbalouti, A.G.; Moghaddam, M. Storage stability of essential oil of cumin (Cuminum cyminum L.) as a function of temperature. Int. J. Food Prop. 2017, 20, 1742–1750. [Google Scholar] [CrossRef]
- Wanner, J.; Bail, S.; Jirovetz, L.; Buchbauer, G.; Schmidt, E.; Gochev, V.; Girova, T.; Atanasova, T.; Stoyanova, A. Chemical composition and antimicrobial activity of cumin oil (Cuminum cyminum, Apiaceae). Nat. Prod. Commun. 2010, 5, 1355–1358. [Google Scholar] [CrossRef] [Green Version]
- Sharifi, A.; Mohammadzadeh, A.; Salehi, T.Z.; Mahmoodi, P.; Nourian, A. Cuminum cyminum L. essential oil: A promising antibacterial and antivirulence agent against multidrug-resistant Staphylococcus aureus. Front. Microbiol. 2021, 12, 667833. [Google Scholar] [CrossRef]
- Akarchariya, N.; Sirilun, S.; Julsrigival, J.; Chansakaowa, S. Chemical profiling and antimicrobial activity of essential oil from Curcuma aeruginosa Roxb., Curcuma glans K. Larsen & J. Mood and Curcuma cf. xanthorrhiza Roxb. collected in Thailand. Asian Pac. J. Trop. Biomed. 2017, 7, 881–885. [Google Scholar] [CrossRef]
- Nikbakht, M.; Rahimi-Nasrabadi, M.; Ahmadi, F.; Gandomi, H.; Abbaszadeh, S.; Batooli, H. The chemical composition and in vitro antifungal activities of essential oils of five Eucalyptus species. J. Essent. Oil Bear. Plants 2015, 18, 666–677. [Google Scholar] [CrossRef]
- Abd El-Gawad, A.M.A.; Elshamy, A.I.; El Gendy, A.E.; Al-Rowaily, S.L.; Assaeed, A.M. Preponderance of oxygenated sesquiterpenes and diterpenes in the volatile oil constituents of Lactuca serriola L. revealed antioxidant and allelopathic activity. Chem. Biodivers. 2019, 16, e1900278. [Google Scholar] [CrossRef] [PubMed]
- Essa, A.F.; El-Hawary, S.S.; Abd-ElGawad, A.M.; Kubacy, T.M.; El-Khrisy, E.E.A.M.; Elshamy, A.I.; Younis, I.Y. Prevalence of diterpenes in essential oil of Euphorbia mauritanica L.: Detailed chemical profile, antioxidant, cytotoxic and phytotoxic activities. Chem. Biodivers. 2021, 18, e2100238. [Google Scholar] [CrossRef]
- Abdelhameed, M.F.; Asaad, G.F.; Ragab, T.I.M.; Ahmed, R.F.; El Gendy, A.E.-N.G.; El-Rahman, S.S.A.; Elgamal, A.M.; Elshamy, A.I. Oral and topical anti-inflammatory and antipyretic potentialities of Araucaria bidiwillii shoot essential oil and its nanoemulsion in relation to chemical composition. Molecules 2021, 26, 5833. [Google Scholar] [CrossRef]
- Verma, R.S.; Padalia, R.C.; Goswami, P.; Verma, S.K.; Chauhan, A.; Darokar, M.P. Chemical composition and antibacterial activity of foliage and resin essential oils of Araucaria cunninghamii Aiton ex D.Don and Araucaria heterophylla (Salisb.) Franco from India. Ind. Crop. Prod. 2014, 61, 410–416. [Google Scholar] [CrossRef]
- Elshamy, A.I.; Abd-ElGawad, A.M.; El-Amier, Y.A.; El Gendy, A.E.G.; Al-Rowaily, S.L. Interspecific variation, antioxidant and allelopathic activity of the essential oil from three Launaea species growing naturally in heterogeneous habitats in Egypt. Flavour Fragr. J. 2019, 34, 316–328. [Google Scholar] [CrossRef]
- Abd-ElGawad, A.; El Gendy, A.E.-N.; El-Amier, Y.; Gaara, A.; Omer, E.; Al-Rowaily, S.; Assaeed, A.; Al-Rashed, S.; Elshamy, A. Essential oil of Bassia muricata: Chemical characterization, antioxidant activity, and allelopathic effect on the weed Chenopodium murale. Saudi J. Biol. Sci. 2020, 27, 1900–1906. [Google Scholar] [CrossRef] [PubMed]
- Mulyaningsih, S.; Sporer, F.; Zimmermann, S.; Reichling, J.; Wink, M. Synergistic properties of the terpenoids aromadendrene and 1,8-cineole from the essential oil of Eucalyptus globulus against antibiotic-susceptible and antibiotic-resistant pathogens. Phytomedicine 2010, 17, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Tak, J.-H.; Jovel, E.; Isman, M.B. Comparative and synergistic activity of Rosmarinus officinalis L. essential oil constituents against the larvae and an ovarian cell line of the cabbage looper, Trichoplusia ni (Lepidoptera: Noctuidae). Pest Manag. Sci. 2015, 72, 474–480. [Google Scholar] [CrossRef]
- Saha, S.; Walia, S.; Kundu, A.; Sharma, K.; Singh, J.; Tripathi, B.; Raina, A. Compositional and functional difference in cumin (Cuminum cyminum) essential oil extracted by hydrodistillation and SCFE. Cogent Food Agric. 2016, 2, 1143166. [Google Scholar] [CrossRef]
- Karakaya, S.; Yilmaz, S.V.; Özdemir, Ö.; Koca, M.; Pınar, N.M.; Demirci, B.; Yıldırım, K.; Sytar, O.; Turkez, H.; Baser, K.H.C. A caryophyllene oxide and other potential anticholinesterase and anticancer agent in Salvia verticillata subsp. amasiaca (Freyn & Bornm.) Bornm. (Lamiaceae). J. Essent. Oil Res. 2020, 32, 512–525. [Google Scholar] [CrossRef]
- Abd-ElGawad, A.M.; Elgamal, A.M.; Ei-Amier, Y.A.; Mohamed, T.A.; El Gendy, A.E.-N.G.; Elshamy, A.I. Chemical composition, allelopathic, antioxidant, and anti-inflammatory activities of sesquiterpenes rich essential oil of Cleome amblyocarpa Barratte & Murb. Plants 2021, 10, 1294. [Google Scholar] [PubMed]
- Abd El-Gawad, A.M.A.; El-Amier, Y.A.; Bonanomi, G. Essential oil composition, antioxidant and allelopathic activities of Cleome droserifolia (Forssk) Delile. Chem. Biodivers. 2018, 15, e1800392. [Google Scholar] [CrossRef] [PubMed]
- Fayed, E.M.; Abd-ElGawad, A.M.; Elshamy, A.I.; El-Halawany, E.F.; El-Amier, Y.A.E. Essential oil of Deverra tortuosa aerial parts: Detailed chemical profile, allelopathic, antimicrobial, and antioxidant activities. Chem. Biodivers. 2021, 18, e2000914. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Jubeh, B.; Karaman, R. Resistance of Gram-negative bacteria to current antibacterial agents and approaches to resolve it. Molecules 2020, 25, 1340. [Google Scholar] [CrossRef] [Green Version]
- ElShamy, A.; Abd-ElGawad, A.; Mohamed, T.; El Gendy, A.E.; El Aty, A.A.A.; Saleh, I.; Moustafa, M.F.; Hussien, T.A.; Pare, P.W.; Hegazy, M.F. Extraction development for antimicrobial and phytotoxic essential oils from Asteraceae species: Achillea fragrantissima, Artemisia judaica and Tanacetum sinaicum. Flavour Fragr. J. 2021, 36, 352–364. [Google Scholar] [CrossRef]
- Saleh, I.; Abd-ElGawad, A.; El Gendy, A.E.-N.; Abd El Aty, A.; Mohamed, T.; Kassem, H.; Aldosri, F.; ElShamy, A.; Hegazy, M.-E.F. Phytotoxic and antimicrobial activities of Teucrium polium and Thymus decussatus essential oils extracted using hydrodistillation and microwave-assisted techniques. Plants 2020, 9, 716. [Google Scholar] [CrossRef]
- Abd El-Gawad, A.; El Gendy, A.; Elshamy, A.; Omer, E. Chemical composition of the essential oil of Trianthema portulacastrum L. aerial parts and potential antimicrobial and phytotoxic activities of its extract. J. Essent. Oil Bear. Plants 2016, 19, 1684–1692. [Google Scholar] [CrossRef]
- de Sousa, J.P.; de Azerêdo, G.A.; de Araújo Torres, R.; da Silva Vasconcelos, M.A.; da Conceição, M.L.; de Souza, E.L. Synergies of carvacrol and 1,8-cineole to inhibit bacteria associated with minimally processed vegetables. Int. J. Food Microbiol. 2012, 154, 145–151. [Google Scholar] [CrossRef]
- Schmidt, E.; Bail, S.; Friedl, S.M.; Jirovetz, L.; Buchbauer, G.; Wanner, J.; Denkova, Z.; Slavchev, A.; Stoyanova, A.; Geissler, M. Antimicrobial activities of single aroma compounds. Nat. Prod. Commun. 2010, 5, 1365–1368. [Google Scholar] [CrossRef] [Green Version]
- Azaz, D.; Demirci, F.; Satıl, F.; Kürkçüoğlu, M.; Hüsnü, K.; Bașerb, C. Antimicrobial activity of some Satureja essential oils. Z. Nat. C 2002, 57, 817–821. [Google Scholar] [CrossRef]
- Jassal, K.; Kaushal, S.; Rashmi; Rani, R. Antifungal potential of guava (Psidium guajava) leaves essential oil, major compounds: Beta-caryophyllene and caryophyllene oxide. Arch. Phytopathol. Plant Prot. 2021, 54, 2034–2050. [Google Scholar] [CrossRef]
- Ghaffari, T.; Kafil, H.S.; Asnaashari, S.; Farajnia, S.; Delazar, A.; Baek, S.C.; Hamishehkar, H.; Kim, K.H. Chemical composition and antimicrobial activity of essential oils from the aerial parts of Pinus eldarica grown in Northwestern Iran. Molecules 2019, 24, 3203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammed, H.A.; Al-Omar, M.S.; Aly, M.S.A.; Hegazy, M.M. Essential oil constituents and biological activities of the halophytic plants, Suaeda Vermiculata Forssk and Salsola cyclophylla Bakera growing in Saudi Arabia. J. Essent. Oil Bear. Plants 2019, 22, 82–93. [Google Scholar] [CrossRef]
- Kiashi, F.; Hadjiakhoondi, A.; Tofighi, Z.; Khanavi, M.; Ajani, Y.; Koulaei, S.A.; Yassa, N. Compositions of essential oils and some biological properties of Stachys laxa Boiss. & Buhse and S. byzantina K. Koch. Res. J. Pharmacogn. 2021, 8, 5–15. [Google Scholar] [CrossRef]
- Wang, Q.-H.; Xu, Y.-H.; Bao, W.-Q.; Pa, B.-L.; Hao, J.-S. Structure elucidation and antimicrobial activities of five compounds from Artemisia integrifolia L. Z. Nat. C 2019, 74, 275–278. [Google Scholar] [CrossRef]
- Tackholm, V. Students’ Flora of Egypt; Cairo University: Cairo, Egypt, 1974. [Google Scholar]
- Elgamal, A.M.; Ahmed, R.F.; Abd-ElGawad, A.M.; El Gendy, A.E.-N.G.; Elshamy, A.I.; Nassar, M.I. Chemical profiles, anticancer, and anti-aging activities of essential oils of Pluchea dioscoridis (L.) DC. and Erigeron bonariensis L. Plants 2021, 10, 667. [Google Scholar] [CrossRef]
- Miguel, M.G. Antioxidant activity of medicinal and aromatic plants. A review. Flavour Fragr. J. 2010, 25, 291–312. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Lorian, V. Antibiotics in Laboratory Medicine; Lippincott Williams & Wilkins: Baltimore, MD, USA, 2005. [Google Scholar]





| No | Rt a | Conc.% b | Compound | Formula | KI c |
|---|---|---|---|---|---|
| Oxygenated monoterpenes | |||||
| 1 | 6.39 | 1.31 ± 0.03 | α-Terpineol | C10H18O | 1186 |
| 2 | 9.82 | 0.55 ± 0.01 | α-Linalool | C10H18O | 1095 |
| 3 | 11.92 | 21.99 ± 0.21 | Cuminic aldehyde | C10H12O | 1239 |
| 4 | 13.42 | 2.17 ± 0.06 | p-Cymen-7-ol | C10H14O | 1287 |
| 5 | 13.87 | 1.45 ± 0.04 | Carvacrol | C10H14O | 1298 |
| 6 | 16.07 | 1.51 ± 0.04 | Eugenol | C10H12O2 | 1356 |
| 7 | 17.26 | 0.84 ± 0.02 | 10-(acetylmethyl)-3-Carene | C13H20O | 1380 |
| Monoterpene hydrocarbons | |||||
| 8 | 10.82 | 0.63 ± 0.03 | 2-Bornene | C10H16 | 1165 |
| Oxygenated sesquiterpenes | |||||
| 9 | 19.68 | 0.65 ± 0.03 | Neryl acetone | C13H22O | 1436 |
| 10 | 21.81 | 0.25 ± 0.01 | trans-Nerolidol | C15H26O | 1561 |
| 11 | 24.02 | 0.43 ± 0.01 | trans-Sesquisabinene hydrate | C15H26O | 1577 |
| 12 | 24.16 | 0.97 ± 0.02 | Spathulenol | C15H24O | 1577 |
| 13 | 25.38 | 0.95 ± 0.02 | Isoaromadendrene epoxide | C15H24O | 1579 |
| 14 | 24.59 | 17.34 ± 0.11 | Caryophyllene oxide | C15H24O | 1582 |
| 15 | 25.19 | 0.42 ± 0.01 | Carotol | C15H26O | 1594 |
| 16 | 25.58 | 1.24 ± 0.03 | Widdrol | C15H26O | 1599 |
| 17 | 26.8 | 2.70 ± 0.06 | Humulene epoxide | C15H24O | 1608 |
| 18 | 27.05 | 0.43 ± 0.03 | Clov-2-ene-9α-ol | C15H24O | 1616 |
| 19 | 27.53 | 3.84 ± 0.07 | Aromadendrene oxide-(2) | C15H24O | 1631 |
| 20 | 28.14 | 8.51 ± 0.20 | ar-Turmerone | C15H20O | 1669 |
| 22 | 34.21 | 1.85 ± 0.04 | trans-Z-α-Bisabolene epoxide | C15H24O | 1675 |
| 22 | 36.28 | 0.53 ± 0.02 | (E,E)-Farnesyl acetone | C18H30O | 1915 |
| Sesquiterpene hydrocarbons | |||||
| 23 | 17.72 | 1.07 ± 0.03 | Longicyclene | C15H24 | 1374 |
| 24 | 18.24 | 1.97 ± 0.04 | Isocaryophillene | C15H24 | 1408 |
| 25 | 19.42 | 0.39 ± 0.02 | trans-Caryophyllene | C15H24 | 1417 |
| 26 | 20.32 | 0.57 ± 0.01 | β-Farnesene | C15H24 | 1442 |
| 27 | 20.53 | 0.31 ± 0.01 | ar-Curcumene | C15H22 | 1480 |
| 28 | 22.85 | 0.26 ± 0.01 | α-Calacorene | C15H20 | 1544 |
| Oxygenated diterpenes | |||||
| 29 | 29.86 | 0.33 ± 0.02 | trans-Geranylgeraniol | C20H34O | 2201 |
| Carotenoid derived compounds | |||||
| 30 | 12.77 | 1.21 ± 0.04 | dihydroedulan II | C13H22O | 1284 |
| 31 | 13.13 | 0.56 ± 0.02 | Theaspirane A | C13H22O | 1298 |
| 32 | 16.84 | 0.72 ± 0.03 | β-Damascenone | C13H18O | 1384 |
| 33 | 34.02 | 11.74 ± 0.13 | Hexahydrofarnesyl acetone | C18H36O | 1845 |
| Others | |||||
| 34 | 28.24 | 2.32 ± 0.06 | Benzyl acetylacetate | C11H12O3 | 1486 |
| 35 | 32.11 | 0.30 ± 0.01 | n-Octadecyl chloride | C18H57Cl | 1399 |
| 36 | 35.48 | 0.27 ± 0.01 | n-Nonadecane | C17H34O2 | 1900 |
| 37 | 36.77 | 0.78 ± 0.02 | Methyl palmitate | C21H44 | 1921 |
| 38 | 41.77 | 0.93 ± 0.03 | n-Heneicosane | C17H34O2 | 2100 |
| 39 | 43.03 | 0.50 ± 0.02 | 9,12-Octadecadienoic acid | C18H32O2 | 2085 |
| 40 | 44.69 | 0.26 ± 0.01 | 2-Nonadecanone | C19H38O | 2106 |
| 41 | 47.52 | 0.78 ± 0.02 | n-Docosane | C22H46 | 2200 |
| 42 | 48.14 | 0.54 ± 0.01 | n-Tetracosane | C24H50 | 2400 |
| 43 | 57.76 | 0.99 ± 0.04 | n-Octacosane | C28H58 | 2800 |
| Total | 97.36 | ||||
| Microbes | K. aegyptiaca (10 mg mL−1) | MIC (10 mg mL−1) | Cephradin | Tetracycline | Azithromycin | Ampicillin |
|---|---|---|---|---|---|---|
| Gram-negative bacteria | ||||||
| Escherichia coli | 22.04 ± 0.74 C,# | 0.031 | 15.67 ± 0.42 E | 20.11 ± 0.55 B | 18.08 ± 0.44 C | 20.97 ± 0.75 C |
| Pseudomonas aeruginosa | 13.67 ± 0.91 E | 0.044 | 0.00 G | 0.00 E | 12.57 ± 0.31 D | 0.00 F |
| Salmonella typhimurium | 26.08 ± 1.02 A | 0.038 | 0.00 G | 9.47 ± 0.37 D | 0.00 E | 0.00 F |
| Streptococcus epidermis | 0.00 H | 0.00 | 11.05 ± 0.81 F | 21.07 ± 0.98 A | 20.36 ± 0.77 A | 10.57 ± 0.57 D |
| Gram-positive bacteria | ||||||
| Bacillus cereus | 23.11 ± 0.58 B | 0.031 | 19.6 ± 0.43 C | 9.68 ± 0.27 D | 20.15 ± 0.33 A | 6.45 ± 0.36 E |
| Staphylococcus aureus | 16.17 ± 0.51 D | 0.052 | 20.17 ± 0.79 B | 18.51 ± 0.65 C | 20.48 ± 0.49 A | 29.14 ± 1.20 A |
| Staphylococcus haemolyticus | 6.24 ± 0.11 G | 0.562 | 24.17 ± 0.66 A | 20.30 ± 1.01 B | 19.19 ± 0.61 B | 20.95 ± 0.94 C |
| Staphylococcus xylosus | 11.61 ± 0.32 F | 0.092 | 18.34 ± 0.77 D | 18.48 ± 0.88 C | 18.75 ± 0.73 B | 24.66 ± 0.68 B |
| LSD0.05 | 0.51 *** | 0.52 *** | 0.49 *** | 0.45 *** | 0.44 *** | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abd-ElGawad, A.M.; El-Amier, Y.A.; Bonanomi, G.; Gendy, A.E.-N.G.E.; Elgorban, A.M.; Alamery, S.F.; Elshamy, A.I. Chemical Composition of Kickxia aegyptiaca Essential Oil and Its Potential Antioxidant and Antimicrobial Activities. Plants 2022, 11, 594. https://doi.org/10.3390/plants11050594
Abd-ElGawad AM, El-Amier YA, Bonanomi G, Gendy AE-NGE, Elgorban AM, Alamery SF, Elshamy AI. Chemical Composition of Kickxia aegyptiaca Essential Oil and Its Potential Antioxidant and Antimicrobial Activities. Plants. 2022; 11(5):594. https://doi.org/10.3390/plants11050594
Chicago/Turabian StyleAbd-ElGawad, Ahmed M., Yasser A. El-Amier, Giuliano Bonanomi, Abd El-Nasser G. El Gendy, Abdallah M. Elgorban, Salman F. Alamery, and Abdelsamed I. Elshamy. 2022. "Chemical Composition of Kickxia aegyptiaca Essential Oil and Its Potential Antioxidant and Antimicrobial Activities" Plants 11, no. 5: 594. https://doi.org/10.3390/plants11050594