Impact of Harvest Time and Pruning Technique on Total CBD Concentration and Yield of Medicinal Cannabis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cultivation Methods
2.1.1. Harvest Time
2.1.2. Pruning Techniques
2.2. Sampling and Laboratory Analysis
2.3. Calculations
2.4. Statistical Analysis
3. Results and Discussion
3.1. Effect of Harvest Time (HT)
3.1.1. Biomass Accumulation by Harvest Time
3.1.2. Cannabinoid Concentration and Yield by Harvest Time
3.2. Effect of Pruning Technique (PT)
3.2.1. Biomass Allocation by PT
3.2.2. Cannabinoid Concentration and Total CBD Yield by PT
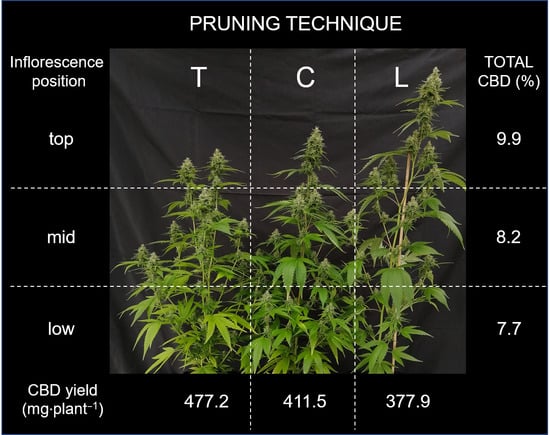
3.2.3. Inflorescence Position by PT—Inner Plant Variability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HT | Harvest Time |
| PT | Pruning technique |
| C | Control |
| T | Topping |
| L | Lollipop |
| DAP | Days after planting |
| THC | Δ9-Tetrahydrocannabinol |
| CBD | Cannabidiol |
| CBDA | Cannabidiolic acid |
| THCA | Δ9-Tetrahydrocannabinolic acid |
| CBGA | Cannabigerolic acid |
| CBNA | Cannabinolic acid |
References
- Prohibition Partners. The Global Cannabis Report 2019; New Frontier Data: Washington, DC, USA, 2019. [Google Scholar]
- Prohibition Partners. CBD—A Supply Overview 2020. Available online: https://prohibitionpartners.com/reports/cbd-a-supply-overview/ (accessed on 30 December 2021).
- Adamek, K.; Jones, A.M.P.; Torkamaneh, D. Accumulation of Somatic Mutations Leads to Genetic Mosaicism in Cannabis. bioRxiv 2021. [Google Scholar] [CrossRef]
- Andre, C.M.; Hausman, J.-F.; Guerriero, G. Cannabis Sativa: The Plant of the Thousand and One Molecules. Front. Plant Sci. 2016, 7, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuardi, A.W. History of Cannabis as a Medicine: A Review. Braz. J. Psychiatry 2006, 28, 153–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jugl, S.; Okpeku, A.; Costales, B.; Morris, E.J.; Alipour-Haris, G.; Hincapie-Castillo, J.M.; Stetten, N.E.; Sajdeya, R.; Keshwani, S.; Joseph, V.; et al. A Mapping Literature Review of Medical Cannabis Clinical Outcomes and Quality of Evidence in Approved Conditions in the USA from 2016 to 2019. MCA 2021, 4, 21–42. [Google Scholar] [CrossRef]
- Whiting, P.F.; Wolff, R.F.; Deshpande, S.; Di Nisio, M.; Duffy, S.; Hernandez, A.V.; Keurentjes, J.C.; Lang, S.; Misso, K.; Ryder, S.; et al. Cannabinoids for Medical Use: A Systematic Review and Meta-Analysis. JAMA 2015, 313, 2456–2473. [Google Scholar] [CrossRef] [PubMed]
- ElSohly, M.A. (Ed.) Marijuana and the Cannabinoids; Forensic Science and Medicine; Humana Press: Totowa, NJ, USA, 2007; ISBN 978-1-58829-456-2. [Google Scholar]
- Jin, D.; Dai, K.; Xie, Z.; Chen, J. Secondary Metabolites Profiled in Cannabis Inflorescences, Leaves, Stem Barks, and Roots for Medicinal Purposes. Sci. Rep. 2020, 10, 3309. [Google Scholar] [CrossRef]
- Esposito, L.G.A.; Overbaugh, E.; Xiong, J.; Rathinasabapathy, T.; Komarnytsky, S.; da Silva, D.J.H.; Esposito, D.A. Immune Responses Are Differentially Regulated by Root, Stem, Leaf, and Flower Extracts of Female and Male CBD Hemp (Cannabis Sativa L.) Plants. Immuno 2021, 1, 369–379. [Google Scholar] [CrossRef]
- European Monitoring Centre for Drugs and Drug Addiction. Medical Use of Cannabis and Cannabinoids: Questions and Answers for Policymaking; Publications Office of the European Union: Lisbon, Portugal, 2018. [Google Scholar]
- Chandra, S.; Lata, H.; ElSohly, M.A.; Walker, L.A.; Potter, D. Cannabis Cultivation: Methodological Issues for Obtaining Medical-Grade Product. Epilepsy Behav. 2017, 70, 302–312. [Google Scholar] [CrossRef]
- Malík, M.; Velechovský, J.; Tlustoš, P. The Overview of Existing Knowledge on Medical Cannabis Plants Growing. Plant Soil Environ. 2021, 67, 425–442. [Google Scholar] [CrossRef]
- Reichel, P.; Munz, S.; Hartung, J.; Präger, A.; Kotiranta, S.; Burgel, L.; Schober, T.; Graeff-Hönninger, S. Impact of Three Different Light Spectra on the Yield, Morphology and Growth Trajectory of Three Different Cannabis Sativa L. Strains. Plants 2021, 10, 1866. [Google Scholar] [CrossRef]
- Burgel, L.; Hartung, J.; Pflugfelder, A.; Graeff-Hönninger, S. Impact of Growth Stage and Biomass Fractions on Cannabinoid Content and Yield of Different Hemp (Cannabis Sativa L.) Genotypes. Agronomy 2020, 10, 372. [Google Scholar] [CrossRef] [Green Version]
- Yang, R.; Berthold, E.C.; McCurdy, C.R.; da Silva Benevenute, S.; Brym, Z.T.; Freeman, J.H. Development of Cannabinoids in Flowers of Industrial Hemp (Cannabis Sativa L.): A Pilot Study. J. Agric. Food Chem. 2020, 68, 6058–6064. [Google Scholar] [CrossRef]
- Shiponi, S.; Bernstein, N. The Highs and Lows of P Supply in Medical Cannabis: Effects on Cannabinoids, the Ionome, and Morpho-Physiology. Front. Plant Sci. 2021, 12, 910. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, N.; Gorelick, J.; Zerahia, R.; Koch, S. Impact of N, P, K, and Humic Acid Supplementation on the Chemical Profile of Medical Cannabis (Cannabis Sativa L). Front. Plant Sci. 2019, 10, 736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aubin, M.-P.; Seguin, P.; Vanasse, A.; Tremblay, G.F.; Mustafa, A.F.; Charron, J.-B. Industrial Hemp Response to Nitrogen, Phosphorus, and Potassium Fertilization. Crop Forage Turfgrass Manag. 2015, 1, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Bevan, L.; Jones, M.; Zheng, Y. Optimisation of Nitrogen, Phosphorus, and Potassium for Soilless Production of Cannabis Sativa in the Flowering Stage Using Response Surface Analysis. Front. Plant Sci. 2021, 12, 2587. [Google Scholar] [CrossRef]
- Danziger, N.; Bernstein, N. Light Matters: Effect of Light Spectra on Cannabinoid Profile and Plant Development of Medical Cannabis (Cannabis Sativa L.). Ind. Crops Prod. 2021, 164, 113351. [Google Scholar] [CrossRef]
- Rodriguez-Morrison, V.; Llewellyn, D.; Zheng, Y. Cannabis Yield, Potency, and Leaf Photosynthesis Respond Differently to Increasing Light Levels in an Indoor Environment. Front. Plant Sci. 2021, 12, 456. [Google Scholar] [CrossRef] [PubMed]
- Westmoreland, F.M.; Kusuma, P.; Bugbee, B. Cannabis Lighting: Decreasing Blue Photon Fraction Increases Yield but Efficacy Is More Important for Cost Effective Production of Cannabinoids. PLoS ONE 2021, 16, e0248988. [Google Scholar] [CrossRef] [PubMed]
- Eichhorn Bilodeau, S.; Wu, B.-S.; Rufyikiri, A.-S.; MacPherson, S.; Lefsrud, M. An Update on Plant Photobiology and Implications for Cannabis Production. Front. Plant Sci. 2019, 10, 296. [Google Scholar] [CrossRef] [PubMed]
- Vanhove, W.; Van Damme, P.; Meert, N. Factors Determining Yield and Quality of Illicit Indoor Cannabis (Cannabis Spp.) Production. Forensic Sci. Int. 2011, 212, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Gorelick, J.; Bernstein, N. Chemical and Physical Elicitation for Enhanced Cannabinoid Production in Cannabis. In Cannabis sativa L.—Botany and Biotechnology; Chandra, S., Lata, H., ElSohly, M.A., Eds.; Springer International Publishing: Basel, Switzerland, 2017; pp. 439–456. ISBN 978-3-319-54564-6. [Google Scholar]
- Caplan, D.; Dixon, M.; Zheng, Y. Increasing Inflorescence Dry Weight and Cannabinoid Content in Medical Cannabis Using Controlled Drought Stress. HortScience 2019, 54, 964–969. [Google Scholar] [CrossRef] [Green Version]
- Danziger, N.; Bernstein, N. Shape Matters: Plant Architecture Affects Chemical Uniformity in Large-Size Medical Cannabis Plants. Plants 2021, 10, 1834. [Google Scholar] [CrossRef] [PubMed]
- Danziger, N.; Bernstein, N. Plant Architecture Manipulation Increases Cannabinoid Standardization in ‘drug-Type’ Medical Cannabis. Ind. Crops Prod. 2021, 167, 113528. [Google Scholar] [CrossRef]
- Madhusoodanan, J. Can Cannabis Go Green? Nature 2019, 572, S8–S9. [Google Scholar] [CrossRef]
- Cervantes, J. Marijuana Horticulture the Indoor/Outdoor Medical Grower’s Bible; Van Patten Publishing: Portland, OR, USA, 2006; ISBN 978-1-878823-23-6. [Google Scholar]
- Janatová, A.; Fraňková, A.; Tlustoš, P.; Hamouz, K.; Božik, M.; Klouček, P. Yield and Cannabinoids Contents in Different Cannabis (Cannabis Sativa L.) Genotypes for Medical Use. Ind. Crops Prod. 2018, 112, 363–367. [Google Scholar] [CrossRef]
- Short, D. Cultivating Exceptional Cannabis: An Expert Breeder Shares His Secrets; Quick Trading Company: Piedmont, CA, USA, 2004; ISBN 978-1-936807-12-3. [Google Scholar]
- Morrow, K. Marijuana Horticulture Fundamentals A Comprehensive Guide to Cannabis Cultivation and Hashish Production; Green Candy Press: San Francisco, CA, USA, 2016; ISBN 978-1-937866-35-8. [Google Scholar]
- De Backer, B.; Maebe, K.; Verstraete, A.G.; Charlier, C. Evolution of the Content of THC and Other Major Cannabinoids in Drug-Type Cannabis Cuttings and Seedlings During Growth of Plants. J. Forensic Sci. 2012, 57, 918–922. [Google Scholar] [CrossRef]
- Grassa, C.J.; Weiblen, G.D.; Wenger, J.P.; Dabney, C.; Poplawski, S.G.; Timothy Motley, S.; Michael, T.P.; Schwartz, C.J. A New Cannabis Genome Assembly Associates Elevated Cannabidiol (CBD) with Hemp Introgressed into Marijuana. New Phytol. 2021, 230, 1665–1679. [Google Scholar] [CrossRef]
- Aizpurua-Olaizola, O.; Soydaner, U.; Öztürk, E.; Schibano, D.; Simsir, Y.; Navarro, P.; Etxebarria, N.; Usobiaga, A. Evolution of the Cannabinoid and Terpene Content during the Growth of Cannabis Sativa Plants from Different Chemotypes. J. Nat. Prod. 2016, 79, 324–331. [Google Scholar] [CrossRef]
- Livingston, S.J.; Quilichini, T.D.; Booth, J.K.; Wong, D.C.J.; Rensing, K.H.; Laflamme-Yonkman, J.; Castellarin, S.D.; Bohlmann, J.; Page, J.E.; Samuels, A.L. Cannabis Glandular Trichomes Alter Morphology and Metabolite Content during Flower Maturation. Plant J. 2020, 101, 37–56. [Google Scholar] [CrossRef] [PubMed]
- Spitzer-Rimon, B.; Duchin, S.; Bernstein, N.; Kamenetsky, R. Architecture and Florogenesis in Female Cannabis Sativa Plants. Front. Plant Sci. 2019, 10, 350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Namdar, D.; Mazuz, M.; Ion, A.; Koltai, H. Variation in the Compositions of Cannabinoid and Terpenoids in Cannabis Sativa Derived from Inflorescence Position along the Stem and Extraction Methods. Ind. Crops Prod. 2018, 113, 376–382. [Google Scholar] [CrossRef]
- Kebrom, T.H. A Growing Stem Inhibits Bud Outgrowth—The Overlooked Theory of Apical Dominance. Front. Plant Sci. 2017, 8, 1874. [Google Scholar] [CrossRef] [PubMed]
- Ambroszczyk, A.M.; Cebula, S.; Sekara, A. The Effect of Plant Pruning on the Light Conditions and Vegetative Development of Eggplant (L.) in Greenhouse Cultivation. J. Fruit Ornam. Plant Res. 2008, 68, 57–70. [Google Scholar] [CrossRef]
- Folina, A.; Kakabouki, I.; Tourkochoriti, E.; Roussis, I.; Pateroulakis, H.; Bilalis, D. Evaluation of the Effect of Topping on Cannabidiol (CBD) Content in Two Industrial Hemp (Cannabis Sativa L.) Cultivars. Bull. UASVM Hortic. 2020, 77, 46–52. [Google Scholar] [CrossRef]
- Jin, D.; Jin, S.; Chen, J. Cannabis Indoor Growing Conditions, Management Practices, and Post-Harvest Treatment: A Review. Am. J. Plant Sci. 2019, 10, 925. [Google Scholar] [CrossRef] [Green Version]
- John, J.A. (Ed.) Row—Column Designs. In Cyclic Designs; Monographs on Statistics and Applied Probability; Springer US: Boston, MA, USA, 1987; pp. 93–115. ISBN 978-1-4899-3326-3. [Google Scholar]
- Wolfinger, R. Covariance Structure Selection in General Mixed Models. Commun. Stat. -Simul. Comput. 1993, 22, 1079–1106. [Google Scholar] [CrossRef]
- Piepho, H.P.; Buchse, A.; Emrich, K. A Hitchhiker’s Guide to Mixed Models for Randomized Experiments. J. Agron. Crop Sci. 2003, 189, 310–322. [Google Scholar] [CrossRef]
- European Monitoring Centre for Drugs and Drug Addiction. Cannabis Production and Markets in Europe; Publications Office: Lisbon, Portugal, 2012. [Google Scholar]
- Hammami, N.; Privé, J.-P.; Joly, D.L.; Moreau, G. Associations between Cannabinoids and Growth Stages of Twelve Industrial Hemp Cultivars Grown Outdoors in Atlantic Canada. Ind. Crops Prod. 2021, 172. [Google Scholar] [CrossRef]
- Ryu, B.R.; Islam, M.J.; Azad, M.O.K.; Go, E.-J.; Rahman, M.H.; Rana, M.S.; Lim, Y.-S.; Lim, J.-D. Conversion Characteristics of Some Major Cannabinoids from Hemp (Cannabis Sativa L.) Raw Materials by New Rapid Simultaneous Analysis Method. Molecules 2021, 26, 4113. [Google Scholar] [CrossRef] [PubMed]
- Tahir, M.N.; Shahbazi, F.; Rondeau-Gagné, S.; Trant, J.F. The biosynthesis of the cannabinoids. J. Cannabis Res. 2021, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Potter, D.J. A Review of the Cultivation and Processing of Cannabis (Cannabis Sativa L.) for Production of Prescription Medicines in the UK. Drug Test. Anal. 2014, 6, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Cline, M.G. Concepts and Terminology of Apical Dominance. Am. J. Bot. 1997, 84, 1064–1069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kocjan Ačko, D.; Flajšman, M.; Trdan, S. Apical Bud Removal Increased Seed Yield in Hemp Cannabis Sativa L. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2019, 69, 317–323. [Google Scholar] [CrossRef]
- Backer, R.; Schwinghamer, T.; Rosenbaum, P.; McCarty, V.; Eichhorn Bilodeau, S.; Lyu, D.; Ahmed, M.B.; Robinson, G.; Lefsrud, M.; Wilkins, O.; et al. Closing the Yield Gap for Cannabis: A Meta-Analysis of Factors Determining Cannabis Yield. Front. Plant Sci. 2019, 10, 495. [Google Scholar] [CrossRef] [Green Version]
- Knight, G.; Hansen, S.; Connor, M.; Poulsen, H.; McGovern, C.; Stacey, J. The Results of an Experimental Indoor Hydroponic Cannabis Growing Study, Using the ‘Screen of Green’ (ScrOG) Method—Yield, Tetrahydrocannabinol (THC) and DNA Analysis. Forensic Sci. Int. 2010, 202, 36–44. [Google Scholar] [CrossRef] [PubMed]



| Harvest Time (Weeks of Flowering) | Cannabinoid Concentration (%) | Total CBD Yield (mg Plant−1) | ||
|---|---|---|---|---|
| CBD | CBDA | Total CBD | ||
| 5 | 0 ± 0 c | 8.73 ± 0.87 | 7.66 ± 0.77 | 415.0 ± 52.9 c |
| 7 | 0.03 ± 0.01 b | 9.91 ± 0.68 | 8.72 ± 0.61 | 785.4 ± 69.2 b |
| 9 | 0.07 ± 0.01 a | 9.87 ± 0.41 | 8.73 ± 0.37 | 1266.6 ± 41.6 a |
| 11 | 0.07 ± 0.01 a | 8.84 ± 0.54 | 7.84 ± 0.48 | 1334.9 ± 127.3 a |
| Source of variation | p-values | |||
| Harvest Time (HT) | <0.0001 | 0.2703 | 0.2767 | <0.0001 |
| Pruning Technique (PT) | 0.1142 | 0.3026 | 0.2972 | 0.0923 |
| HT × PT interactions | 0.5955 | 0.7769 | 0.7842 | 0.6811 |
| Plant Organ | Location | Dry Matter (g·Plant−1) | p-Values for PT | ||
|---|---|---|---|---|---|
| PT | |||||
| C | L | T | |||
| Stems | Total | 4.3 ± 0.4 a | 4.4 ± 0.4 a | 5.5 ± 0.4 a | 0.1005 |
| Main axis | 2.8 ± 0.3 a | 3.1 ± 0.3 a | 2.4 ± 0.3 a | 0.3853 | |
| Side shoots | 1.6 ± 0.2 b | 1.3 ± 0.2 b | 2.9 ± 0.2 a | <0.0001 | |
| Leaves | Total | 7.6 ± 0.4 b | 6.9 ± 0.4 b | 9 ± 0.4 a | 0.0052 |
| Main axis | 2.9 ± 0.1 b | 3.6 ± 0.1 a | 2.6 ± 0.1 b | 0.0013 | |
| Side shoots | 4.7 ± 0.3 b | 3.2 ± 0.3 c | 6.4 ± 0.3 a | <0.0001 | |
| Inflorescences | Total | 16.3 ± 0.6 b | 15.7 ± 0.6 b | 18.5 ± 0.6 a | 0.0117 |
| Main axis | 4.8 ± 0.4 a | 5.4 ± 0.4 a | 4.8 ± 0.4 a | 0.4226 | |
| Side shoots | 11.5 ± 0.6 b | 10.2 ± 0.6 b | 13.7 ± 0.6 a | 0.0036 | |
| Harvest Time (Weeks of Flowering) | Total CBD Yield (mg·Plant−1) | |||
|---|---|---|---|---|
| Means of Harvest Time | PT | |||
| C | L | T | ||
| 5 | 415.0 ± 52.9 c | 517 ± 91.6 | 313.2 ± 91.6 | 414.7 ± 91.6 |
| 7 | 785.4 ± 69.2 b | 835.5 ± 119.9 | 718 ± 119.9 | 802.6 ± 119.9 |
| 9 | 1266.6 ± 41.6 a | 1234.3 ± 72 | 1133.9 ± 72 | 1431.6 ± 72 |
| 11 | 1334.9 ± 127.3 a | 1365.3 ± 220.4 | 1111.4 ± 220.4 | 1528.1 ± 220.4 |
| Source of variation | p-values | |||
| Harvest Time (HT) | <0.0001 | |||
| Pruning Technique (PT) | 0.0923 | |||
| HT × PT interactions | 0.6811 | |||
| Inflorescence Position | Inflorescence Fresh Weight (g·Plant−1) | ||
|---|---|---|---|
| PT | |||
| C | L | T | |
| Top | 23.5 ± 2.4 a B | 33.9 ± 2.4 a | 27.9 ± 2.4 a AB |
| Mid | 30.7 ± 2.4 a AB | 26.7 ± 2.4 a B | 35.9 ± 2.4 a |
| Low | 13.6 ± 2.4 b A | 6.7 ± 2.4 b A | 10.4 ± 2.4 b A |
| Source of variation | p-values | ||
| Pruning Technique (PT) | <0.0001 | ||
| Inflorescence position | 0.2243 | ||
| PT × Inflorescence position interactions | 0.0215 | ||
| Pruning Technique | Total CBD (%) | Total CBD Yield (mg Plant−1) |
|---|---|---|
| C | 8.5 ± 0.6 | 411.5 ± 24.6 ab |
| L | 7.9 ± 0.6 | 377.9 ± 24.6 b |
| T | 9.0 ± 0.6 | 477.2 ± 24.6 a |
| Inflorescence position | ||
| Top | 9.9 ± 0.5 a | 576.5 ± 27.4 a |
| Mid | 8.2 ± 0.5 b | 533.2 ± 27.4 a |
| Low | 7.4 ± 0.5 b | 156.8 ± 27.4 b |
| Source of variation | p-values | |
| Pruning Technique (PT) | 0.5207 | 0.0500 |
| Inflorescence position | 0.0028 | <0.0001 |
| PT × Inflorescence position interactions | 0.7341 | 0.0597 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crispim Massuela, D.; Hartung, J.; Munz, S.; Erpenbach, F.; Graeff-Hönninger, S. Impact of Harvest Time and Pruning Technique on Total CBD Concentration and Yield of Medicinal Cannabis. Plants 2022, 11, 140. https://doi.org/10.3390/plants11010140
Crispim Massuela D, Hartung J, Munz S, Erpenbach F, Graeff-Hönninger S. Impact of Harvest Time and Pruning Technique on Total CBD Concentration and Yield of Medicinal Cannabis. Plants. 2022; 11(1):140. https://doi.org/10.3390/plants11010140
Chicago/Turabian StyleCrispim Massuela, Danilo, Jens Hartung, Sebastian Munz, Federico Erpenbach, and Simone Graeff-Hönninger. 2022. "Impact of Harvest Time and Pruning Technique on Total CBD Concentration and Yield of Medicinal Cannabis" Plants 11, no. 1: 140. https://doi.org/10.3390/plants11010140