Green Synthesis of Metallic Nanoparticles Using Some Selected Medicinal Plants from Southern Africa and Their Biological Applications
Abstract
:1. Introduction
2. MNPs and Their Application
2.1. Silver NPs (AgNPs)
2.2. Gold NPs (AuNPs)
2.3. Metal Oxide NPs
3. Synthesis of MNPs
3.1. The Advent of Green Nanotechnology
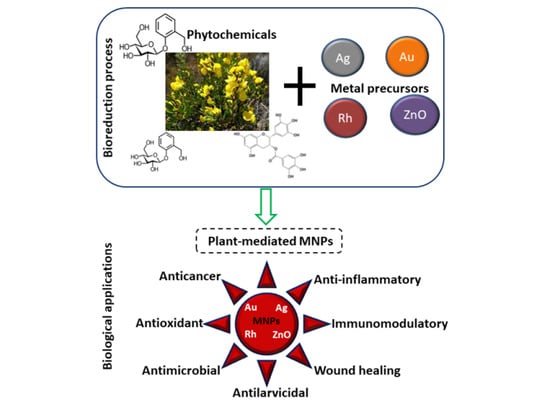
3.2. The Synthesis of Plant-Mediated MNPs
4. Biological Application of MNPs Synthesized from Some Selected South African Medicinal Plants
4.1. South African Medicinal Plant Biodiversity
4.2. MNPs Synthesized from Indigenous SA Plants and Their Application
4.2.1. Cyclopia intermedia
4.2.2. Sutherlandiafructecens
4.2.3. Hypoxis hemerocallidea
4.2.4. Eucomisautumnalis
4.2.5. Plumbago auriculata
4.2.6. Catharanthus roseus
4.2.7. Aspalathus linearis
4.2.8. Indigofera tinctoria
4.2.9. Artemisia herba-alba
4.2.10. Cantella asiatica
4.2.11. Galenia africana
4.2.12. Sclerocarya birrea
4.3. Preclinical and Clinical Application of MNPs
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dikshit, P.; Kumar, J.; Das, A.; Sadhu, S.; Sharma, S.; Singh, S.; Gupta, P.; Kim, B. Green Synthesis of Metallic Nanoparticles: Applications and Limitations. Catalysts 2021, 11, 902. [Google Scholar] [CrossRef]
- Salem, S.S.; Fouda, A. Green Synthesis of Metallic Nanoparticles and Their Prospective Biotechnological Applications: An Overview. Biol. Trace Elem. Res. 2020, 199, 344–370. [Google Scholar] [CrossRef]
- Abdelbasir, S.M.; Shalan, A.E. An overview of nanomaterials for industrial wastewater treatment. Korean J. Chem. Eng. 2019, 36, 1209–1225. [Google Scholar] [CrossRef]
- Patra, J.K.; Baek, K. Green Nanotechnology: Factors Affecting Synthesis and Characterization. J. Nanomater. 2014, 2014, 1417305. [Google Scholar] [CrossRef] [Green Version]
- Ovais, M.; Khalil, A.T.; Ayaz, M.; Ahmad, I.; Nethi, S.K.; Mukherjee, S. Biosynthesis of metal nanoparticles via microbial enzymes: A mechanistic approach. Int. J. Mol. Sci. 2018, 19, 100. [Google Scholar] [CrossRef] [Green Version]
- Dube, P.; Meyer, S.; Madiehe, A.; Meyer, M. Antibacterial activity of biogenic silver and gold nanoparticles synthesized from Salvia africana-lutea and Sutherlandia frutescens. Nanotechnology 2020, 31, 505607. [Google Scholar] [CrossRef]
- Singh, K.; Naidoo, Y.; Mocktar, C.; Baijnath, H. Biosynthesis of silver nanoparticles using Plumbago auriculata leaf and calyx extracts and evaluation of their antimicrobial activities. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018, 9, 035004. [Google Scholar] [CrossRef]
- Elbagory, A.M.; Cupido, C.N.; Meyer, M.; Hussein, A.A. Large scale screening of southern African plant extracts for the green synthesis of gold nanoparticles using microtitre-plate method. Molecules 2016, 21, 1498. [Google Scholar] [CrossRef] [Green Version]
- Bhagyanathan, N.K.; Thoppil, J.E. Plant-mediated synthesis of Silver nanoparticles by two species of Cynanchum L. (Apocynaceae): A comparative approach on its physical characteristics. Int. J. Nano Dimens. 2018, 9, 104–111. [Google Scholar]
- Madkour, L.H. Ecofriendly green biosynthesized of metallic nanoparticles: Bio-reduction mechanism, characterization and pharmaceutical applications in biotechnology industry. Glob. Drugs Ther. 2017, 3, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Bar, H.; Bhui, D.K.; Sahoo, G.P.; Sarkar, P.; De, S.P.; Misra, A. Green synthesis of silver nanoparticles using latex of Jatropha curcas. Colloids Surf. A Physicochem. Eng. Asp. 2009, 339, 134–139. [Google Scholar] [CrossRef]
- Ali, M.A.; Ahmed, T.; Wu, W.; Hossain, A.; Hafeez, R.; Masum, M.M.I.; Wang, Y.; An, Q.; Sun, G.; Li, B. Advancements in plant and microbe-based synthesis of metallic nanoparticles and their antimicrobial activity against plant pathogens. Nanomaterials 2020, 10, 1146. [Google Scholar] [CrossRef]
- Das, R.K.; Pachapur, V.L.; Lonappan, L.; Naghdi, M.; Pulicharla, R.; Maiti, S.; Cledon, M.; Dalila, L.M.A.; Sarma, S.J.; Brar, S.K. Biological synthesis of metallic nanoparticles: Plants, animals and microbial aspects. Nanotechnol. Environ. Eng. 2017, 2, 18. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Singh, P.; Kim, Y.; Zhang, D. Biological Synthesis of Nanoparticles from Plants and Microorganisms Biological Synthesis of Nanoparticles from Plants and Microorganisms. Trends Biotechnol. 2016, 34, 588–599. [Google Scholar] [CrossRef]
- El-Seedi, H.R.; El-Shabasy, R.M.; Khalifa, S.A.M.; Saeed, A.; Shah, A.; Shah, R.; Iftikhar, F.J.; Abdel-Daim, M.M.; Omri, A.; Hajrahand, N.H.; et al. Metal Nanoparticles Fabricated by Green Chemistry Using Natural Extracts: Biosynthesis, Mechanisms, and Applications. RSC Adv. 2019, 9, 24539–24559. [Google Scholar] [CrossRef] [Green Version]
- Ovais, M.; Khalil, A.T.; Islam, N.U.; Ahmad, I.; Ayaz, M.; Saravanan, M.; Shinwari, Z.K.; Mukherjee, S. Role of Plant Phytochemicals and Microbial Enzymes in Biosynthesis of Metallic Nanoparticles. Appl. Microbiol. Biotechnol. 2018, 102, 6799–6814. [Google Scholar] [CrossRef]
- Ahmeda, A.; Zangeneh, A.; Zangeneh, M.M. Green synthesis and chemical characterization of gold nanoparticle synthesized using Camellia sinensis leaf aqueous extract for the treatment of acute myeloid leukemia in comparison to daunorubicin in a leukemic mouse model. Appl. Organomet. Chem. 2020, 34, e5290. [Google Scholar] [CrossRef]
- Ahmed, S.; Saifullah; Ahmad, M.; Swami, B.L.; Ikram, S. Green synthesis of silver nanoparticles using Azadirachta indica aqueous leaf extract. J. Radiat. Res. Appl. Sci. 2016, 9, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Majoumouo, M.S.; Remaliah, N.; Sibuyi, S.; Tincho, M.B.; Boyom, F.F. Synthesis of Biogenic Gold Nanoparticles From. Molecules 2020, 25, 4469. [Google Scholar] [CrossRef] [PubMed]
- Aboyewa, J.A.; Sibuyi, N.R.S.; Meyer, M.; Oguntibeju, O.O. Gold nanoparticles synthesized using extracts of Cyclopia intermedia, commonly known as honeybush, amplify the cytotoxic effects of doxorubicin. Nanomaterials 2021, 11, 132. [Google Scholar] [CrossRef] [PubMed]
- Mahomoodally, M.F. Traditional medicines in Africa: An appraisal of ten potent African medicinal plants. Evid. Based Complement. Altern. Med. 2013, 2013, 617459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mordeniz, C. Introductory Chapter: Traditional and Complementary Medicine. Intech 2019, 395, 116–124. [Google Scholar] [CrossRef] [Green Version]
- Van Wyk, B.E. The potential of South African plants in the development of new medicinal products. S. Afr. J. Bot. 2011, 77, 812–829. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, N.; Mohd, S.; Rizvi, D.; Sahai, N.; Dutta, R. Biosynthesis, Characterization of Gold Nanoparticles Using M. indica Leaf Extract and Their Anticancer Activity. Int. J. Nanomed. 2016, 2, 7–11. [Google Scholar]
- Elbagory, A.M.; Meyer, M.; Cupido, C.N.; Hussein, A.A. Inhibition of Bacteria Associated with Wound Infection by Biocompatible Inhibition of Bacteria Associated with Wound Infection by Biocompatible Green Synthesized Gold Nanoparticles from South African Plant Extracts. Nanomaterials 2017, 7, 417. [Google Scholar] [CrossRef] [Green Version]
- Rajagopal, T.; Ponmanickam, P.; Ayyanar, M. Synthesis of silver nanoparticles using Catharanthus roseus root extract and its larvicidal effects. J. Environ. Biol. 2015, 36, 1283–1289. [Google Scholar]
- Elbagory, M.A.; Hussein, A.A.; Meyer, M. The In Vitro Immunomodulatory Effects Of Gold Nanoparticles Synthesized From Hypoxis hemerocallidea Aqueous Extract And Hypoxoside On Macrophage And Natural Killer Cells. Int. J. Nanomed. 2019, 14, 9007–9018. [Google Scholar] [CrossRef] [Green Version]
- Tyavambiza, C.; Elbagory, A.M.; Madiehe, A.M.; Meyer, M.; Meyer, S. The Antimicrobial and Anti-Inflammatory Effects of Silver Nanoparticles Synthesised from Cotyledon orbiculata Aqueous Extract. Nanomaterials 2021, 11, 1343. [Google Scholar] [CrossRef]
- Ismail, E.; Kenfouch, M.; Dhlamini, M.; Dube, S.; Maaza, M. Green Biosynthesis of Rhodium Nanoparticles Via Aspalathus linearis Natural Extract. J. Nanomater. Mol. Nanotechnol. 2017, 6. [Google Scholar] [CrossRef]
- Castro, L.; Blázquez, M.L.; Ángel Muñoz, J.; González, F.G.; Ballester, A. Mechanism and Applications of Metal Nanoparticles Prepared by Bio-Mediated Process. Rev. Adv. Sci. Eng. 2014, 3, 199–216. [Google Scholar] [CrossRef]
- Mittal, A.K.; Chisti, Y.; Banerjee, U.C. Synthesis of metallic nanoparticles using plant extracts. Biotechnol. Adv. 2013, 31, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.D.; Regulacio, M.D.; Ye, E.; Han, M.Y. Chemical routes to top-down nanofabrication. Chem. Soc. Rev. 2013, 42, 6006–6018. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.F.; Garcia, A.C.; Ferreira, E.B.; Pires, C.; Oliveira, V.L.; Tremiliosi-Filho, G.; Gasparotto, L.H.S. New insights into the formation mechanism of Ag, Au and AgAu nanoparticles in aqueous alkaline media: Alkoxides from alcohols, aldehydes and ketones as universal reducing agents. Phys. Chem. Chem. Phys. 2015, 17, 21683–21693. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Ingle, A.; Gupta, I.; Birla, S.; Yadav, A.; Abd-Elsalam, K. Potential Role of Biological Systems in Formation of Nanoparticles: Mechanism of Synthesis and Biomedical Applications. Curr. Nanosci. 2013, 9, 576–587. [Google Scholar] [CrossRef]
- Qais, F.A.; Shafiq, A.; Khan, H.M.; Husain, F.M.; Khan, R.A.; Alenazi, B.; Alsalme, A.; Ahmad, I. Antibacterial Effect of Silver Nanoparticles Synthesized Using Murraya koenigii (L.) against Multidrug-Resistant Pathogens. Bioinorg. Chem. Appl. 2019, 2019, 4649506. [Google Scholar] [CrossRef] [Green Version]
- Mishra, J.; Drummond, J.; Quazi, S.H.; Karanki, S.S.; Shaw, J.J.; Chen, B.; Kumar, N. Prospective of colon cancer treatments and scope for combinatorial approach to enhanced cancer cell apoptosis. Crit. Rev. Oncol. Hematol. 2013, 86, 232–250. [Google Scholar] [CrossRef] [Green Version]
- Deshmukh, S.P.; Patil, S.M.; Mullani, S.B.; Delekar, S.D. Silver Nanoparticles as an Effective Disinfectant: A Review. Mater. Sci. Eng. C 2019, 97, 954–965. [Google Scholar] [CrossRef]
- Salleh, A.; Naomi, R.; Utami, N.D.; Mohammad, A.W.; Mahmoudi, E.; Mustafa, N.; Fauzi, M.B. The potential of silver nanoparticles for antiviral and antibacterial applications: A mechanism of action. Nanomaterials 2020, 10, 1566. [Google Scholar] [CrossRef]
- Chamakura, K.; Perez-Ballestero, R.; Luo, Z.; Bashir, S.; Liu, J. Comparison of bactericidal activities of silver nanoparticles with common chemical disinfectants. Colloids Surf. B Biointerfaces 2011, 84, 88–96. [Google Scholar] [CrossRef]
- Vo, T.T.; Nguyen, T.T.N.; Huynh, T.T.T.; Vo, T.T.T.; Nguyen, T.T.N.; Nguyen, D.T.; Dang, V.S.; Dang, C.H.; Nguyen, T.D. Biosynthesis of silver and gold nanoparticles using aqueous extract from crinum latifolium leaf and their applications forward antibacterial effect and wastewater treatment. J. Nanomater. 2019, 2019, 8385935. [Google Scholar] [CrossRef] [Green Version]
- Al-Shmgani, H.S.A.; Mohammed, W.H.; Sulaiman, G.M.; Saadoon, A.H. Biosynthesis of silver nanoparticles from Catharanthus roseus leaf extract and assessing their antioxidant, antimicrobial, and wound-healing activities. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1234–1240. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, Y.; Ding, T.; Liu, J.; Zhao, H. Multifunctional Gold Nanoparticles: A Novel Nanomaterial for Various Medical Applications and Biological Activities. Front. Bioeng. Biotechnol. 2020, 8, 990. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, S.; Yasemi, M.; Safaie-Qamsari, E.; Rashidiani, J.; Abkar, M.; Hassani, M.; Mirhosseini, S.A.; Kooshki, H. Using gold nanoparticles in diagnosis and treatment of melanoma cancer. Artif. Cells Nanomed. Biotechnol. 2018, 46, 462–471. [Google Scholar] [CrossRef] [Green Version]
- Singh, P.; Pandit, S.; Mokkapati, V.R.S.S.; Garg, A.; Ravikumar, V.; Mijakovic, I. Gold nanoparticles in diagnostics and therapeutics for human cancer. Int. J. Mol. Sci. 2018, 19, 1979. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, G.M.; Waheeb, H.M.; Jabir, M.S.; Khazaal, S.H.; Dewir, Y.H.; Naidoo, Y. Hesperidin Loaded on Gold Nanoparticles as a Drug Delivery System for a Successful Biocomparticle, Anti-Cancer, Anti-Inflammatory and Phagocytosis Inducer Model. Sci. Rep. 2020, 10, 9362. [Google Scholar] [CrossRef]
- Mugaka, B.P.; Hu, Y.; Ma, Y.; Ding, Y. Surface Modification of Nanoparticles for Targeted Drug Delivery; Springer Nature: Cham, Switzerland, 2019; pp. 391–403. [Google Scholar] [CrossRef]
- Shikha, S.; Chaudhuri, S.R.; Bhattacharyya, M.S. Facile One Pot Greener Synthesis of Sophorolipid Capped Gold Nanoparticles and its Antimicrobial Activity having Special Efficacy Against Gram Negative Vibrio cholerae. Sci. Rep. 2020, 10, 1463. [Google Scholar] [CrossRef]
- Wongyai, K.; Wintachai, P.; Maungchang, R.; Rattanakit, P. Exploration of the Antimicrobial and Catalytic Properties of Gold Nanoparticles Greenly Synthesized by Cryptolepis buchanani Roem. And Schult Extract. J. Nanomater. 2020, 2020, 1320274. [Google Scholar] [CrossRef]
- Katas, H.; Lim, C.S.; Nor Azlan, A.Y.H.; Buang, F.; Mh Busra, M.F. Antibacterial activity of biosynthesized gold nanoparticles using biomolecules from Lignosus rhinocerotis and chitosan. Saudi Pharm. J. 2019, 27, 283–292. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [Green Version]
- Patra, N.; Dehury, N.; Pal, A.; Behera, A.; Patra, S. Preparation and mechanistic aspect of natural xanthone functionalized gold nanoparticle. Mater. Sci. Eng. C 2018, 90, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Kang, M.H.; Qasim, M.; Kim, J.H. Nanoparticle-mediated combination therapy: Two-in-one approach for cancer. Int. J. Mol. Sci. 2018, 19, 3264. [Google Scholar] [CrossRef] [Green Version]
- Sibuyi, N.R.S. Peptide-functionalized nanoparticles for the selective induction of apoptosis in target cells. Nanomedicine 2018, 12, 1631–1645. [Google Scholar] [CrossRef]
- Wang, L.; Xu, J.; Yan, Y.; Liu, H.; Li, F. Synthesis of gold nanoparticles from leaf Panax notoginseng and its anticancer activity in pancreatic cancer PANC-1 cell lines. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1216–1223. [Google Scholar] [CrossRef] [Green Version]
- Anadozie, S.O.; Adewale, O.B.; Meyer, M. In vitro anti-oxidant and cytotoxic activities of gold nanoparticles In vitro anti-oxidant and cytotoxic activities of gold nanoparticles synthesized from an aqueous extract of the Xylopia aethiopica fruit. Nanotechnology 2021, 32, 315101. [Google Scholar]
- Hernández-Hernández, A.A.; Aguirre-Álvarez, G.; Cariño-Cortés, R.; Mendoza-Huizar, L.H.; Jiménez-Alvarado, R. Iron oxide nanoparticles: Synthesis, functionalization, and applications in diagnosis and treatment of cancer. Chem. Pap. 2020, 74, 3809–3824. [Google Scholar] [CrossRef]
- Singh, J.; Dutta, T.; Kim, K.H.; Rawat, M.; Samddar, P.; Kumar, P. “Green” synthesis of metals and their oxide nanoparticles: Applications for environmental remediation. J. Nanobiotechnol. 2018, 16, 84. [Google Scholar] [CrossRef] [PubMed]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Silva, B.F.; Pérez, S.; Gardinalli, P.; Singhal, R.K.; Mozeto, A.A.; Barceló, D. Analytical chemistry of metallic nanoparticles in natural environments. TrAC Trends Anal. Chem. 2011, 30, 528–540. [Google Scholar] [CrossRef]
- Shah, M.; Fawcett, D.; Sharma, S.; Tripathy, S.K. Green Synthesis of Metallic Nanoparticles via Biological Entities Green Synthesis of Metallic Nanoparticles via Biological Entities. Materials 2015, 8, 7278–7308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smitha, S.L.; Philip, D.; Gopchandran, K.G. Green synthesis of gold nanoparticles using Cinnamomum zeylanicum leaf broth. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2009, 74, 735–739. [Google Scholar] [CrossRef]
- Espinosa, J.C.M.; Cerritos, R.C.; Morales, M.A.R.; Guerrero, K.P.S.; Contreras, R.A.S.; Macías, J.H. Characterization of silver nanoparticles obtained by a green route and their evaluation in the bacterium of pseudomonas aeruginosa. Crystals 2020, 10, 395. [Google Scholar] [CrossRef]
- Thakkar, K.N.; Mhatre, S.S.; Parikh, R.Y. Biological synthesis of metallic nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 257–262. [Google Scholar] [CrossRef]
- Ahmad, T.; Bustam, M.A.; Irfan, M.; Moniruzzaman, M.; Asghar, H.M.A.; Bhattacharjee, S. Mechanistic investigation of phytochemicals involved in green synthesis of gold nanoparticles using aqueous Elaeis guineensis leaves extract: Role of phenolic compounds and flavonoids. Biotechnol. Appl. Biochem. 2019, 66, 698–708. [Google Scholar] [CrossRef]
- Philip, D. Rapid green synthesis of spherical gold nanoparticles using Mangifera indica leaf. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2010, 77, 807–810. [Google Scholar] [CrossRef]
- Malik, P.; Shankar, R.; Malik, V.; Sharma, N.; Mukherjee, T.K. Green Chemistry Based Benign Routes for Nanoparticle Synthesis Nanoparticles: A Glance. J. Nanopart. 2014, 2014, 302429. [Google Scholar] [CrossRef] [Green Version]
- Marslin, G.; Siram, K.; Maqbool, Q.; Selvakesavan, R.K.; Kruszka, D.; Kachlicki, P.; Franklin, G. Secondary metabolites in the green synthesis of metallic nanoparticles. Materials 2018, 11, 940. [Google Scholar] [CrossRef] [Green Version]
- Maroyi, A. Diversity of use and local knowledge of wild and cultivated plants in the Eastern Cape province, South Africa. J. Ethnobiol. Ethnomed. 2017, 13, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Street, R.A.; Prinsloo, G. Commercially important medicinal plants of South Africa: A review. J. Chem. 2013, 2013, 205048. [Google Scholar] [CrossRef]
- Van Wyk, A.S.; Prinsloo, G. Medicinal plant harvesting, sustainability and cultivation in South Africa. Biol. Conserv. 2018, 227, 335–342. [Google Scholar] [CrossRef]
- Plants, M.; Van Wyk, B.B.; Van Oudtshoorn, B. Turning folklore into an ethnomedicinal catalogue. S. Afr. J. Sci. 2009, 105, 250. [Google Scholar]
- Singh, S.; Krishna, T.H.A.; Kamalraj, S.; Kuriakose, G.C.; Valayil, J.M.; Jayabaskaran, C. Phytomedicinal importance of Saraca asoca ( Ashoka ): An exciting past, an emerging present and a promising future. Curr. Sci. 2015, 109, 1790–1801. [Google Scholar] [CrossRef] [Green Version]
- Tavakoli, J.; Miar, S.; Zadehzare, M.M.; Akbari, H. Evaluation of effectiveness of herbal medication in cancer care: A review study. Iran. J. Cancer Prev. 2012, 5, 144–156. [Google Scholar] [PubMed]
- Khoobchandani, M.; Katti, K.K.; Karikachery, A.R.; Thipe, V.C.; Srisrimal, D.; Dhurvas Mohandoss, D.K.; Darshakumar, R.D.; Joshi, C.M.; Katti, K.V. New Approaches in Breast Cancer Therapy Through Green Nanotechnology and Nano-Ayurvedic Medicine Pre-Clinical and Pilot Human Clinical Investigations. Int. J. Nanomed. 2020, 15, 181–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Yasiri, A.Y.; Khoobchandani, M.; Cutler, C.S.; Watkinson, L.; Carmack, T.; Smith, C.J.; Kuchuk, M.; Loyalka, S.K.; Lugão, A.B.; Katti, K.V. Mangiferin functionalized radioactive gold nanoparticles (MGF-198AuNPs) in prostate tumor therapy: Green nanotechnology for production,: In vivo tumor retention and evaluation of therapeutic efficacy. Dalton Trans. 2017, 46, 14561–14571. [Google Scholar] [CrossRef] [PubMed]
- Manisha, D.R.; Alwala, J.; Kudle, K.R.; Rudra, M.P.P. Biosynthesis of Silver nanoparticles using flower extracts of Catharanthus roseus and evaluation of its antibacterial efficacy. World J. Pharm. Pharm. Sci. 2014, 3, 669–677. [Google Scholar]
- Thipe, V.C.; Amiri, K.P.; Bloebaum, P.; Karikachery, A.R.; Khoobchandani, M.; Katti, K.K.; Jurisson, S.S.; Katti, K.V. Development of resveratrol-conjugated gold nanoparticles: Interrelationship of increased resveratrol corona on anti-tumor efficacy against breast, pancreatic and prostate cancers. Int. J. Nanomed. 2019, 14, 4413–4428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Journal, A.I.; Hembram, K.C.; Kumar, R.; Kandha, L.; Parhi, P.K.; Kundu, C.N.; Bindhani, B.K. Therapeutic prospective of plant-induced silver nanoparticles: Application as antimicrobial and anticancer agent. Artif. Cells Nanomed. Biotechnol. 2018, 46, S38–S51. [Google Scholar] [CrossRef] [Green Version]
- Ajuwon, O.R.; Ayeleso, A.O.; Adefolaju, G.A. The potential of South African herbal tisanes, rooibos and honeybush in the management of type 2 diabetes mellitus. Molecules 2018, 23, 3207. [Google Scholar] [CrossRef] [Green Version]
- Dube, P.; Meyer, S.; Marnewick, J.L. Antimicrobial and antioxidant activities of different solvent extracts from fermented and green honeybush (Cyclopia intermedia) plant material. S. Afr. J. Bot. 2016, 110, 184–193. [Google Scholar] [CrossRef]
- Aboyade, O.M.; Styger, G.; Gibson, D.; Hughes, G. Sutherlandia frutescens: The meeting of science and traditional knowledge. J. Altern. Complement. Med. 2014, 20, 71–76. [Google Scholar] [CrossRef] [Green Version]
- Mbita, Z.; Ntsendwana, B.; Mathipa, M.M.; Mketo, N. ZnO nanoparticles via Sutherlandia frutescens plant extract: Physical and biological properties ZnO nanoparticles via Sutherlandia frutescens plant extract: Physical and biological properties. Mater. Res. Express 2019, 6, 085006. [Google Scholar]
- Owira, P.M.O.; Ojewole, J.A.O. ‘African Potato’ (Hypoxis hemerocallidea corm): A Plant-Medicine for Modern and 21st Century Diseases of Mankind ? —A Review. Phytother. Res. 2018, 23, 147–152. [Google Scholar] [CrossRef]
- Oguntibeju, O.O.; Meyer, S.; Aboua, Y.G.; Goboza, M. Hypoxis hemerocallidea Significantly Reduced Hyperglycaemia and Hyperglycaemic-Induced Oxidative Stress in the Liver and Kidney Tissues of Streptozotocin-Induced Diabetic Male Wistar Rats. Evid. Based Complement. Altern. Med. 2016, 2016, 8934362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alaribe, F.N.; Maepa, M.J.; Mkhumbeni, N.; Motaung, S.C.K.M. Possible roles of Eucomis autumnalis in bone and cartilage regeneration: A review. Trop. J. Pharm. Res. 2018, 17, 741–749. [Google Scholar] [CrossRef] [Green Version]
- Masondo, N.A.; Aremu, A.O.; Finnie, J.F.; Van Staden, J. Growth and phytochemical levels in micropropagated Eucomis autumnalis subspecies autumnalis using different gelling agents, explant source, and plant growth regulators. In Vitro Cell. Dev. Biol. Plant 2015, 51, 102–110. [Google Scholar] [CrossRef]
- Lediga, M.E.; Malatjie, T.S.; Olivier, D.K.; Ndinteh, D.T.; Vuuren, S.F. Biosynthesis and characterisation of antimicrobial silver nanoparticles from a selection of fever-reducing medicinal plants of South Africa. S. Afr. J. Bot. 2018, 119, 172–180. [Google Scholar] [CrossRef]
- Karishma, S.; Yougasphree, N.; Baijnath, H. A Comprehensive Review on the Genus Plumbago With Focus on Plumbago (Plumbaginaceae). Afr. J.Tradit. Complement. Altern. Med. 2018, 15, 199–215. [Google Scholar]
- Sumsakul, W.; Plengsuriyakarn, T.; Chaijaroenkul, W.; Viyanant, V.; Karbwang, J.; Na-Bangchang, K. Antimalarial activity of plumbagin in vitro and in animal models. BMC Complement. Altern. Med. 2014, 14, 15. [Google Scholar] [CrossRef] [Green Version]
- Oguntibeju, O.O.; Aboua, Y.; Goboza, M. Vindoline—A Natural Product from Catharanthus roseus Reduces Hyperlipidemia and Renal Pathophysiology in Experimental Type 2 Diabetes. Biomedicines 2019, 7, 59. [Google Scholar] [CrossRef] [Green Version]
- Gajalakshmi, S.; Vijayalakshmi, S.; Rajeswari, V. Pharmacological Activities of Catharanthus roseus : A Perspective Review. Int. J. Pharm. Bio Sci. 2013, 4, 431–439. [Google Scholar]
- Goboza, M.; Aboua, Y.G.; Chegou, N.; Oguntibeju, O.O. Biomedicine & Pharmacotherapy Vindoline effectively ameliorated diabetes-induced hepatotoxicity by docking oxidative stress, inflammation and hypertriglyceridemia in type 2 diabetes-induced male Wistar rats. Biomed. Pharmacother. 2019, 112, 108638. [Google Scholar] [CrossRef]
- Joubert, E.; de Beer, D. Phenolic content and antioxidant activity of rooibos food ingredient extracts. J. Food Compos. Anal. 2012, 27, 45–51. [Google Scholar] [CrossRef]
- Joubert, E.; Gelderblom, W.C.A.; Louw, A.; de Beer, D. South African herbal teas: Aspalathus linearis, Cyclopia spp. and Athrixia phylicoides-A review. J. Ethnopharmacol. 2008, 119, 376–412. [Google Scholar] [CrossRef] [PubMed]
- Thipe, V.C.; Njobeh, P.B.; Mhlanga, S.D. Optimization of Commercial Antibiotic Agents Using Gold Nanoparticles Against Toxigenic Aspergillus spp. Mater. Today Proc. 2015, 2, 4136–4148. [Google Scholar] [CrossRef]
- Li, S.; Cunningham, A.B.; Fan, R.; Wang, Y. Identity blues: The ethnobotany of the indigo dyeing by Landian Yao (Iu Mien) in Yunnan, Southwest China. J. Ethnobiol. Ethnomed. 2019, 15, 13. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.R.; Boothapandi, M.; Madhavarani, A. Preliminary Phytochemical Screening and In Vitro Antioxidant Activities of Aqueous Extract of Indigofera tinctoria and Indigofera astragalina. Int. J. Drug Res. Technol. 2014, 4, 46–54. [Google Scholar]
- Vijayan, R.; Joseph, S.; Mathew, B. Indigofera tinctoria leaf extract mediated green synthesis of silver and gold nanoparticles and assessment of their anticancer, antimicrobial, antioxidant and catalytic properties catalytic properties. Artif. Cells Nanomed. Biotechnol. 2018, 46, 861–871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boudjelal, A.; Siracusa, L.; Henchiri, C.; Sarri, M.; Abderrahim, B.; Baali, F.; Ruberto, G. Antidiabetic Effects of Aqueous Infusions of Artemisia herba-alba and Ajuga iva in Alloxan-Induced Diabetic Rats. Planta Med. 2015, 81, 696–704. [Google Scholar] [CrossRef]
- Segal, R.; Feuerstein, I.; Danin, A. Chemotypes of Artemisia herba-alba in Israel based on their sesquiterpene lactone and essential oil constitution. Biochem. Syst. Ecol. 1987, 15, 411–416. [Google Scholar] [CrossRef]
- Thabiani, A.; Ali, M.; Panneerselvam, C.; Murugan, K.; Trivedi, S.; Mahyoub, J.A.; Hassan, M.; Maggi, F.; Sut, S.; Dall, S.; et al. Journal of Photochemistry & Photobiology, B : Biology The desert wormwood (Artemisia herba-alba)—From Arabian folk medicine to a source of green and e ff ective nanoinsecticides against mosquito vectors. J. Photochem. Photobiol. B Biol. 2018, 180, 225–234. [Google Scholar] [CrossRef]
- Orhan, I.E. Centella asiatica (L.) Urban: From traditional medicine to modern medicine with neuroprotective potential. Evid. Based Complement. Altern. Med. 2012, 2012, 946259. [Google Scholar] [CrossRef] [Green Version]
- Rout, A.; Jena, P.K.; Parida, U.K.; Bindhani, B.K. Green synthesis of silver nanoparticles using leaves extract of Centella asiatica L. for studies against human pathogens. Int. J. Pharma Bio Sci. 2013, 4, 661–674. [Google Scholar]
- Ng’uni, T.; Klaasen, J.A.; Fielding, B.C. Acute toxicity studies of the South African medicinal plant Galenia africana. Toxicol. Rep. 2018, 5, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Mativandlela, S.P.N.; Muthivhi, T.; Kikuchi, H.; Oshima, Y.; Hamilton, C.; Hussein, A.A.; Van Der Walt, M.L.; Houghton, P.J.; Lall, N. Antimycobacterial flavonoids from the leaf extract of Galenia africana. J. Nat. Prod. 2009, 72, 2169–2171. [Google Scholar] [CrossRef] [PubMed]
- Mocheki, T.A.; Ligavha-Mbelengwa, M.H.; Tshisikhawe, M.P.; Swelankomo, N.; Tshivhandekano, T.R.; Mokganya, M.G.; Ramovha, L.I.; Masevhe, N.A. Comparative population ecology of Sclerocarya birrea (A. rich.) hochst. subspecies caffra (sond) in two rural villages of limpopo province, South Africa. Pak. J. Bot. 2018, 50, 2339–2345. [Google Scholar]
- Virginie, A.; Dago Pierre, K.; Francois, M.G.; Franck, A.M. Hytochemical Screening of Sclerocarya birrea (Anacardiaceae) and Khaya senegalensis (Meliaceae), Antidiabetic Plants. Int. J. Pharm. Chem. 2016, 2, 1–5. [Google Scholar] [CrossRef]
- Joubert, E.; Joubert, M.E.; Bester, C.; de Beer, D.; De Lange, J.H. Honeybush (Cyclopia spp.): From local cottage industry to global markets—The catalytic and supporting role of research. S. Afr. J. Bot. 2011, 77, 887–907. [Google Scholar] [CrossRef]
- Marnewick, J.L.; van der Westhuizen, F.H.; Joubert, E.; Swanevelder, S.; Swart, P.; Gelderblom, W.C.A. Chemoprotective properties of rooibos (Aspalathus linearis), honeybush (Cyclopia intermedia) herbal and green and black (Camellia sinensis) teas against cancer promotion induced by fumonisin B1 in rat liver. Food Chem. Toxicol. 2009, 47, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Magcwebeba, T.U.; Swart, P.; Swanevelder, S.; Joubert, E.; Gelderblom, W.C.A. In vitro chemopreventive properties of green tea, rooibos and honeybush extracts in skin cells. Molecules 2016, 21, 1622. [Google Scholar] [CrossRef] [Green Version]
- Mills, E.; Cooper, C.; Seely, D.; Kanfer, I. African herbal medicines in the treatment of HIV: Hypoxis and Sutherlandia. An overview of evidence and pharmacology. Nutr. J. 2005, 4, 19. [Google Scholar] [CrossRef] [Green Version]
- Stander, A.; Marais, S.; Stivaktas, V.; Vorster, C.; Albrecht, C.; Lottering, M.L.; Joubert, A.M. In vitro effects of Sutherlandia frutescens water extracts on cell numbers, morphology, cell cycle progression and cell death in a tumorigenic and a non-tumorigenic epithelial breast cell line. J. Ethnopharmacol. 2009, 124, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Chadwick, W.A.; Roux, S.; van de Venter, M.; Louw, J.; Oelofsen, W. Anti-diabetic effects of Sutherlandia frutescens in Wistar rats fed a diabetogenic diet. J. Ethnopharmacol. 2007, 109, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Aremu, O.S.; Ogunleye, T.Q.; Seru, L.K.; Mkhize, Z. Synergistic broad-spectrum antibacterial activity of Hypoxis hemerocallidea-derived silver nanoparticles and streptomycin against respiratory pathobionts. Sci. Rep. 2021, 11, 15222. [Google Scholar] [CrossRef]
- Mishra, J.N.; Verma, N.K. A brief study on Catharanthus roseus : A review A brief study on Catharanthus roseus : A review. Int. J. Res. Pharm. Pharm. Sci. 2017, 2, 20–23. [Google Scholar]
- Tolambiya, P.; Mathur, S. A study on potential phytopharmaceuticals assets in Catharanthus roseus L. (Alba). Int. J. Life Sci. Biotechnol. Pharma Res. 2016, 5, 1–6. [Google Scholar] [CrossRef]
- Mosina, G.K.E.; Maroyi, A.; Potgieter, M.J. Comparative analysis of plant use in peri-urban domestic gardens of the Limpopo Province, South Africa. J. Ethnobiol. Ethnomed. 2014, 10, 35. [Google Scholar] [CrossRef] [Green Version]
- Padmaa Paarakh, M.; Swathi, S.; Taj, T.; Tejashwini, V.; Tejashwini, B. Catharanthus roseus Linn—A Review. Acta Sci. Pharm. Sci. 2019, 3, 19–24. [Google Scholar] [CrossRef]
- Sutrisna, E. Catharanthus roseus (Tapak dara): “A controversial” medicinal plant in Indonesia. Int. J. Res. Ayurveda Pharm. 2015, 6, 630–633. [Google Scholar] [CrossRef]
- Verma, A.K.; Singh, R.R. Induced dwarf mutant in Catharanthus roseus with enhanced antibacterial activity. Indian J. Pharm. Sci. 2010, 72, 655–657. [Google Scholar] [CrossRef] [Green Version]
- Nayak, B.S.; Anderson, M.; Pinto Pereira, L.M. Evaluation of wound-healing potential of Catharanthus roseus leaf extract in rats. Fitoterapia 2007, 78, 540–544. [Google Scholar] [CrossRef]
- Diallo, A.; Beye, A.C.; Doyle, T.B.; Park, E.; Maaza, M. Green synthesis of Co3O4 nanoparticles via Aspalathus linearis: Physical properties. Green Chem. Lett. Rev. 2015, 8, 30–36. [Google Scholar] [CrossRef] [Green Version]
- Purnama, H.; Hidayati, N.; Safitri, D.S.; Rahmawati, S. Effect of initial treatment in the preparation of natural indigo dye from Indigofera tinctoria. AIP Conf. Proc. 2017, 1855, 020022. [Google Scholar] [CrossRef] [Green Version]
- Sharopov, F.; Zhang, H.; Wink, M.; Setzer, W. Aromatic Medicinal Plants from Tajikistan (Central Asia). Medicines 2015, 2, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alzweiri, M.; Al, A.; Mansi, K.; Hudaib, M.; Aburjai, T. Ethnopharmacological survey of medicinal herbs in Jordan, the Northern Badia region. J. Ethnopharmacol. 2011, 137, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Laid, M.; Hegazy, M.E.F.; Ahmed, A.A.; Ali, K.; Belkacemi, D.; Ohta, S. Sesquiterpene lactones from Algerian Artemisia herba-alba. Phytochem. Lett. 2008, 1, 85–88. [Google Scholar] [CrossRef]
- Yogeswaran, L.; Norazzila, O.; Puzi, N.N.A.; Saim, A.; Idrus, R.H. Recent Updates in Neuroprotective and Neuroregenerative Potential of Centella asiatica. Malays. J. Med. Sci. 2016, 23, 4–14. [Google Scholar]
- Somboonwong, J.; Kankaisre, M.; Tantisira, B.; Tantisira, M.H. Wound healing activities of different extracts of Centella asiatica in incision and burn wound models : An experimental animal study. BMC Complement. Altern. Med. 2012, 12, 103. [Google Scholar] [CrossRef] [Green Version]
- Hossain, M.; Polash, S.A.; Takikawa, M.; Shubhra, R.D. Investigation of the Antibacterial Activity and in vivo Cytotoxicity of Biogenic Silver Nanoparticles as Potent Therapeutics. Front. Bioeng. Biotechnol. 2019, 7, 239. [Google Scholar] [CrossRef]
- Velidandi, A.; Dahariya, S.; Pabbathi, N.P.P.; Kalivarathan, D.; Baadhe, R.R. A review on synthesis, applications, toxicity, risk assessment and limitations of plant extracts synthesized silver nanoparticles. NanoWorld J. 2020, 6, 35–60. [Google Scholar] [CrossRef]
- Alphandéry, E. Natural metallic nanoparticles for application in nano-oncology. Int. J. Mol. Sci. 2020, 21, 4412. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, J.; Zeng, J.; Li, Z.; Zuo, H.; Huang, C.; Zhao, X. Nano-gold loaded with resveratrol enhance the anti-hepatoma effect of resveratrol in vitro and in vivo. J. Biomed. Nanotechnol. 2019, 15, 288–300. [Google Scholar] [CrossRef] [PubMed]




| Plant Species | Indigenous Application | Major Phytochemicals | MNPs | MNP Size (nm) | MNP Bio-Activity |
|---|---|---|---|---|---|
| Cyclopia intermedia | Treat constipation, nervousness, cough, eczema, epilepsy and regulate blood pressure [21] | Mangiferin (MGF), isomangiferin, hesperidin and isosakuranetin [78,79] | AuNPs | 20 | Anticancer [20] |
| Sutherlandiafructecens | Treat wounds, cancer, diabetes, skin diseases, rheumatism, urinary tract infection, fever, gonorrhoea, kidney and liver problems [6,80] | Saponins, pinitols, flavonoids, triterpenoids, Cannavanine, cycloartane glycosides, flavonol glycosides, and aminobutyric acid [80] | ZnONPs AgNPs | 5–25 15–20 | Antimicrobial [81] Antibacterial and anticancer [6] |
| Hypoxishemerocallidea | Immune booster, purgative, and laxative tonic Treat tuberculosis, urinary tract infection, infertility, cancer, diabetes, and wounds [82,83] | Sterols, norlignane, daucosterols, stanols, hypoxide, sterolins, and β-sitosterol [82] | AuNPs | 9–27 | Antibacterialand anti-inflammatory [25,27] |
| Eucomisautumnalis | Reduce fever, urinary diseases, stomach, lower backaches, syphilis and sometimes used to induce labour [84,85] | Homoisoflavanones, terpenoids, and diben-α-pyrones [85] | AgNPs | 56 | Antimicrobial [86] |
| Plumbago auriculata | Treat headaches, warts, skin infection, wounds, and fracture [87,88] | Tannins, phenols, alkaloids, saponins, flavonoids, plumbagin, α-amyrin, capensisone, and diomuscinone [87] | AgNPs | 15.22 | Antimicrobial [7] |
| Catharanthus roseus | Treat rheumatism, venereal diseases, skin infections, high blood pressure, and diabetes [89,90] | Vinblastine, deoxyvinblastin, vincoline, cathanranthamine, rosicine, leurosine, vindoline, vincristine [91] | AgNPs AgNPs AgNPs | 49 35.55 6–25 | Antimicrobial and wound healing [41] Larvicidal [26] Antimicrobial [75] |
| Aspalathus linearis | Treat insomnia, stomach cramps, allergies, digestive problems as well as improve appetite [22] | Spalathin, orientin, isoquercitrin, luteolin hyperoside [92,93] | AuNPs RhNPs | 44 1.2 | Antimicrobial [94] |
| Indigofera tinctoria | Epilepsy, asthma, stomach ache, bronchitis, and some skin diseases [95] | Saponins, alkaloids, flavonoids, phenolic compounds [96] | AuNPs | 6–29 | Antibacterial, antifungal, and anticancer [97] |
| Artemisia herba-alba | Treat anorexia, indigestion, and gastrointestinal problems [98,99] | 1,8-cineole, alpha, and beta-thujone, davanone, chrysanthenone, cis-chrysanthenol [98,100,101] | AgNPs | 6–29 | Antibacterial and mosquito repellant [102] |
| Centella asiatica | Treat fever, leprosy, syphilis, tuberculosis, leprosy, asthma, epilepsy, mental disorder, minor wounds Consumed as a vegetable and used as a spice [103] | Triterpenoids, centellose, medacassoside, triaponosides, flavonoid quercetin, rutin, kaemferol, patuletin, apigenin, polyacetylenes, phenolic acids, sterols [103] | AgNPs | 30–50 | Antimicrobial [104] |
| Galenia africana | Treat venereal sores, eye infections, asthma, tuberculosis, cough, wounds, skin infections and relieve toothache [105] | Trihydroxyflavanone, trihydroxychalcone, dihydroxychalcone, trihydroxy-3-methoxychalcone [106] | AuNPs | 9–27 | Antibacterial [25] |
| Sclerocarya birrea | Treat dysentery, rheumatism, malaria, and diarrhea [107,108] | Glucosides, steroids, glycosides, flavonoids, fatty oils, alkaloids, phenols, resins, calcium, phosphorus [107,108] | AgNPs | 112 | Antimicrobial [86] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aboyewa, J.A.; Sibuyi, N.R.S.; Meyer, M.; Oguntibeju, O.O. Green Synthesis of Metallic Nanoparticles Using Some Selected Medicinal Plants from Southern Africa and Their Biological Applications. Plants 2021, 10, 1929. https://doi.org/10.3390/plants10091929
Aboyewa JA, Sibuyi NRS, Meyer M, Oguntibeju OO. Green Synthesis of Metallic Nanoparticles Using Some Selected Medicinal Plants from Southern Africa and Their Biological Applications. Plants. 2021; 10(9):1929. https://doi.org/10.3390/plants10091929
Chicago/Turabian StyleAboyewa, Jumoke A., Nicole R. S. Sibuyi, Mervin Meyer, and Oluwafemi O. Oguntibeju. 2021. "Green Synthesis of Metallic Nanoparticles Using Some Selected Medicinal Plants from Southern Africa and Their Biological Applications" Plants 10, no. 9: 1929. https://doi.org/10.3390/plants10091929