Controlled Release Fertilizers: A Review on Coating Materials and Mechanism of Release
Abstract
:1. Introduction
2. Limitations of Conventional Fertilizers
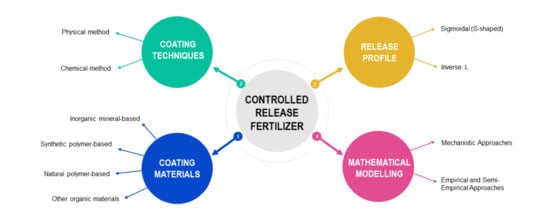
3. Controlled Release Fertilizer (CRF)
3.1. Advantages of CRF
3.2. Disadvantages of CRF
4. Coating Materials
4.1. Inorganic Material-Based Coatings
4.2. Synthetic Polymer-Based Coatings
4.3. Natural Polymer-Based Coatings
4.4. Other Organic Material Coatings
5. Coating Techniques
6. Important Factors Affecting CRFs
6.1. Temperature
6.2. pH
6.3. Ionic Strength
6.4. Granule Radius and Coating Thickness
7. Mechanism of Release
8. Predicting Nutrient Release of CRFs with Modelling
8.1. Mechanistic Approaches
8.2. Empirical and Semi-Empirical Approaches
8.2.1. General Neural Network Model (GRNN)
8.2.2. Higuchi Model
8.2.3. Ritger–Peppas and Korsmeyer–Peppas Models (Power Law)
9. Commercial Uses
10. Conclusions and Future Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- HLEF. Global Agriculture Towards 2050. Available online: http://www.fao.org/fileadmin/templates/wsfs/docs/Issues_papers/HLEF2050_Global_Agriculture.pdf (accessed on 29 April 2020).
- Ain, N.U.; Naveed, M.; Hussain, A.; Mumtaz, M.Z.; Rafique, M.; Bashir, M.A.; Alamri, S.; Siddiqui, M.H. Impact of Coating of Urea with Bacillus-Augmented Zinc Oxide on Wheat Grown under Salinity Stress. Plants 2020, 9, 1375. [Google Scholar] [CrossRef] [PubMed]
- Xiaoyu, N.; Yuejin, W.; Zhengyan, W.; Lin, W.; Guannan, Q.; Lixiang, Y. A novel slow-release urea fertiliser: Physical and chemical analysis of its structure and study of its release mechanism. Biosyst. Eng. 2013, 115, 274–282. [Google Scholar] [CrossRef]
- Versino, F.; Urriza, M.; García, M.A. Eco-compatible cassava starch films for fertilizer controlled-release. Int. J. Biol. Macromol. 2019, 134, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Gil-Ortiz, R.; Naranjo, M.Á.; Ruiz-Navarro, A.; Caballero-Molada, M.; Atares, S.; García, C.; Vicente, O. New Eco-Friendly Polymeric-Coated Urea Fertilizers Enhanced Crop Yield in Wheat. Agronomy 2020, 10, 438. [Google Scholar] [CrossRef] [Green Version]
- Savci, S. Investigation of effect of chemical fertilizers on environment. APCBEE Procedia 2012, 1, 287–292. [Google Scholar] [CrossRef] [Green Version]
- Gil-Ortiz, R.; Naranjo, M.Á.; Ruiz-Navarro, A.; Atares, S.; García, C.; Zotarelli, L.; San Bautista, A.; Vicente, O. Enhanced Agronomic Efficiency Using a New Controlled-Released, Polymeric-Coated Nitrogen Fertilizer in Rice. Plants 2020, 9, 1183. [Google Scholar] [CrossRef]
- Messiga, A.J.; Dyck, K.; Ronda, K.; Van Baar, K.; Haak, D.; Yu, S.; Dorais, M. Nutrients Leaching in Response to Long-Term Fertigation and Broadcast Nitrogen in Blueberry Production. Plants 2020, 9, 1530. [Google Scholar] [CrossRef]
- Fan, L.-T.; Singh, S.K. Introduction. In Controlled Release: A Quantitative Treatment, 1st ed.; Fan, L.-T., Singh, S.K., Eds.; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; Volume 13, pp. 1–8. [Google Scholar]
- Law, J.W.-F.; Ser, H.-L.; Khan, T.M.; Chuah, L.-H.; Pusparajah, P.; Chan, K.-G.; Goh, B.-H.; Lee, L.-H. The potential of Streptomyces as biocontrol agents against the rice blast fungus, Magnaporthe oryzae (Pyricularia oryzae). Front. Microbiol. 2017, 8, 3. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.; Tan, L.T.-H.; Fang, Y.-L.; Jin, Z.-J.; Zhou, L.; Goh, B.-H.; Lee, L.-H.; Zhou, J.; He, Y.-W. Overexpression of oxyR Increases Phenazine-1-Carboxylic Acid Biosynthesis via Small RNA phrS in the Rhizobacterium Strain Pseudomonas PA1201. Mol. Plant Microbe Interact. 2020, 33, 488–498. [Google Scholar] [CrossRef]
- Law, J.W.-F.; Chan, K.-G.; He, Y.-W.; Khan, T.M.; Ab Mutalib, N.-S.; Goh, B.-H.; Lee, L.-H. Diversity of Streptomyces spp. from mangrove forest of Sarawak (Malaysia) and screening of their antioxidant and cytotoxic activities. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Ser, H.-L.; Law, J.W.-F.; Tan, W.-S.; Yin, W.-F.; Chan, K.-G.; Lee, L.-H. Genome sequence of bioactive streptomycete isolated from mangrove forest in East Malaysia, Streptomyces monashensis MUSC 1JT. Prog. Drug Discov. Biomed. Sci. 2019, 2. [Google Scholar] [CrossRef]
- Mangzira Kemung, H.; Tan, L.T.-H.; Chan, K.-G.; Ser, H.-L.; Law, J.W.-F.; Lee, L.-H.; Goh, B.-H. Streptomyces sp. Strain musc 125 from mangrove soil in malaysia with anti-mrsa, anti-biofilm and antioxidant activities. Molecules 2020, 25, 3545. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, M.; Ni, B.; Xie, L. κ-Carrageenan–sodium alginate beads and superabsorbent coated nitrogen fertilizer with slow-release, water-retention, and anticompaction properties. Ind. Chem. Eng. Res. 2012, 51, 1413–1422. [Google Scholar] [CrossRef]
- Cole, J.C.; Smith, M.W.; Penn, C.J.; Cheary, B.S.; Conaghan, K.J. Nitrogen, phosphorus, calcium, and magnesium applied individually or as a slow release or controlled release fertilizer increase growth and yield and affect macronutrient and micronutrient concentration and content of field-grown tomato plants. Sci. Hortic. 2016, 211, 420–430. [Google Scholar] [CrossRef] [Green Version]
- Cong, Z.; Yazhen, S.; Changwen, D.; Jianmin, Z.; Huoyan, W.; Xiaoqin, C. Evaluation of waterborne coating for controlled-release fertilizer using Wurster fluidized bed. Ind. Eng. Chem. Res. 2010, 49, 9644–9647. [Google Scholar] [CrossRef]
- IFA. Executive summary fertilizer outlook 2019–2023. In Proceedings of the IFA Annual Conference, Montreal, QC, Canada, 14–15 May 2019; p. 2. [Google Scholar]
- Wu, Y. Chemical fertilizer use efficiency and its determinants in China’s farming sector. China Agric. Econ. Rev. 2011, 3, 117–130. [Google Scholar] [CrossRef]
- Iftime, M.M.; Ailiesei, G.L.; Ungureanu, E.; Marin, L. Designing chitosan based eco-friendly multifunctional soil conditioner systems with urea controlled release and water retention. Carbohydr. Polym. 2019, 223, 115040. [Google Scholar] [CrossRef]
- Hermida, L.; Agustian, J. Slow release urea fertilizer synthesized through recrystallization of urea incorporating natural bentonite using various binders. Environ. Technol. Inno. 2019, 13, 113–121. [Google Scholar] [CrossRef]
- Ravishankara, A.; Daniel, J.S.; Portmann, R.W. Nitrous oxide (N2O): The dominant ozone-depleting substance emitted in the 21st century. Science 2009, 326, 123–125. [Google Scholar] [CrossRef] [Green Version]
- Trenkel, M.E. Slow-and Controlled-Release and Stabilized Fertilizers: An Option for Enhancing Nutrient Use Efficiency in Agriculture; IFA, International Fertilizer Industry Association: Berlin, Germany, 2010. [Google Scholar]
- Shaviv, A. Controlled release fertilizers. In Proceedings of the IFA International Workshop on Enhanced-Efficiency Fertilizers, Frankfurt, Germany, 28–30 June 2005. [Google Scholar]
- AAPFCO. Official Publication No. 48; Association of American Plant Food Control Officials, Inc.: West Lafayette, IA, USA, 1995. [Google Scholar]
- Shaviv, A. Advances in controlled-release fertilizers. Adv. Agron. 2001, 71, 1–49. [Google Scholar]
- Sempeho, S.I.; Kim, H.T.; Mubofu, E.; Hilonga, A. Meticulous overview on the controlled release fertilizers. Adv. Chem. 2014, 2014, 363071. [Google Scholar] [CrossRef]
- Lammel, J. Cost of the different options available to the farmers: Current situation and prospects. In Proceedings of the IFA International Workshop on Enhanced-Efficiency Fertilizers, Frankfurt, Germany, 28–30 June 2005. [Google Scholar]
- Mumtaz, I.; Majeed, Z.; Ajab, Z.; Ahmad, B.; Khurshid, K.; Mubashir, M. Optimized tuning of rosin adduct with maleic anhydride for smart applications in controlled and targeted delivery of urea for higher plant’s uptake and growth efficiency. Ind. Crops. Prod. 2019, 133, 395–408. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, H.; Wang, X.; Ma, Z.; Li, X.; Li, R.; Li, Q.; Wang, R.; Jia, X. Spout Fluidized Bed Assisted Preparation of Poly (tannic acid)-Coated Urea Fertilizer. ACS Omega 2020, 5, 1127–1133. [Google Scholar] [CrossRef] [Green Version]
- Shi, W.; Ju, Y.; Bian, R.; Li, L.; Joseph, S.; Mitchell, D.R.; Munroe, P.; Taherymoosavi, S.; Pan, G. Biochar bound urea boosts plant growth and reduces nitrogen leaching. Sci. Total Environ. 2020, 701, 134424. [Google Scholar] [CrossRef] [PubMed]
- Vashishtha, M.; Dongara, P.; Singh, D. Improvement in properties of urea by phosphogypsum coating. Int. J. Chemtech. Res. 2010, 2, 36–44. [Google Scholar]
- Ibrahim, K.R.M.; Babadi, F.E.; Yunus, R. Comparative performance of different urea coating materials for slow release. Particuology 2014, 17, 165–172. [Google Scholar] [CrossRef]
- Babadi, F.E.; Yunus, R.; Rashid, S.A.; Salleh, M.A.M.; Ali, S. New coating formulation for the slow release of urea using a mixture of gypsum and dolomitic limestone. Particuology 2015, 23, 62–67. [Google Scholar] [CrossRef]
- Yu, X.; Li, B. Release mechanism of a novel slow-release nitrogen fertilizer. Particuology 2019, 45, 124–130. [Google Scholar] [CrossRef]
- Dixon, J. Roles of clays in soils. Appl. Clay. Sci. 1991, 5, 489–503. [Google Scholar] [CrossRef]
- Kottegoda, N.; Sandaruwan, C.; Priyadarshana, G.; Siriwardhana, A.; Rathnayake, U.A.; Berugoda Arachchige, D.M.; Kumarasinghe, A.r.; Dahanayake, D.; Karunaratne, V.; Amaratunga, G.A. Urea-hydroxyapatite nanohybrids for slow release of nitrogen. ACS Nano 2017, 11, 1214–1221. [Google Scholar] [CrossRef]
- Elhassani, C.E.; Essamlali, Y.; Aqlil, M.; Nzenguet, A.M.; Ganetri, I.; Zahouily, M. Urea-impregnated HAP encapsulated by lignocellulosic biomass-extruded composites: A novel slow-release fertilizer. Environ. Technol. Inno. 2019, 15, 100403. [Google Scholar] [CrossRef]
- Dubey, A.; Mailapalli, D.R. Zeolite coated urea fertilizer using different binders: Fabrication, material properties and nitrogen release studies. Environ. Technol. Inno. 2019, 16, 100452. [Google Scholar] [CrossRef]
- Pereira, E.I.; Da Cruz, C.C.; Solomon, A.; Le, A.; Cavigelli, M.A.; Ribeiro, C. Novel slow-release nanocomposite nitrogen fertilizers: The impact of polymers on nanocomposite properties and function. Ind. Chem. Eng. Res. 2015, 54, 3717–3725. [Google Scholar] [CrossRef]
- Ni, B.; Liu, M.; Lu, S.; Xie, L.; Wang, Y. Environmentally friendly slow-release nitrogen fertilizer. J. Agric. Food. Chem. 2011, 59, 10169–10175. [Google Scholar] [CrossRef] [PubMed]
- Azeem, B.; Kushaari, K.; Man, Z.B.; Basit, A.; Thanh, T.H. Review on materials & methods to produce controlled release coated urea fertilizer. J. Control. Release 2014, 181, 11–21. [Google Scholar]
- Yang, Y.-C.; Zhang, M.; Li, Y.; Fan, X.-H.; Geng, Y.-Q. Improving the quality of polymer-coated urea with recycled plastic, proper additives, and large tablets. J. Agric. Food. Chem. 2012, 60, 11229–11237. [Google Scholar] [CrossRef]
- Li, L.; Sun, Y.; Cao, B.; Song, H.; Xiao, Q.; Yi, W. Preparation and performance of polyurethane/mesoporous silica composites for coated urea. Mater. Des. 2016, 99, 21–25. [Google Scholar] [CrossRef]
- Dai, C.; Yang, L.; Xie, J.; Wang, T.-J. Nutrient diffusion control of fertilizer granules coated with a gradient hydrophobic film. Colloids Surf. A 2020, 588, 124361. [Google Scholar] [CrossRef]
- Emami, N.; Razmjou, A.; Noorisafa, F.; Korayem, A.H.; Zarrabi, A.; Ji, C. Fabrication of smart magnetic nanocomposite asymmetric membrane capsules for the controlled release of nitrate. Environ. Nanotechnol. Monit. Manag. 2017, 8, 233–243. [Google Scholar] [CrossRef]
- Ye, H.-M.; Li, H.-F.; Wang, C.-S.; Yang, J.; Huang, G.; Meng, X.; Zhou, Q. Degradable polyester/urea inclusion complex applied as a facile and environment-friendly strategy for slow-release fertilizer: Performance and mechanism. Chem. Eng. J. 2020, 381, 122704. [Google Scholar] [CrossRef]
- Bi, S.; Barinelli, V.; Sobkowicz, M.J. Degradable Controlled Release Fertilizer Composite Prepared via Extrusion: Fabrication, Characterization, and Release Mechanisms. Polymers 2020, 12, 301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Jia, C.; Zhang, X.; Jiang, Y.; Zhang, M.; Lu, P.; Chen, H. Synthesis and performance of bio-based epoxy coated urea as controlled release fertilizer. Prog. Org. Coat 2018, 119, 50–56. [Google Scholar] [CrossRef]
- Sarkar, K.; Sen, K. Polyvinyl alcohol based hydrogels for urea release and Fe (III) uptake from soil medium. J. Environ. Chem. Eng. 2018, 6, 736–744. [Google Scholar] [CrossRef]
- Chen, S.; Yang, M.; Ba, C.; Yu, S.; Jiang, Y.; Zou, H.; Zhang, Y. Preparation and characterization of slow-release fertilizer encapsulated by biochar-based waterborne copolymers. Sci. Total. Environ. 2018, 615, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Lubkowski, K.; Smorowska, A.; Grzmil, B.; Kozłowska, A. Controlled-release fertilizer prepared using a biodegradable aliphatic copolyester of poly (butylene succinate) and dimerized fatty acid. J. Agric. Food. Chem. 2015, 63, 2597–2605. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Tong, Z.; Geng, Y.; Li, Y.; Zhang, M. Biobased polymer composites derived from corn stover and feather meals as double-coating materials for controlled-release and water-retention urea fertilizers. J. Agric. Food. Chem. 2013, 61, 8166–8174. [Google Scholar] [CrossRef]
- Bortoletto-Santos, R.; Ribeiro, C.; Polito, W.L. Controlled release of nitrogen-source fertilizers by natural-oil-based poly (urethane) coatings: The kinetic aspects of urea release. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Uzoh, C.F.; Onukwuli, O.D.; Ozofor, I.H.; Odera, R.S. Encapsulation of urea with alkyd resin-starch membranes for controlled N2 release: Synthesis, characterization, morphology and optimum N2 release. Process. Saf. Environ. 2019, 121, 133–142. [Google Scholar] [CrossRef]
- Bortoletto-Santos, R.; Cavigelli, M.A.; Montes, S.E.; Schomberg, H.H.; Le, A.; Thompson, A.I.; Kramer, M.; Polito, W.l.; Ribeiro, C. Oil-based polyurethane-coated urea reduces nitrous oxide emissions in a corn field in a Maryland loamy sand soil. J. Clean. Prod. 2020, 249, 119329. [Google Scholar] [CrossRef]
- Liu, L.; Shen, T.; Yang, Y.; Gao, B.; Li, Y.C.; Xie, J.; Tang, Y.; Zhang, S.; Wang, Z.; Chen, J. Bio-based Large Tablet Controlled-Release Urea: Synthesis, Characterization, and Controlled-Released Mechanisms. J. Agric. Food Chem. 2018, 66, 11265–11272. [Google Scholar] [CrossRef] [Green Version]
- Dong Feng, G.; Ma, Y.; Zhang, M.; You Jia, P.; Hong Hu, L.; Guo Liu, C.; Hong Zhou, Y. Polyurethane-coated urea using fully vegetable oil-based polyols: Design, nutrient release and degradation. Prog. Org. Coat 2019, 133, 267–275. [Google Scholar] [CrossRef]
- Liu, J.; Yang, Y.; Gao, B.; Li, Y.C.; Xie, J. Bio-based elastic polyurethane for controlled-release urea fertilizer: Fabrication, properties, swelling and nitrogen release characteristics. J. Clean. Prod. 2019, 209, 528–537. [Google Scholar] [CrossRef]
- Liu, L.; Ni, Y.; Zhi, Y.; Zhao, W.; Pudukudy, M.; Jia, Q.; Shan, S.; Zhang, K.; Li, X. Sustainable and Biodegradable Copolymers from SO2 and Renewable Eugenol: A Novel Urea Fertilizer Coating Material with Superio Slow Release Performance. Macromolecules 2020, 53, 936–945. [Google Scholar] [CrossRef]
- Yang, L.; An, D.; Wang, T.-J.; Kan, C.; Jin, Y. Swelling and diffusion model of a hydrophilic film coating on controlled-release urea particles. Particuology 2017, 30, 73–82. [Google Scholar] [CrossRef]
- Riyajan, S.-A.; Sasithornsonti, Y.; Phinyocheep, P. Green natural rubber-g-modified starch for controlling urea release. Carbohydr. Polym. 2012, 89, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Xiang, Y.; Xu, Y.; Wei, J.; Zhang, Z.; Li, L.; Li, J. Poly-acrylic acid grafted natural rubber for multi-coated slow release compound fertilizer: Preparation, properties and slow-release characteristics. Int. J. Biol. Macromol. 2020, 146, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Rychter, P.; Kot, M.; Bajer, K.; Rogacz, D.; Sišková, A.; Kapuśniak, J. Utilization of starch films plasticized with urea as fertilizer for improvement of plant growth. Carbohydr. Polym. 2016, 137, 127–138. [Google Scholar] [CrossRef]
- Niu, Y.; Li, H. Controlled release of urea encapsulated by starch-g-poly (vinyl acetate). Ind. Eng. Chem. Res. 2012, 51, 12173–12177. [Google Scholar] [CrossRef]
- Giroto, A.S.; Guimarães, G.G.; Colnago, L.A.; Klamczynski, A.; Glenn, G.; Ribeiro, C. Controlled release of nitrogen using urea-melamine-starch composites. J. Clean. Prod. 2019, 217, 448–455. [Google Scholar] [CrossRef]
- Jin, S.; Wang, Y.; He, J.; Yang, Y.; Yu, X.; Yue, G. Preparation and properties of a degradable interpenetrating polymer networks based on starch with water retention, amelioration of soil, and slow release of nitrogen and phosphorus fertilizer. J. Appl. Polym. Sci. 2013, 128, 407–415. [Google Scholar] [CrossRef]
- Xiao, X.; Yu, L.; Xie, F.; Bao, X.; Liu, H.; Ji, Z.; Chen, L. One-step method to prepare starch-based superabsorbent polymer for slow release of fertilizer. Chem. Eng. J. 2017, 309, 607–616. [Google Scholar] [CrossRef] [Green Version]
- Wen, P.; Han, Y.; Wu, Z.; He, Y.; Ye, B.-C.; Wang, J. Rapid synthesis of a corncob-based semi-interpenetrating polymer network slow-release nitrogen fertilizer by microwave irradiation to control water and nutrient losses. Arab. J. Chem. 2017, 10, 922–934. [Google Scholar] [CrossRef]
- Salimi, M.; Motamedi, E.; Motesharezedeh, B.; Hosseini, H.M.; Alikhani, H.A. Starch-g-poly (acrylic acid-co-acrylamide) composites reinforced with natural char nanoparticles toward environmentally benign slow-release urea fertilizers. J. Environ. Chem. Eng. 2020, 8, 103765. [Google Scholar] [CrossRef]
- Qiao, D.; Liu, H.; Yu, L.; Bao, X.; Simon, G.P.; Petinakis, E.; Chen, L. Preparation and characterization of slow-release fertilizer encapsulated by starch-based superabsorbent polymer. Carbohydr. Polym. 2016, 147, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Patil, M.D.; Patil, V.D.; Sapre, A.A.; Ambone, T.A.; Torris At, A.; Shukla, P.G.; Shanmuganathan, K. Tuning controlled release behavior of starch granules using nanofibrillated cellulose derived from waste sugarcane bagasse. ACS Sustain. Chem. Eng. 2018, 6, 9208–9217. [Google Scholar] [CrossRef]
- Xie, L.; Liu, M.; Ni, B.; Wang, Y. Utilization of wheat straw for the preparation of coated controlled-release fertilizer with the function of water retention. J. Agric. Food. Chem. 2012, 60, 6921–6928. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, Q.; Su, Y.; Yue, Q.; Gao, B.; Su, Y. A novel wheat straw cellulose-based semi-IPNs superabsorbent with integration of water-retaining and controlled-release fertilizers. J. Taiwan Inst. Chem. Eng. 2015, 55, 170–179. [Google Scholar] [CrossRef]
- Bortolin, A.; Aouada, F.A.; Mattoso, L.H.; Ribeiro, C. Nanocomposite PAAm/methyl cellulose/montmorillonite hydrogel: Evidence of synergistic effects for the slow release of fertilizers. J. Agric. Food. Chem. 2013, 61, 7431–7439. [Google Scholar] [CrossRef]
- Wen, P.; Wu, Z.; He, Y.; Ye, B.-C.; Han, Y.; Wang, J.; Guan, X. Microwave-assisted synthesis of a semi-interpenetrating polymer network slow-release nitrogen fertilizer with water absorbency from cotton stalks. ACS Sustain. Chem. Eng. 2016, 4, 6572–6579. [Google Scholar] [CrossRef]
- Olad, A.; Zebhi, H.; Salari, D.; Mirmohseni, A.; Tabar, A.R. Slow-release NPK fertilizer encapsulated by carboxymethyl cellulose-based nanocomposite with the function of water retention in soil. Mater. Sci. Eng. C 2018, 90, 333–340. [Google Scholar] [CrossRef]
- Mulder, W.; Gosselink, R.; Vingerhoeds, M.; Harmsen, P.; Eastham, D. Lignin based controlled release coatings. Ind. Crops. Prod. 2011, 34, 915–920. [Google Scholar] [CrossRef]
- Tønnesen, H.H.; Karlsen, J. Alginate in drug delivery systems. Drug Dev. Ind. Pharm. 2002, 28, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Rashidzadeh, A.; Olad, A. Slow-released NPK fertilizer encapsulated by NaAlg-g-poly (AA-co-AAm)/MMT superabsorbent nanocomposite. Carbohydr. Polym. 2014, 114, 269–278. [Google Scholar] [CrossRef] [PubMed]
- De Matos, M.; Mattos, B.D.; Tardy, B.L.; Rojas, o.J.; Magalhães, W.L. Use of Biogenic Silica in Porous Alginate Matrices for Sustainable Fertilization with Tailored Nutrient Delivery. ACS Sustain. Chem. Eng. 2018, 6, 2716–2723. [Google Scholar] [CrossRef]
- Araújo, B.R.; Romão, L.P.; Doumer, M.E.; Mangrich, A.S. Evaluation of the interactions between chitosan and humics in media for the controlled release of nitrogen fertilizer. J. Environ. Manag. 2017, 190, 122–131. [Google Scholar] [CrossRef]
- Huey, C.E.; Yahya, W.Z.N.; Mansor, N. Allicin incorporation as urease inhibitor in a chitosan/starch based biopolymer for fertilizer application. In Proceedings of the Conference on Biomedical and Advanced Materials, The Bayview Hotel Langkawi, Langkawi, Malaysia, 28–29 November 2017; pp. 2187–2196. [Google Scholar]
- Adlim, M.; Zarlaida, F.; Rahmayani, R.F.I.; Wardani, R. Nutrient release properties of a urea–magnesium–natural rubber composite coated with chitosan. Environ. Technol. Innov. 2019, 16, 100442. [Google Scholar] [CrossRef]
- Yang, D.; Yunguo, L.; Shaobo, L.; Huang, x.; Zhongwu, L.; Xiaofei, T.; Guangming, Z.; Lu, Z. Potential benefits of biochar in agricultural soils: A review. Pedosphere 2017, 27, 645–661. [Google Scholar]
- Wen, P.; Wu, Z.; Han, Y.; Cravotto, G.; Wang, J.; Ye, B.-C. Microwave-assisted synthesis of a novel biochar-based slow-release nitrogen fertilizer with enhanced water-retention capacity. ACS Sustain. Chem. Eng. 2017, 5, 7374–7382. [Google Scholar] [CrossRef]
- Sun, Y.-M.; Huang, W.-F.; Chang, C.-C. Spray-coated and solution-cast ethylcellulose pseudolatex membranes. J. Membr. Sci. 1999, 157, 159–170. [Google Scholar] [CrossRef]
- Beig, B.; Niazi, M.B.K.; Jahan, Z.; Hussain, A.; Zia, M.H.; Mehran, M.T. Coating materials for slow release of nitrogen from urea fertilizer: A review. J. Plant. Nutr. 2020, 43, 1510–1533. [Google Scholar] [CrossRef]
- Hede, P.D.; Bach, P.; Jensen, A.D. Fluidized-Bed Coating with Sodium Sulfate and PVA− TiO2, 1. Review and Agglomeration Regime Maps. Ind. Eng. Chem. Res. 2009, 48, 1893–1904. [Google Scholar] [CrossRef]
- Nickerson, M.; Yan, C.; Cloutier, S.; Zhang, W. Protection and masking of omega-3 and-6 oils via microencapsulation. In Microencapsulation in the Food Industry; Gaonkar, A.G., Vasisht, N., Khare, A.R., Sobel, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 485–500. [Google Scholar]
- Teunou, E.; Poncelet, D. Batch and continuous fluid bed coating–review and state of the art. J. Food Eng. 2002, 53, 325–340. [Google Scholar] [CrossRef]
- Popov, B.N. Organic coatings. In Corrosion Engineering: Principles and Solved Problems; Popov, B.N., Ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 557–579. [Google Scholar]
- Uzma, N.; Khaja Mohinuddin Salar, B.; Kumar, B.S.; Aziz, N.; David, M.A.; Reddy, V.D. Impact of organic solvents and environmental pollutants on the physiological function in petrol filling workers. Int. J. Environ. Res. 2008, 5, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Lokensgard, E. Industrial Plastics: Theory and Applications, 6th ed.; Cengage Learning: Farmington Hills, MI, USA, 2016. [Google Scholar]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, S.; Nawwar, G.A.; Sultan, M. Development of bio-based polymeric hydrogel: Green, sustainable and low cost plant fertilizer packaging material. J. Environ. Chem. Eng. 2016, 4, 203–210. [Google Scholar] [CrossRef]
- Du, C.-W.; Zhou, J.-M.; Shaviv, A. Release characteristics of nutrients from polymer-coated compound controlled release fertilizers. J. Polym. Environ. 2006, 14, 223–230. [Google Scholar] [CrossRef]
- Basu, S.; Kumar, N.; Srivastava, J. Modeling NPK release from spherically coated fertilizer granules. Simul. Model. Pract. Theory 2010, 18, 820–835. [Google Scholar] [CrossRef]
- Shaviv, A.; Raban, S.; Zaidel, E. Modeling controlled nutrient release from polymer coated fertilizers: Diffusion release from single granules. Environ. Sci. Technol. 2003, 37, 2251–2256. [Google Scholar] [CrossRef]
- Irfan, S.A.; Razali, R.; Kushaari, K.; Mansor, N.; Azeem, B.; Versypt, A.N.F. A review of mathematical modeling and simulation of controlled-release fertilizers. J. Control. Release 2018, 271, 45–54. [Google Scholar] [CrossRef]
- Bruschi, M.L. Mathematical and physiochemical models of drug release. In Strategies to Modify the Drug Release from Pharmaceutical Systems, 1st ed.; Bruschi, M.L., Ed.; Woodhead Publishing: Sawston/Cambridge, UK, 2015; pp. 63–86. [Google Scholar]
- Jarrell, W.; Boersma, L. Release of urea by granules of sulfur-coated urea. Soil Sci. Soc. Am. J. 1980, 44, 418–422. [Google Scholar] [CrossRef]
- Glasser, V.; Stajer, P.; Vidensky, J.; Svandova, P.; Knor, V. Urea-formaldehyde resins as packaging materials for industrial fertilisers with protracted action. Part 4. Int. Polym. Sci. Technol. 1987, 14, 127. [Google Scholar]
- Lu, S.; Lee, S. Slow release of urea through latex film. J. Control. Release 1992, 18, 171–180. [Google Scholar] [CrossRef]
- Lu, S.; Chang, S.-L.; Ku, W.-Y.; Chang, H.-C.; Wang, J.-Y.; Lee, D.-J. Urea release rate from a scoop of coated pure urea beads: Unified extreme analysis. J. Chin. Inst. Chem. Eng. 2007, 38, 295–302. [Google Scholar] [CrossRef]
- Trinh, T.H.; Kushaari, K.; Basit, A.; Azeem, B.; Shuib, A. Use of multi-diffusion model to study the release of urea from urea fertilizer coated with polyurethane-like coating (PULC). APCBEE Procedia 2014, 8, 146–150. [Google Scholar] [CrossRef] [Green Version]
- Shen, Y.Z.; Du, C.W.; Zhou, J.M.; Ma, F. Modeling Nutrient Release fromSwelling Polymer-Coated Urea. Appl. Eng. Agric. 2015, 31, 247–254. [Google Scholar]
- Shaviv, A.; Raban, S.; Zaidel, E. Modeling controlled nutrient release from a population of polymer coated fertilizers: Statistically based model for diffusion release. Environ. Sci. Technol. 2003, 37, 2257–2261. [Google Scholar] [CrossRef]
- Trinh, T.H.; Kushaari, K.; Shuib, A.A.; Ismail, L.; Azeem, B. Modelling the release of nitrogen from controlled release fertiliser: Constant and decay release. Biosyst. Eng. 2015, 130, 34–42. [Google Scholar] [CrossRef]
- Trinh, T.H.; Kushaari, K.; Basit, A. Modeling the release of nitrogen from controlled-release fertilizer with imperfect coating in soils and water. Ind. Chem. Eng. Res. 2015, 54, 6724–6733. [Google Scholar] [CrossRef]
- Du, C.; Tang, D.; Zhou, J.; Wang, H.; Shaviv, A. Prediction of nitrate release from polymer-coated fertilizers using an artificial neural network model. Biosyst. Eng. 2008, 99, 478–486. [Google Scholar] [CrossRef]
- Ambrose, R.P.K.; Wassgren, C.R.; Pai, D.; Chen, Y. Layer-Wise Agglomerated Urea Granules. U.S. Patent 16/704,342, 11 June 2020. [Google Scholar]
- Adhikari, R.; Muster, T.H.; Freischmidt, G. Controlled Release Granular Fertiliser. U.S. Patent 16/337,608, 30 January 2020. [Google Scholar]
- Li, J.; Jingling, Z.; Song, X.; Ong, C.N.; Loh, C.S.; Tan, W.K. Production of Nutrigel Materials from Soya Waste. U.S. Patent 16/643,177, 20 August 2020. [Google Scholar]
- Kannan, G.; Posada, C.; Haigh, J.; Harper, T.; Kanagalingam, S. Coated Granular Fertilizers, Methods of Manufacture Thereof, and Uses Thereof. U.S. Patent 10,233,133, 19 March 2019. [Google Scholar]
- Flores, J.; Bosley, M.A.; Rifai, S.; Mahoney, R.P.; Hajduk, D.; Casado Portilla, R.; Newton, T.; Soane, D.S. Nontoxic Coating Concentrates for Agricultural Uses. U.S. Patent 16/696,029, 23 July 2020. [Google Scholar]
- Venkatramesh, M.; Kendirgi, F.; Sanders, S.D.; Sanders, A.Z.; Hasinoff, M.P.; Pursell, J.T. Microbial Coating of Controlled-Release Fertilizers. U.S. Patent 16/784,812, 13 August 2020. [Google Scholar]
- Nave, B.; Pasda, G.; Wissemeier, A.; Staal, M.; Schneider, K.-H.; Schmid, M.; Zerulla, W.; Lohe, D.; Zhu, S.S. Mixtures Comprising a Biopesticide and a Nitrification Inhibitor. U.S. Patent 16/630,092, 28 May 2020. [Google Scholar]







| Sulfur-Based | ||||
|---|---|---|---|---|
| Material | Modifier | Research Findings | Release Duration a | References |
| Gypsum | Sulfur/paraffin; ground magnesium lime//polyol | Addition of hydrophobic sealant slows down release but still faster than commercial CRF. | NA | [33,34] |
| Phospho- gypsum | Paraffin wax/Span− 80 | Addition of emulsifier significantly reduces the release rate due to enhanced paraffin adhesion. | 10 days | [35] |
| Mineral-based | ||||
| Hydroxyapatite (HA) | Lignocellulosic biomass | Urea adsorption due to chemical bond with HA results in slow release. It can be further enhanced with the addition of hydrophobic filler. | 5 min–3 days | [37,38] |
| Zeolite | Corn and potato starch, bentonite, white cement, acrylic polymer | Suitable binder type can slow down the release rate. | 8 h (>8 h for acrylic polymer) b | [39] |
| Bentonite | Starch, hydroxypropyl methylcellulose (HPMC); hydrophilic polymer (polyacrylamide); hydrophobic polymer (polycaprolactone) | Nanocomposite provides a superior controlled release. The urea release rate is affected by binder type and slowed down due to adsorption by bentonite. | 2 days | [3,21,40] |
| Attapulgite (APT) | Ethyl cellulose (EC) and sodium carboxymethyl cellulose/hydroxyethyl cellulose hydrogel | Urea release slowed down due to adsorption by APT. Optimum. Carboxymethyl cellulose and hydroxymethyl cellulose (CMC/HEC) and crosslinker content are also important factors. | 5 days | [41] |
| Synthetic Polymer-Based | ||||
|---|---|---|---|---|
| Material | Modifier | Research Findings | Release Duration a | References |
| Poly- styrene | Wax; Polyurethane | Wax is brittle and cannot prevent water penetration into the coating. Increasing size slows down release and reduces coating material required. | 70 days | [43] |
| Poly- urethane (PU) | Mesoporous silica; Hydroxypropyl-terminated Polydimethylsiloxane (HP-PDMS) | Filler morphology affects the release rate. Implementation of hydrophobic gradient layer increases urea diffusion resistance. | 55–70 days | [44,45] |
| Polyether sulfone | Fe2O3 nanoparticles (NPs) | A new class of CRF. Fe2O3 NP increases coating thickness and reduces release rate. It also allows the carrier to be recovered and recycled. | NA | [46] |
| Biodegradable Synthetic Polymer-based | ||||
| Aliphatic Polyester | - | The increasing size of CRF but using smaller urea crystals slows down degradability and release rate. | 1 day | [47,48] |
| Bio-based Epoxy | Different ratio of liquified bagasse (LB) to bisphenol-A diglycidyl ether (BDE) | Optimum BDE amount increases compactness and hydrophobicity and retards release rate. | 10–30 days | [49] |
| Polyvinyl Alcohol | PEG and Na2SO4; biochar | High water swelling rate and only 15–20% release on the first day. Improves water retention in soil and can adsorb Fe(III) ions which reduces toxicity to plants. Biochar improves mechanical strength, degradability and slows down release rate. | >30 days b | [50,51] |
| Natural Polymer-Based | ||||
|---|---|---|---|---|
| Material | Modifier | Research Findings | Release Duration a | References |
| Biobased Polyurethane (PU) | Isocyanate, acrylonitrile modification, superabsorbent from chicken feather meal; nano fumed silica | Double layer polymer coating significantly retards the release rate. Castor oil-based PU has better adherence as the coating material. Nano fumed silica reduces porosity and pore size. Isocyanate affects the structure of PU which affects the release rate. | 14–77 days | [53,54,56,57,58,59] |
| Bio-based modified alkyd resin | Cassava Starch | Using castor oil reduces coating requirement compared to rubber oil. | NA | [55] |
| Polysulfone (SO2 and eugenol based) | - | Increasing Mw of polymer reduces the rate of degradation, slowing down the release. | 3–30 days | [60] |
| Latex | - | Urea content affects swelling degree which greatly affects the release rate. | 30 days | [61] |
| Natural rubber | Cassava starch; attapulgite/NR and NR-g-Polyacrylic acid | Hydrophobic NR can retard release rate with enhanced hydrophilicity through grafting. Multicoated CRF with NR and hydrogel shows great controlled release. | >24 h b | [62,63] |
| Starch | bentonite; cellulose nanofibril from bagasse; natural char NP; bagasse, melamine, polyvinylacetate; EC | Urea can act as a plasticizer. Modification of starch to increase hydrophobicity and the use of reinforcing agent can improve controlled release. Starch-based hydrogel shows excellent water holding capacity and retention in soil. Using an appropriate filler creates interactions which slow down the release. | 6–30 days | [4,64,65,66,67,68,69,70,71,72] |
| Cellulose | Silica NP, bentonite, montmorillonite (MMT) | Incorporation of filler into cellulose-based coating material promotes tortuous path and compactness which slows down diffusion. | 6 days–30 days; >30 days (w/MMT)c | [73,74,75,76,77] |
| Lignin | Alkenyl succinic anhydride | Water-repelling properties shows great potential to retard nutrient release | 10–30 min | [75] |
| Alginate | Κ-Carrageenan/celite superabsorbent; MMT; biogenic silica | Incorporation of filler increases porosity which improves water absorption and slows down the release. | 18–50 days; >60 days (w/MMT) | [15,80,81] |
| Chitosan | Humic substances; starch+allicin; salicylaldehyde; magnesium+natural rubber | Smaller urea crystals can be better encapsulated in the matrix for slow release. Chitosan does not provide strong effects but incorporation with other materials may promote interactions that retard release. | 7–13 days | [20,82,83,84] |
| Other Organic Materials | ||||
|---|---|---|---|---|
| Material | Modifier | Research Findings | Release Duration a | References |
| Biochar | Bentonite, sepiolite | Good urea sorption capability by biochar and mineral binder to slow down the release. | 30 days | [31,86] |
| Rosin Adduct | Maleic anhydride | The effective barrier for urea release due to the covalent bond between maleic anhydride and urea. Works effectively under different soil texture. | 4 days b | [29] |
| Coating Techniques | Advantages | Disadvantages |
|---|---|---|
| Physical Method | ||
| Rotary Drum | • Can be a continuous process, low operating cost, easily scaled | • Requires a large number of materials to achieve a uniform coating |
| Pan Coating | • Can be a continuous process, low operating cost, easily scaled | • High air temperature for drying • Poor maintenance of humidity level results in a defective structure |
| Fluidized Bed | • Can be a continuous process, low operating cost, easily scaled • Can achieve a more uniform coating • A more extensive selection of materials | • Expensive equipment • Long residence time • Prone to filter blocking • Higher chance of solvent explosion • Lower performance with larger granule size |
| Melting and Extrusion | • Solvent-free • Simple and cheap | • Hot melts are involved • Expensive equipment |
| Chemical Method | ||
| Solution Polymerization/crosslinking | • Solvent reduces viscosity which makes it easier to process. • Crosslinking density can be controlled by varying monomer, initiator and cross-linking agent content | • Lower rate of reaction results in possible loss of compound • Difficult to recover solvent from its final form. |
| Inverse Suspension Polymerization | • Crosslinking density can be controlled by varying monomer, initiator and cross-linking agent content • Higher efficiency due to high reaction rate • The solvent can be recovered which reduces the cost | • Prone to contamination by the suspension • Must perform separation to purify polymer |
| Microwave Irradiation | • Simple and low energy consumption | • Not widely implemented in CRF preparation |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lawrencia, D.; Wong, S.K.; Low, D.Y.S.; Goh, B.H.; Goh, J.K.; Ruktanonchai, U.R.; Soottitantawat, A.; Lee, L.H.; Tang, S.Y. Controlled Release Fertilizers: A Review on Coating Materials and Mechanism of Release. Plants 2021, 10, 238. https://doi.org/10.3390/plants10020238
Lawrencia D, Wong SK, Low DYS, Goh BH, Goh JK, Ruktanonchai UR, Soottitantawat A, Lee LH, Tang SY. Controlled Release Fertilizers: A Review on Coating Materials and Mechanism of Release. Plants. 2021; 10(2):238. https://doi.org/10.3390/plants10020238
Chicago/Turabian StyleLawrencia, Dora, See Kiat Wong, Darren Yi Sern Low, Bey Hing Goh, Joo Kheng Goh, Uracha Rungsardthong Ruktanonchai, Apinan Soottitantawat, Learn Han Lee, and Siah Ying Tang. 2021. "Controlled Release Fertilizers: A Review on Coating Materials and Mechanism of Release" Plants 10, no. 2: 238. https://doi.org/10.3390/plants10020238